Abstract
Vasoactive intestinal peptide (VIP) treatment induced mRNA expression of Prolactin (PRL) in the chicken anterior pituitary gland. VIP responsive element (VRE) of the PRL promoter was identified in the various bird species. However, transcription factor, which binds to VRE, has not yet been identified. Prolactin regulatory element-binding protein (PREB) gene cloned as a candidate transcription factor binds to VRE. Increases of mRNA levels of PRL and PREB during embryogenesis were identified. However, whether VIP affects levels of PRL and PREB mRNA during embryogenesis remains unknown. The effects of VIP and forskolin on mRNA expression of PRL and PREB in the embryonic anterior pituitary gland were assessed. Furthermore, administration of VIP to laying hens was conducted to examine the relationship between VIP and PREB mRNA expression. At day 14 of the embryonic growth stage, VIP treatment did not affect mRNA levels of either PRL or PREB, whereas forskolin treatment induced the increase of these mRNA levels. At day 20, both VIP and forskolin induced an increase of PRL and PREB mRNA levels. The administration of VIP significantly increased mRNA levels of PRL and PREB in the anterior pituitary gland of White Leghorn and Nagoya. These results indicate that the effects of VIP on PRL and PREB mRNA expression levels of VIP receptor may in turn affect PRL and PREB mRNA levels in the chicken anterior pituitary gland.
Keywords: forskolin, PREB, PRL, VIP
Introduction
The bird prolactin regulatory element-binding protein (PREB) gene encodes a 1.4-kb transcript, which is translated into a transcription factor and contains three WD motifs (Hiyama et al., 2015, 2016). These WD repeats are considered to be involved in the binding of PREB to the prolactin (PRL) promoter to regulate mRNA expression. PREB was primarily isolated by Southwestern screening on the basis of its binding capacity to a Pit-1 binding site (1P) proximal to PRL promoter in rats (Fliss et al., 1999). Pit-1 plays important roles in the mRNA expression of anterior pituitary hormones, and Pit-1 mutations have been shown to induce a deficiency of PRL-, GH-, and TSH-producing cells, leading to dwarfism (Li et al., 1990; Radovick et al., 1992). Additive effects of Pit-1 and PREB on the rat PRL promoter have been reported (Fliss et al., 1999). This additive effect indicates that Pit-1 and PREB independently exert actions on the 1P element. Savage et al. (2003) suggest the requirement of additional transcription factor other than Pit-1 for high levels of PRL mRNA expression. These results strongly support the function of PREB for the mRNA expression of PRL in the rat anterior pituitary gland.
In chicken, Pit-1 binding sites are located proximal to the promoter of the PRL gene. Vasoactive intestinal polypeptide (VIP) is a physiological releasing factor of PRL in birds (Macnamee et al., 1986), and both plasma PRL and PRL mRNA levels in the anterior pituitary gland are highly correlated to the hypothalamic content of VIP or levels of VIP receptor mRNA in the anterior pituitary gland (Sharp et al., 1989; Chaiseha and El Halawani 1999; Kansaku et al., 2001; Chaiseha et al., 2004). The presence of VIP response element (VRE) near the Pit-1 binding site in the proximal promoter of the PRL gene was originally identified in the turkey (Kang et al., 2004). Conservation of VRE in the proximal promoter of the PRL gene has been reported in various avian species including chicken (Kansaku et al., 2008). Thus, the effects of VIP are generally mediated through VRE. Interestingly, the core sequence of the avian VRE shows high similarity to the PREB binding motif of the mammalian PRL promoter (Hiyama et al., 2009). This similarity may suggest that possibility of binding of avian PREB to the VRE to regulate PRL mRNA expression in anterior pituitary glands.
