Abstract
Chickens display a wide spectrum of phenotypic variations in quantitative traits such as egg-related traits. Quantitative trait locus (QTL) analysis is a statistical method used to understand the relationship between phenotypic (trait measurements) and genotypic data (molecular markers). We have performed QTL analyses for egg-related traits using an original resource population based on the Japanese Large Game (Oh-Shamo) and the White Leghorn breeds of chickens. In this article, we summarize the results of our extensive QTL analyses for 11 and 66 traits for egg production and egg quality, respectively. We reveal that at least 30 QTL regions on 17 different chromosomes affect phenotypic variation in egg-related traits. Each locus had an age-specific effect on traits, and a variety in effects was also apparent, such as additive, dominance, and epistatic-interaction effects. Although genome-wide association study (GWAS) is suitable for gene-level resolution mapping of GWAS loci with additive effects, QTL mapping studies enable us to comprehensively understand genetic control, such as chromosomal regions, genetic contribution to phenotypic variance, mode of inheritance, and age-specificity of both common and rare alleles. QTL analyses also describe the relationship between genotypes and phenotypes in experimental populations. Accumulation of QTL information, including GWAS loci, is also useful for studies of population genomics approached without phenotypic data in order to validate the identified genomic signatures of positive selection. The combination of QTL studies and next-generation sequencing techniques with uncharacterized genetic resources will enhance current understanding of the relationship between genotypes and phenotypes in livestock animals.
Keywords: chickens, egg production trait, egg quality trait, epistasis, genotype-phenotype relationship, quantitative trait loci
Introduction
Animals show phenotypic variation in a large number of traits (Darwin, 1868). In chickens, phenotypic variation is also seen not only in traits with Mendelian inheritance such as plumage color, but also in quantitative traits such as egg-related traits. Presently, chickens that display high egg production abilities have been developed by intensive artificial selection in the egg layer industry. In fact, the breeding strategy of the layer industry has resulted in the current high performance commercial chickens that are used for egg production worldwide. Although significant genetic changes must occur through selective breeding, the relationship between genotypes and phenotypes is not well characterized. Quantitative trait locus (QTL) analysis (Lander and Botstein, 1989; Haley and Knott, 1992) is a statistical method used to understand the relationship between phenotypic (trait measurements) and genotypic data (molecular markers) (Miles and Wayne, 2008). In egg-production and egg-quality traits of chickens, over 890 QTLs have been reported to date and listed in the Animal QTLdb (Hu et al., 2016). These studies have been conducted using several types of F2 and/or back-cross populations based on breeds such as White Leghorn, Rhode Island Red, Cornish, and White Plymouth Rock (e.g., Tuiskula-Haavisto et al., 2002, 2011; Sasaki et al., 2004; Hansen et al., 2005). Accumulation of QTL information will contribute toward a holistic understanding of the relationship between genotypes and phenotypes.
Genetic analysis using original resource populations is advantageous to understand how phenotypic variation of quantitative traits is influenced by common and rare alleles in the experimental populations. It is well known that genome-wide association study (GWAS) enables GWAS loci to be specified at gene-level resolution (Ozaki et al., 2002). However, GWAS usually focuses on common variants only, because genetic markers (single nucleotide polymorphisms; SNPs) that show minorallele frequencies, lower than 0.05, are generally excluded from the analysis. For QTL analysis with a segregating population, both common and rare alleles can be analyzed experimentally. In addition, QTL analysis can estimate chromosomal regions, genetic contribution to the phenotypic variance, and effects (additive, dominance, and epistatic-interaction effects) of loci in the population. These parameters are essential to understand the genotype-phenotype relationship in heterogeneous populations such as livestock animals.
In Hiroshima University, Japan, Tsudzuki (the last author of this article) and colleagues have constructed a large experimental F2 resource population in chickens for QTL analysis. The resource population was produced by crossing males of the Japanese Large Game (Oh-Shamo) breed, known for cockfighting, with females of the White Leghorn breeds, and consists of three sub-populations: a population for growth- and meat-related traits (Tsudzuki et al., 2007; Yoshida et al., 2013), a population for growth- and egg-related traits (Goto et al., 2011, 2014a, b), and a population for growth-related traits. Of note, the population for growth and egg-related traits has 421 F2 females and well-documented phenotypes that describe a wide range of egg production stages. As a result of QTL analyses, we were able to successfully detect many QTLs affecting egg production traits (Goto et al., 2011), external egg traits (Goto et al., 2014a), and internal egg traits (Goto et al., 2014b) throughout the chicken genome. In this article, we summarized the results of our extensive QTL analyses for egg-related traits with an original resource population created with the Oh-Shamo and White Leghorn breeds of chickens. We will focus on current progress and future prospects toward the main goal of understanding the genetic architecture of quantitative traits in animals.