Changes of mRNA levels of PREB in the anterior pituitary gland during embryogenesis have been shown in chickens and turkeys (Hiyama et al., 2015, 2016). An increase in levels of PREB mRNA has been found to precede those of PRL mRNA during embryogenesis. Moreover, similar changes of PREB mRNA levels to PRL mRNA levels were identified at different reproductive stages (Hiyama et al., 2015). Levels of mRNA of the VIP receptor were reported in the chicken embryonic anterior pituitary gland (Kansaku et al., 2001) and turkey anterior pituitary gland during the reproductive cycle (Chaiseha et al., 2004). Although regulatory mechanism of chicken PREB mRNA expression had not been identified, changes of mRNA levels suggest the functional relationship between VIP and PREB. Because higher contents of VIP were identified in the hypothalamus of incubating stages than laying stages, one could speculate that higher levels of PREB mRNA during the incubation stages are induced by hypothalamic VIP. However, whether VIP affects both PRL and PREB mRNA of the embryonic anterior pituitary gland remains unknown. Since increase of PRL mRNA levels observed both late stage of embryonic day and laying stage to incubation stage of adult hens, investigation of effects of VIP on expression of PRL mRNA and PREB mRNA expression in the anterior pituitary gland may provide possible involvement of PREB for the PRL mRNA expression.
Accordingly, in this study, we aimed to examine the effects of VIP on the mRNA expression of PRL and PREB in the anterior pituitary glands of developing embryos and laying hens. Forskolin was used to test weather VIP induce mRNA expression of PRL and PREB under the low levels of VIP receptor mRNA.
Materials and Methods
Culture of Embryonic Anterior Pituitary Glands
In total, 100 fertilized eggs of White Leghorn hens were obtained from a local hatchery (Chiba Hatchery Co., Togane, Chiba, Japan) and incubated at 37°C in a humidified incubator. Handling of eggs and sampling of embryonic anterior pituitary glands were in accordance with the guidelines for animal experimentation of Azabu University. Following decapitation, anterior pituitary glands were harvested from developing embryos on days 14 and 20. Anterior pituitary glands were primarily incubated in M199 (Life Technologies Corporation, Carlsbad, CA, USA) supplemented with 10 mM HEPES (Wako Pure Chemical Industries Ltd, Osaka) and 0.1% bovine serum albumin (Sigma, St Louis, MO) for 60 min in a humidified incubator (37°C, 5% CO2 and 95% air). After primal incubation, five pituitaries of day 14 embryos (N=4 for each treatment) and three anterior pituitaries of day 20 embryos (N=4 for each treatment) were incubated with synthetic chicken VIP (10−7 M) or forskolin (10−4 M) (Wako) for 60 min at 37°C. After incubation with VIP or forskolin, pituitaries were snap frozen in liquid nitrogen and stored at −80°C until RNA extraction.
VIP Injection to the Laying Hen
Laying White Leghorn hens (106 months old, N=5 for each treatment) and Nagoya hens (87 months old, N=5 for each treatment) from the Institute's flocks of Aichi Agricultural Research Center were maintained in battery cages exposed to 14 h light/day with freely available food and water. Synthetic chicken VIP (75 µg/kg) was intravenously injected. The dose of VIP was chosen based on that previously described by Talbot et al. (1991). Control hens were injected with a saline vehicle. Chickens were killed 60 min after injection for the collection of anterior pituitary glands.
Extraction of RNA from the Anterior Pituitary Gland and Measurement of mRNA
Extraction of total RNA was conducted using a RNA extraction kit (NucleoSpin RNAII, Takara). After measurement of RNA amount by O.D., 1 µg of total RNA was reverse-transcribed and subjected to PCR amplification of PREB, PRL, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Measurement of PRL and PREB mRNA levels was conducted as previously described (Hiyama et al., 2015). Fragments of PREB cDNA from the anterior pituitary gland of embryos and laying hens were amplified by 28 and 25 cycles of PCR, respectively. The amplification profile of PREB was 2 min denaturation at 94°C for the first cycle and 20 s per cycle thereafter, 30 s annealing at 58°C, 30 s extension at 68°C for the first 25 or 28 cycles, and 5 min extension on the final cycle. The amplification profiles of PRL and GAPDH were 2 min denaturation at 94°C for the first cycle and 20 s per cycle thereafter, 30 s annealing at 59°C, and 30 s extension at 72°C for the first 24 cycles, and 5 min extension on the final cycle. After electrophoresis of PCR products, the signal intensity of each PCR product was determined using a LAS4000 imaging system (Ge Healthcare, Little Chalfont, United Kingdom). Levels of mRNA of PREB and PRL were expressed as values relative to the signal intensity of GAPDH.
Analysis of Results
The proportions of PREB and PRL to GAPDH were analyzed by one-way analysis of variance. The significance of the differences between means was assessed using a least-significant difference test. Statistical analyses were conducted using the commercially available package of the Statistical Analysis System (SAS Institute) software. P<0.05 was considered significant.
Results and Discussion
Levels of mRNA of PRL and PREB in the anterior pituitary gland of the day 14 embryos did not change by VIP stimulation; however, those of the day 20 embryos showed a significant increase compared with the control group. On the other hand, forskolin stimulation induced increases in the mRNA levels of PRL and PREB in both day 14 and 20 embryos (Fig. 1). Levels of mRNAs of PRL and PREB of the laying hens from both White Leghorn and Nagoya were significantly increased by VIP injection (Fig. 2).
Fig. 1.
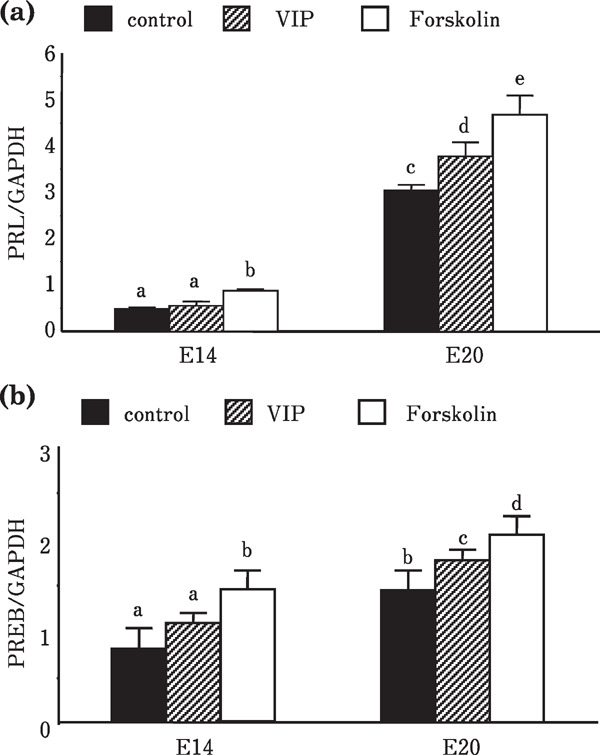
Effects of VIP and forskolin treatment on the expression of PRL and PREB mRNAs of the embryonic anterior pituitary gland. (a) PRL mRNA and (b) PREB mRNA.
Fig. 2.
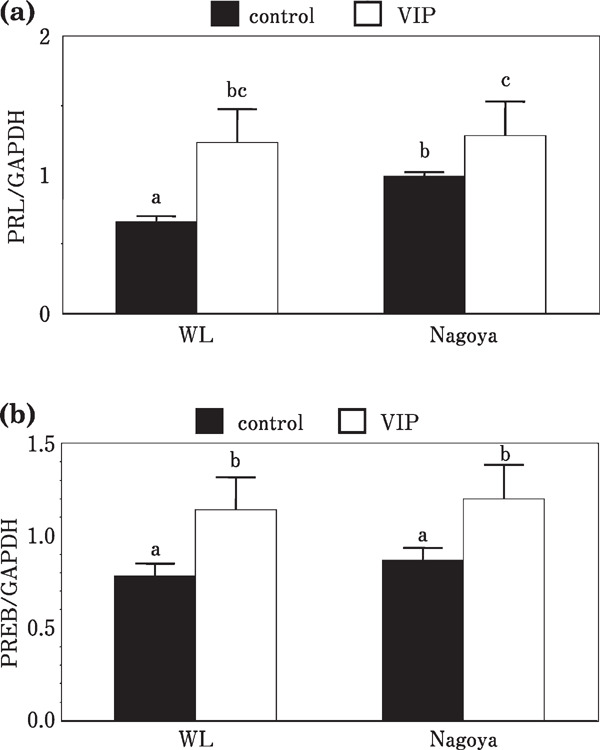
Effects of VIP injection on the expression of PRL and PREB mRNAs of the anterior pituitary gland of laying hens. (a) PRL mRNA and (b) PREB mRNA.