Resource Population (Fig. 1)
Fig. 1.
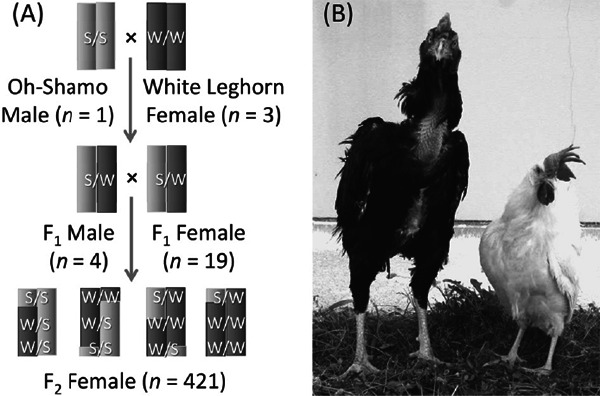
Resource population based on the Oh-Shamo and White Leghorn breeds of chickens for QTL mapping. (A) An Oh-Shamo male was crossed to three White Leghorn females to produce F1 offspring. F2 females (n=421) were produced by full-sib mating between four F1 males and 19 F1 females. Light and dark gray sections in the chromosomes of the F2 females indicate the chromosomal fragments derived from the Oh-Shamo and White Leghorn breeds, respectively. S and W indicate the alleles derived from the Oh-Shamo and White Leghorn breeds, respectively. Segregating alleles in the F2 individuals were confirmed by DNA marker genotyping of individuals in P, F1, and F2 generations. (B) An Oh-Shamo male (left) and a White Leghorn female (right). Morphological differences are obvious between the two breeds.
We selected Japanese Large Game (Oh-Shamo) and White Leghorn (CB strain) (Reynaud et al., 1987) as parental breeds for establishing a QTL mapping resource population. It is well known in Japan that the typical cockfighting breed Oh-Shamo yields a large amount of good quality meat (Tsudzuki, 2003), whereas White Leghorn is known as a traditional layer breed and its females are able to produce many white-shelled eggs (Roberts, 1997). In addition, Oh- Shamo chickens display heavier body weights, lower growth rates, longer time to reach sexual maturity (age at the first egg), and less egg production (brownish-shelled) than White Leghorn chickens. The large phenotypic differences in growth-, meat-, and egg-related traits between the two breeds suggested the existence of significant phenotypic and genotypic variations in the segregating F2 population. Furthermore, Osman et al. (2006) confirmed a large genetic difference between the two breeds. Therefore, it was expected before commencing QTL analyses that polymorphic genetic markers would be found easily throughout the genome, supporting QTL mapping of traits using the generated resource population.
Other resource populations for egg-related traits have been constructed by several research groups to date. Many QTL studies for egg traits have been conducted using resource populations created by crossing various breeds, including White Leghorn×Red Jungle-fowl (Kerje et al., 2003; Wright et al., 2006; Johnsson et al., 2016), Green-legged Partridgenous (a native Polish breed)×Rhode Island Red (Wardeck et al., 2003), White Leghorn×Rhode Island Red (Tuiskula-Haavisto et al., 2002, 2004; Sasaki et al., 2004; Honkatukia et al., 2005, 2013), White Leghorn×Cornish (Hansen et al., 2005), White Leghorn×White Plymouth Rock (Tuiskula-Haavisto et al., 2011), White Leghorn×New Hampshire (Goraga et al., 2012), layer×broiler (Schreiweis et al., 2006; Podisi et al., 2011), and layer×layer (Honkatukia et al., 2011). The importance of differences in genetic backgrounds for understanding the genetic basis of quantitative traits has been discussed in the fields of human (Chow et al., 2016) and mouse genetics (Yalcin and Flint, 2012; Goto et al., 2015a; Churchill, 2016). That is, because the effect of a QTL depends on its genetic background, the QTL effect on phenotypic variation can be often validated using segregating genetic material (e.g., an F2 population), but not using material with a fixed genetic background (e.g., a congenic strain). Moreover, since genetically heterogeneous populations allow us to identify the effect of alleles derived from several strains (Koide et al., 2012), use of these varying genetic resources will enhance our further understanding of the genotype-phenotype relationship.
Egg-related Traits (Fig. 2)
Fig. 2.
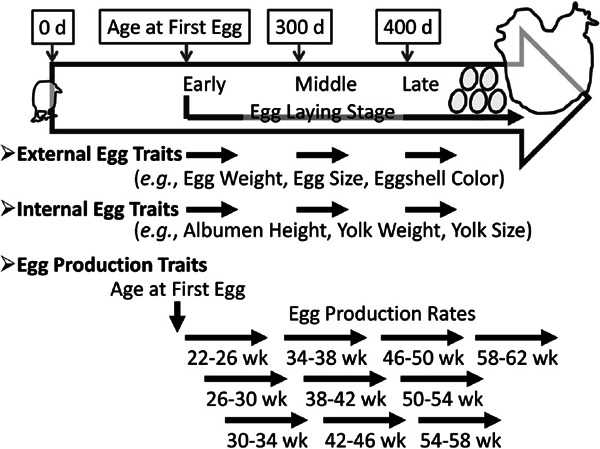
Illustration of the egg laying stage and egg-related traits investigated. Chickens were reared over the course of a year from hatching. Eggs were inspected for external and internal traits at early (first egg age), middle (300-days-old), and late (400-days-old) egg laying stages. Egg production rates were calculated every four weeks for 22- to 62-week-old.
In our study, a large number of eggs were collected over the course of a year and analyzed for many traits (Goto et al., 2011, 2014a, b). Egg traits are classified into three categories: egg production traits, external egg traits, and internal egg traits (Roberts, 2004). Egg production traits include the age at the first egg (AFE) and the egg production rates (EPRs). AFE is defined as the age of hens when they produce an egg for the first time, and is therefore an indicator of sexual maturity, the timing of which is quite important for chicken industry. We recorded the AFE for all hens in our resource population. EPRs are defined as the number of eggs laid per day. We calculated the EPR for 22- to 62-week old hens at every four weeks, which created a total of 10 individual traits for EPR, referred to as EPR1 (22–26 weeks of age) up to EPR10 (58–62 weeks of age). In total, we collected data for 11 egg production traits, i.e., AFE and 10 EPRs (Goto et al., 2011).
In addition, data for 11 kinds of external and internal egg traits were collected at three different egg production stages, namely early (E- first egg), middle (M- 300 days old), and late (L- 400 days old) stages. At the E stage, we collected 10 eggs following the first egg. At the M and L stages, we collected eggs laid during a two-week period from 300- and 400- day-old hens, respectively. External egg traits included egg weight (EW), lengths of the long and short axes of the egg (LLA and LSA, respectively), eggshell strength (SS), eggshell weight (SW), eggshell thickness of the narrow end, blunt end, and equator of the egg (STN, STB, and STE, respectively), and lightness, redness, and yellowness of the eggshell color (SCL, SCR, and SCY, respectively). Internal egg traits included albumen weight (AW), albumen height (AH), lengths of the long and short axes of the thick albumen (LTA and STA, respectively), yolk weight (YW), yolk height (YH), lengths of the long and short axes of the yolk (YL and YS, respectively), and lightness, redness, and yellowness of the yolk color(YCL, YCR, and YCY, respectively). We used 3–10 eggs perhen to analyze each egg quality trait. Mean values of the traits were calculated and treated as individual phenotypic values. In total, we collected data for 33 external and 33 internal egg traits through the E, M, and L egg production stages (Goto et al., 2014a, b).