The effects of VIP on the expression of PRL and PREB mRNA may provide interesting relation of transcription factor and target hormone gene. The effects of VIP on PRL secretion from the anterior pituitary gland are well documented in various adult birds (Lea and Vowles, 1986; Macnamee et al., 1986; Mauro et al., 1989; Cloues et al., 1990; Talbot et al., 1991; Vleck and Patrick, 1999). Moreover, the effect of VIP on PRL secretion and PRL cell differentiation has been reported (Woods and Porter 1998). However, there are only few studies that show the effects on the expression of PRL mRNA in the adult anterior pituitary gland (Talbot et al., 1991; Kansaku et al., 1995, 1998). Thus, whether VIP induces the expression of PRL mRNA in the embryonic anterior pituitary gland remains unclear. In this study, levels of PRL mRNA from day 14 embryos were not changed by VIP stimulation; however, they significantly increased in day 20 embryos. Acquirement of responsiveness to VIP is partially explained by changes in the levels of VIP receptor mRNA. Comparison of mRNA levels of VIP receptor was conducted. Significantly, higher mRNA levels of VIP receptor were detected by PCR analysis from the day 20 of embryo compare to day 14 of embryo (data not shown). Thus, responsiveness to VIP may be highly related to the expression of VIP receptor mRNA.
The response by the anterior pituitary gland of the day 20 embryos on forskolin treatment observed in this study is consistent with that reported in previous studies. Increase of PRL mRNA levels by forskolin was observed in the perifused anterior pituitary gland of chickens (Kansaku et al., 1995). Culture of anterior pituitary cells with VIP was found to increase intra-cellular cAMP levels (Kansaku et al., 1998). Because forskolin activates adenylate cyclase (Hudson and Fain, 1983), increase of mRNA levels may be induced by increased intra-cellular cAMP, which is produced by the activation of adenylate cyclase. Interestingly, forskolin stimulation induced the mRNA expression of PRL and PREB in day 14 of embryonic pituitary gland development. This response to forskolin stimulation may be explained by the presence of transcription factor. In fact, the expression of Pit-1 and PREB in day 14 of embryonic anterior pituitary gland has been reported (Hiyama et al., 2015). The response to forskolin exhibited by the day 14 embryos may indicate that a minimum set of factors, such as PREB and Pit-1, induce the PRL mRNA expression when cellular cAMP levels were increased or PKA was activated.
Another important observation was changes in the levels of PREB mRNA by VIP stimulation and/or injection. Because the regulatory region of the chicken PREB gene had not been cloned, the detailed mechanism for PREB mRNA expression had not been identified. However, the effects of VIP stimulation on the expression of PREB mRNA at least partially support the changes of mRNA levels at different reproductive stages. Levels of PREB mRNA were found to increase between laying and incubating stages in the Silkie hens (Hiyama et al., 2015). The changes of hypothalamic VIP contents during reproductive cycles are well known in chicken and turkey (Sharp et al., 1989; Mauro et al., 1992). High PREB mRNA levels in the incubating stage may be induced by hypothalamic VIP as PRL mRNA levels.