As described above, our study included analysis of a large amount of phenotypic data (24 kinds of traits; 77 traits in total), including two kinds of egg production traits (AFE and EPRs; 11 traits) in 22- to 62-week-old hens and 22 kinds of egg quality traits (EW, LLA, LSA, SS, SW, STN, STB, STE, SCL, SCR, SCY, AW, AH, LTA, STA, YW, YH, YL, YS, YCL, YCR, and YCY) during three different egg production stages (66 traits in total). Conversely, other studies analyzed 1–2 types of egg production traits and 1–10 kinds of egg quality traits (Tuiskula-Haavisto et al., 2002, 2004, 2011; Kerje et al., 2003; Wardeck et al., 2003; Sasaki et al., 2004; Honkatukia et al., 2005, 2011, 2013; Hansen et al., 2005; Wright et al., 2006; Schreiweis et al., 2006; Goraga et al., 2012; Podisi et al., 2011; Johnsson et al., 2016). A notable feature of our QTL studies is the large number of phenotypic traits that were characterized. In addition, a comparable study in which many kinds of egg quality traits are measured at more than three different stages of egg production does not exist. Our study facilitates a thorough understanding of age-specific genetic control of egg-related traits (Goto et al., 2014a, b).
Genetic Makers and Linkage Map
We selected and genotyped 147 microsatellite DNA markers on 26 autosomes and the Z chromosome (Goto et al., 2014a). The criteria for selecting markers were set as follows: (i) there are no shared alleles between an Oh-Shamo male and three White Leghorn females which are parental individuals in the F2 resource population and (ii) markers are distributed throughout the chicken genome as widely as possible. We genotyped microsatellite markers that showed more than two base pairs difference between the two breeds by fluorescence-based DNA-fragment analysis using automated sequencers with samples from three generations (P, F1, and F2). Therefore, we can comprehensively trace chromosomal regions in F2 individuals to their parental breed origins. We annotated each marker in the F2 individuals indicating whether alleles were homozygous derived from Oh-Shamo or White Leghorn (A and B, respectively) or heterozygous (H). A linkage map was constructed using the Kosambi map function of the Map Manager QTX software (Manly et al., 2001), and marker order was referred to the consensus map 2005 (Schmid et al., 2005) listed in the ArkDB (http://www.thearkdb.org/arkdb/). The resulting map covered approximately 68% of the chicken genome with chromosomes 1–15, 17–20, 23–24, 26–28, 32, Z, and linkage group E50C23 (Goto et al., 2014a).
Recently, high-density SNPs genotyping arrays were made available in chickens (Groenen et al., 2011; Kranis et al., 2013). Although efforts are continuing to comprehensively describe the chicken genome, the assembled sequence does not yet cover chromosomes 29–31 and 33–38 (Gallus_gallus-4.0; Galgal4, GCA_ 000002315. 2) (Schmid et al., 2015). Using many SNPs, GWASs for egg-related traits have been conducted by several research groups (Abasht et al., 2009; Liu et al., 2011; Wolc et al., 2012, 2014; Yi et al., 2015; Zhang et al., 2015; Liao et al., 2016) resulting in the identification of many GWAS loci at gene-level resolution which are accumulated in the Animal QTLdb (Hu et al., 2016). Further efforts including utilization of SNPs and whole genome sequencing information with several kinds of populations are required to comprehensively understand the genotype-phenotype relationship.
QTL Analysis (Fig. 3)
Fig. 3.
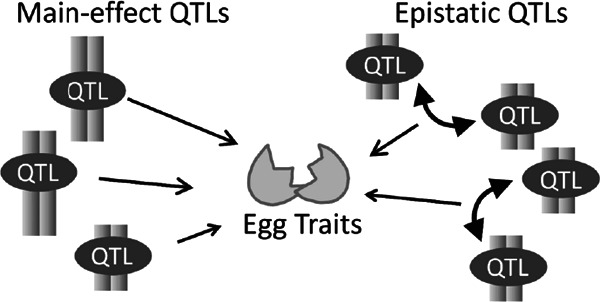
Conceptual diagram of the genetic control of quantitative traits. QTLs are classified into two groups; main-effect QTLs and epistatic QTLs. Main-effect QTLs affect a trait through individual alleles present at a single locus. On the other hand, epistatic QTLs affect a trait through allele combinations between two loci. Conceptually, it is thought that both types of QTLs affect phenotypic variation of egg traits.
QTLs are classified into two groups, namely main-effect and epistatic QTLs. Main-effect QTLs affect a trait through individual alleles (three possible genotypes: homozygous with either paternal or maternal alleles, or heterozygous with both parental alleles) at a locus. Epistatic QTLs affect a trait through allele combinations between two loci (nine possible combinations: three genotypes×three genotypes at each locus). Conceptually, it is thought that many QTLs of both types affect phenotypic variation of egg traits. QTL analyses are conducted to identify the mode-of-action, interaction, number, and precise location of the loci (Miles and Wayne, 2008).
In order to detect main-effect QTLs, we performed a simple interval mapping based on multiple regression analysis (Haley and Knott, 1992) using both phenotypic and genotypic data in the F2 individuals. The analysis enabled us to know the parameter estimates of detected QTLs, including map position, additive effect (half the difference between two homozygotes) and dominance effect (deviation of a heterozygote from the mean of the two homozygotes) (Tsudzuki et al., 2007). After genotypes of marker intervals in the F2 individuals were imputed every 2-cM step along each chromosome (linkage group) from genotypes of flanking markers and the amount of recombination expected (Haley and Knott, 1992), trait values were regressed onto the additive and dominance scores of each position (Kenney-Hunt et al., 2006). Both additive and dominance effects contribute to understanding the mode of inheritance of the locus. For this, the dominance effect/additive effect (d/a) ratio was calculated and used to determine mode of inheritance, defined as overrecessive (d/a<−1.5), recessive (−1.5<d/a<−0.5), additive (−0.5<d/a<0.5), dominance (0.5<d/a<1.5), and over-dominance (1.5<d/a) (Kenney-Hunt et al., 2006).
Chickens have a ZZ/ZW sex chromosome system, and therefore, female and male birds are hemizygote (ZW) and homozygote (ZZ), respectively (Smith and Burt, 1998). In our F2 population, Oh-Shamo male (ZOS ZOS) and White Leghorn females (ZWL WWL) in the parental generation passed their sex chromosomes onto females (ZOSWWL) and males (ZOS ZWL) in the F1 generation. By crossing F1 females (ZOSWWL) and F1 males (ZOSZWL), 421 F2 females (ZOSWWL and ZWLWWL) were produced (Goto et al., 2011). Since this manner is similar to that of autosomal loci in a backcross population (F1×White Leghorn), the locus mode of inheritance is comparable to that in a backcross population (Rance et al., 1997). Therefore, we used a backcross model (Manly et al., 2001) to search QTLs for the Z chromosome.
In order to detect epistatic QTL, all pairs of marker loci were tested based on a linear regression model with a marker-by-marker interaction term and the assumption that a QTL is at a marker locus (Ishikawa et al., 2005). Both the Map Manager QTX software (Manly et al., 2001) and the qtl package (Broman et al., 2003) of R software (http://www.R-project.org/) were used to identify both types of QTLs described above. Genome-wide significant thresholds were determined by permutation tests for taking into account multiple testing (Churchill and Doerge, 1994) for autosomes and sex chromosome separately (Broman et al., 2006).
Detected QTLs for Egg-related Traits (Fig. 4)
Fig. 4.
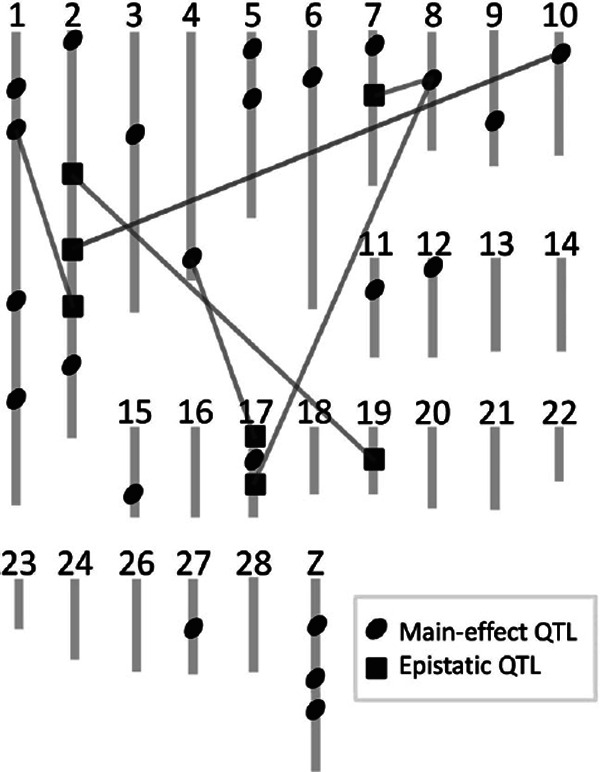
Genetic network influencing phenotypic variation of egg-related traits found in the QTL studies with a single resource population. QTLs detected in our previous studies (Goto et al., 2011; 2014a; 2014b) are summarized in this figure. Detailed information including QTL position, flanking markers, and mode of inheritance is summarized in Table 1. Light gray vertical lines numbered 1–24, 26–28, and Z (above) indicate chromosomes. Dark gray ellipses and squares indicate QTL regions with main and epistatic effects on egg-related traits, respectively. Light gray lines between loci indicate epistatic interactions. Based on this figure, at least 30 QTL regions on 17 different chromosomes control phenotypic variation of egg-related traits, which clearly indicates a complex genetic basis of quantitative traits.