Similar responsiveness of White Leghorn and Nagoya against VIP injection observed in this study was interesting and may provide new insights. Genomic differences between the broody strain and non-broody strain have not been identified. Moreover, the binding of PREB to the PRL proximal promoter has not been demonstrated. Therefore, it is difficult to identify the endocrinological differences of the hypothalamus–pituitary axis between the non-broody strain and broody strain. However, results obtained in this study suggest that changes in the contents of hypothalamic VIP at least in part involve the expression of incubation behavior and the possibility of artificial induction of incubating behavior in the non-broody strain.
In conclusion, the levels of PRL and PREB mRNAs in the anterior pituitary gland from embryo and adult hens were affected by VIP or forskolin treatment. The responsiveness of the anterior pituitary gland to VIP mainly depends on the mRNA expression and presence of VIP receptor.
Acknowledgments
This work was supported, in part, by a Grant-in Aid for Scientific Research from Ministry of Education, Culture, Sport, Science and Technology of Japan (25450401) to N. K. and the Natural Science and Engineering Council of Canada to D.Z.
References
- Chaiseha Y, El Halawani ME. Expression of vasoactive intestinal peptide/peptide histidine isoleucine in several hypothalamic areas during the turkey reproductive cycles: Relationship to prolactin secretion. Neuroendocrinology, 70: 402-412. 1999. [DOI] [PubMed] [Google Scholar]
- Chaiseha Y, Youngren OM, El Halawani ME. Expression of Vasoactive Intestinal Peptide Receptor Messenger RNA in the Hypothalamus and Pituitary Throughout the Turkey Reproductive Cycle. Biology of Reproduction, 70: 593-599. 2004. [DOI] [PubMed] [Google Scholar]
- Cloues R, Ramos C, Silver R. Vasoactive intestinal polypeptide-like immunoreactivity during reproduction in doves: influence of experience and number of offspring. Hormones and Behavior, 24: 215-231. 1990. [DOI] [PubMed] [Google Scholar]
- Fliss MS, Hinkle PM, Bancroft C. Expression cloning and characterization of PREB (prolactin regulatory element binding), a novel WD motif DNA-binding protein with a capacity to regulate prolactin promoter activity. Molecular Endocrinology, 13: 644-657. 1999. [DOI] [PubMed] [Google Scholar]
- Hiyama G, Zadworny D, Kansaku N. Cloning and comparison of prolactin promoter in galliformes. Journal of Poultry Science, 46: 6-12. 2009. [Google Scholar]
- Hiyama G, Kansaku N, Tanaka T, Wakui S, Zadworny D. Characterization of chicken prolactin regulatory element binding protein and its expression in the anterior pituitary gland during embryogenesis and different reproductive stages. Journal of Poultry Science, 52: 42-51. 2015. [Google Scholar]
- Hiyama G, Kansaku N, Wakui S, McQuaid R, Zadworny D. Characterization and Expression of Turkey Prolactin Regulatory Element Binding in the Anterior Pituitary Gland and Pancreas During Embryogenesis. Journal of Poultry Science, 53: 67-75. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson TH, Fain JN. Forskolin-activated adenylate cyclase. Journal of Biological Chemistry, 258: 9755-9761. 1983. [PubMed] [Google Scholar]
- Kang SW, Gazzillo LC, You S, Wong EA, El Halawani ME. Turkey prolactin gene regulation by VIP through 35-bp cis-acting element in the proximal promoter. General and Comparative Endocrinology, 138: 157-165. 2004. [DOI] [PubMed] [Google Scholar]
- Kansaku N, Shimada K, Saito N. Regionalized gene expression of prolactin and growth hormone in the chicken anterior pituitary gland. General and Comparative Endocrinology, 99: 60-68, 1995. [DOI] [PubMed] [Google Scholar]