For egg production traits, main-effect QTLs were detected in three QTL regions, with one located on chromosome 11 and two located on chromosome 1 (Goto et al., 2011). For external egg traits, main-effect QTLs were found in 13 QTL regions, with one located on each of chromosomes 1, 2, 4, 8, 10, 11, 12, and 17, two located on chromosome 5, and three located on the Z chromosome (Goto et al., 2014a). For internal egg traits, main-effect QTLs were discovered in 15 QTL regions, with one located on each of chromosomes 2, 3, 4, 5, 6, 7, 8, 9, 15, and 27, two located on Z chromosome, and three located on chromosome 1 (Goto et al., 2014b).
Epistatic QTLs were detected for egg production and internal egg traits (Goto et al., 2011, 2014b), which are classified into two types. The first epistatic QTL type was those QTLs with both main and interaction effects, shown by dark gray ellipses in Fig. 4. We found this type of loci in four QTL regions, with one located on each of chromosomes 1, 4, 8, and 10. The second epistatic QTL type was those QTLs with an interaction-effect only, shown by dark gray squares in Fig. 4. These interaction effect only loci were found in seven QTL regions, one located on each of chromosomes 7 and 19, two located on chromosome 17, and three located on chromosome 2.
These main and epistatic QTLs accounted for approximately 3–18% of the phenotypic variances (Goto et al., 2011, 2014a, b). However, as described above, differences in genetic backgrounds have a large effect against the expression of quantitative traits (Yalcin and Flint, 2012; Goto et al., 2015a; Chow et al., 2016; Churchill, 2016). The phenotypic variances observed in our F2 resource population are thought to be changeable under different genetic backgrounds. Thus, the effects of our QTLs should be checked using different genetic backgrounds prior to applying the QTLs to actual breeding.
Table 1 shows a summary of the positions, age-specificity, and mode of inheritance of QTL regions detected for egg-related traits in our resource population. Since overlapping QTL regions were described across three past studies (Goto et al., 2011, 2014a, b), it seems that at least 30 QTL regions on 17 different chromosomes affect the phenotypic variation in egg-related traits (Table 1 and Fig. 4). Each locus has an age-specific effect on traits and displays various modes of inheritance including overrecessive, recessive, additive, dominance, and overdominance. Eight QTL regions (QTL2, QTL7, QTL11, QTL17, QTL20, QTL21, QTL28, and QTL30) are corresponding to several QTLs (Tuiskula-Haavisto et al., 2002, 2004, 2011; Kerje et al., 2003; Wardeck et al., 2003; Sasaki et al., 2004; Honkatukia et al., 2013; Hansen et al., 2005; Schreiweis et al., 2006) and GWAS loci (Abasht et al., 2009; Liu et al., 2011; Wolc et al., 2014). Especially, QTL7 on chromosome 2, QTL11 on chromosome 4, and QTL17 on chromosome 8 are similar to the GWAS loci in theirpositions. Comparison of the results from the two analyses may lead to narrower confident intervals of the QTL positions. Moreover, a number of QTL regions affect egg production and external and internal egg quality traits, e.g., QTL2 on chromosome 1, QTL11 on chromosome 4, and QTL17 on chromosome 8, where pleiotropic genes and/or multiples of closely linked genes are likely located. These regions, in particular QTL17 on chromosome 8 which showed the highest LOD scores, require further detailed analysis not only in the present resource population but also in other populations for a deeper understanding of the genotype-phenotype relationship.
Table 1. Summary of the positions, target traits, expression stages, and mode of inheritance of the QTLs found in our studies.
| QTL no. | Chromosome | Markers (position, cM)1 | Trait categories2 | Traits2 | Stages of QTL expression3 |
d/a4 | References5 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E | M | L | |||||||||
| QTL1 | 1 | Around ADL0188 (133) | egg production | AFE | X | −1.1 | recessive | [1] | |||
| EPR | X (26–30 weeks of age) | −1.1 | recessive | [1] | |||||||
| EPR | X (30–34 weeks of age) | −1.3 | recessive | [1] | |||||||
| QTL2 | 1 | MCW0112 (205) - MCW0058 (241) | egg production | EPR | X (54–58 weeks of age) | 0.7 | dominance | [1] | |||
| eggshell color | SCR | X | X | −0.4 | 0.2 | additive | [2] | ||||
| SCY | X | X | −0.2 | 0.4 | additive | [2] | |||||
| albumen height | AH | X | X† | X† | 0.4 | 0.8 | dominance | [3] | |||
| yolk color | YCR | X* | − | [3] | |||||||
| QTL3 | 1 | Around MCW0102 (441)-ADL0245 (459) | albumen height | AH | X‡ | −2.3 | overrecessive | [3] | |||
| QTL4 | 1 | GCT0032 (518)-MCW0107 (565) | yolk color | YCY | X† | 1.0 | dominance | [3] | |||
| QTL5 | 2 | Around LEI0124 (0*) | eggshell color | SCY | X† | 0.7 | dominance | [2] | |||
| QTL6 | 2 | Around MCW0062 (172) | egg production | EPR | X* (42–46 weeks of age) | − | [1] | ||||
| QTL7 | 2 | Around MCW0027 (255) | albumen height | AH | X* | − | [3] | ||||
| QTL8 | 2 | Around LEI0237 (320) | yolk color | YCR | X* | − | [3] | ||||
| QTL9 | 2 | LEI0070 (379)-ADL0146 (403) | albumen height | AH | X† | 13.3 | overdominance | [3] | |||
| QTL10 | 3 | MCW0083 (51)-ADL0229 (111) | yolk color | YCL | X | X | 0.4 | 1.3 | dominance | [3] | |
| QTL11 | 4 | Around MCW00240 (201) and ABR0622 (234*) | egg production | EPR | X* (30–34 weeks of age) | − | [1] | ||||
| egg weight | EW | X† | 0.4 | additive | [2] | ||||||
| eggshell color | SCR | X | 1.0 | dominance | [2] | ||||||
| SCY | X | 1.0 | dominance | [2] | |||||||
| albumen weight | AW | X | X | 0.3 | 0.3 | additive | [3] | ||||
| yolk height | YH | X† | 0.5 | additive | [3] | ||||||
| yolk color | YCL | X† | 2.1 | overdominance | [3] | ||||||
| YCY | X | X | −0.5 | −0.1 | additive | [3] | |||||
| QTL12 | 5 | MCW0038 (71)-MCW0214 (88) | eggshell thickness | STN | X† | 1.5 | dominance | [2] | |||
| QTL13 | 5 | MCW0233(123)-ADL0233(151) | eggshell color | SCL | X | X | 0.0 | 0.3 | additive | [2] | |
| SCR | X | X | X | −0.4 | 0.5 | additive | [2] | ||||
| SCY | X | X | X | −0.2 | 0.4 | additive | [2] | ||||
| albumen height | AH | X | 3.1 | overdominance | [3] | ||||||
| QTL14 | 6 | LEI0092 (59)-LE10196 (110) | albumen height | AH | X† | 0.9 | dominance | [3] | |||
| QTL15 | 7 | MCW0120(44)-ABR0397 (50*) | yolk height | YH | X | 1.3 | dominance | [3] | |||
| QTL16 | 7 | Around LEI0158(122) | egg production | AFE | X* | − | [1] | ||||
| QTL17 | 8 | Around MCW0305 (15) and MCW0095 (26) | egg production | AFE | X* | − | [1] | ||||
| egg weight | EW | X | X | X | −1.9 | −1.8 | overrecessive | [2] | |||