- Kansaku N, Shimada K, Saito N, Hidaka H. Effects of protein kinase A inhibitor (H-89) on VIP-and GRF-induced release and mRNA expression of prolactin and growth hormone in the chicken pituitary gland. Comparative Biochemistry and Physiology, 119C: 89-95. 1998. [DOI] [PubMed] [Google Scholar]
- Kansaku N, Shimada K, Ohkubo T, Saito N, Suzuki T, Matsuda Y, Zadworny D. Molecular Cloning of Chicken Vasoactive Intestinal Polypeptide Receptor Complementary DNA, Tissue Distribution and Chromosomal Localization. Biology of Reproduction, 64: 1575-1581. 2001. [DOI] [PubMed] [Google Scholar]
- Kansaku N, Hiyama G, Sasanami T, Zadworny D. Prolactin and growth hormone in bird: protein structure, gene structure, and genetic variation. Journal of Poultry Science, 45: 1-6. 2008. [Google Scholar]
- Lea RW, Vowles DM. Vasoactive intestinal polypeptide stimulates prolactin release in vivo in the ring dove (Streptopelia risoria). Experientia, 42: 420-422. 1986. [DOI] [PubMed] [Google Scholar]
- Li S, Crenshaw EB, III, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature, 347: 528-533. 1990. [DOI] [PubMed] [Google Scholar]
- Macnamee MC, Sharp PJ, Lea RW, Stering RJ, Harvey S. Evidence that vasoactive intestinal polypeptide is a physiological prolactin-releasing factor in the bantam hen. General and Comparative Endocrinology, 62: 470-478. 1986. [DOI] [PubMed] [Google Scholar]
- Mauro LJ, Elde RP, Youngren OM, Phillips RE, El Halawani ME. Alterations in hypothalamic vasoactive intestinal peptide-like immunoreactivity are associated with reproduction and prolactin release in the female turkey. Endocrinology, 125: 1795-180. 1989. [DOI] [PubMed] [Google Scholar]
- Mauro LJ, Youngren OM, Proudman JA, Phillips RE, El Halawani ME. Effects of reproductive status, ovariectomy, and photoperiod on vasoactive intestinal peptide in the female turkey hypothalamus. General and Comparative Endocrinology, 87: 481-493. 1992. [DOI] [PubMed] [Google Scholar]
- Radovick S, Nations M, Du Y, Berg LA, Weintraub BD, Wondisford FE. A mutation in the POU-homeodomain of Pit-1 responsible for combined pituitary hormone deficiency. Science, 257: 1115-1118. 1992. [DOI] [PubMed] [Google Scholar]
- SAS Institute SAS User Guide: Statistics, 8th edition. Cary, NC, USA, 1999. [Google Scholar]
- Savage JJ, Yaden BC, Kiratipranon P, Rhodes SJ. Transcriptional control during mammalian anterior pituitary development. Gene, 319: 1-19. 2003. [DOI] [PubMed] [Google Scholar]
- Sharp PJ, Sterling RJ, Talbot RT, Huskisson NS. The role of hypothalamic vasoactive intestinal polypeptide in the maintenance of prolactin secretion in incubating bantam hens: observations using passive immunization, radioimmunoassay and immunohistochemistry. Journal of Endocrinology, 122: 5-13. 1989. [DOI] [PubMed] [Google Scholar]
- Talbot RT, Hanks MC, Sterling RJ, Sang HM, Sharp PJ. Pituitary prolactin messenger ribonucleic acid levels in incubating and laying hens: Effects of manipulating plasma levels of vasoactive intestinal polypeptide. Endocrinology, 129: 496-502. 1991. [DOI] [PubMed] [Google Scholar]
- Vleck CM, Patrick DJ. Effects of vasoactive intestinal peptide on prolactin secretion in three species of passerine birds. General and Comparative Endocrinology, 113: 146-154. 1999. [DOI] [PubMed] [Google Scholar]
- Woods KL, Porter TM. Ontogeny of prolactin-secreting cells during chick embryonic development: effects of vasoactive intestinal peptide. General and Comparative Endocrinology, 112: 240-246. 1998. [DOI] [PubMed] [Google Scholar]