| eggshell thickness | STN | X | X | −7.0 | −2.3 | overrecessive | [2] | ||||
| STB | X | X | −2.0 | 0.9 | overrecessive | [2] | |||||
| STE | X | −0.2 | additive | [2] | |||||||
| albumen weight | AW | X | X | X† | −3.2 | −2.6 | overrecessive | [3] | |||
| albumen size | LTA | X | X | −1.7 | −0.9 | recessive | [3] | ||||
| STA | X | X | −2.0 | −1.2 | recessive | [3] | |||||
| yolk weight | YW | X | X | X | –1.8 | −1.3 | recessive | [3] | |||
| yolk height | YH | X | X | X | −2.2 | −0.9 | recessive | [3] | |||
| yolk size | YL | X | X | X | −1.4 | −1.2 | recessive | [3] | |||
| YS | X | X | X,X* | −1.7 | −0.9 | recessive | [3] | ||||
| yolk color | YCL | X | 0.1 | additive | [3] | ||||||
| YCY | X | 0.4 | additive | [3] | |||||||
| QTL18 | 9 | Around ABR0526 (79*) | albumen height | AH | X† | X | 0.8 | 1.5 | dominance | [3] | |
| yolk color | YCL | X† | 0.7 | dominance | [3] | ||||||
| QTL19 | 10 | ADL0272 (44) - Around ADL0106 (88) | egg size | LCE | X† | 0.4 | additive | [2] | |||
| albumen height | AH | X* | − | [3] | |||||||
| QTL20 | 11 | ADL0210 (54)-MCW0066 (69) | egg production | EPR | X (30–34 weeks ot age) | 1.3 | dominance | [1] | |||
| eggshell color | SCY | X | 0.6 | dominance | [2] | ||||||
| QTL21 | 12 | Around ADL0372 (0) | egg size | LLE | X | −0.1 | additive | [2] | |||
| LSE | X | 0.2 | additive | [2] | |||||||
| eggshell strength | SS | X | 1.2 | dominance | [2] | ||||||
| QTL22 | 15 | Around MCW0211 (49) | yolk height | YH | X† | −0.1 | additive | [3] | |||
| QTL23 | 17 | Around ADL0293 (26) | eggshell thickness | STN | X | 1.5 | dominance | [2] | |||
| STE | X | 0.4 | additive | [2] | |||||||
| QTL24 | 17 | Around ABR0530 (0*) | egg production | EPR | X* (30–34 weeks of age) | − | [1] | ||||
| eggshell weight | SW | X† | −0.4 | additive | [2] | ||||||
| QTL25 | 17 | Around ABR0387 (55*) | yolk size | YS | X* | − | [3] | ||||
| QTL26 | 19 | Around ABR0180 (24*) | egg production | EPR | X* (42–46 weeks of age) | − | [1] | ||||
| QTL27 | 27 | Around MCW0328 (47) | albumen size | STA | X | 0.2 | additive | [3] | |||
| yolk color | YCL | X† | 0.5 | additive | [3] | ||||||
| QTL28 | Z | Around ADL0273 (73) | albumen height | AH | X | − | [3] | ||||
| yolk height | YH | X | X | − | [3] | ||||||
| eggshell strength | SS | X | − | [2] | |||||||
| QTL29 | Z | Around MCW0154 (95) | egg size | LLE | X | − | [2] | ||||
| LSE | X | X | − | [2] | |||||||
| QTL30 | Z | Around ABR0119 (135*) | eggshell weight | SW | X | X | X | − | [2] | ||
| eggshell thickness | STN | X | − | [2] | |||||||
| STB | X | X | − | [2] | |||||||
| STE | X | − | [2] | ||||||||
| yolk weight | YW | X | X | − | [3] | ||||||
Markers close to QTL and their reference positions in the consensus map 2005 (http://www.thearkdb.org/arkdb/).
indicates approximate position because the marker has no reference map position.
Traits were classified into 14 categories, i.e. egg production (AFE and EPR), egg weight (EW), egg size (LLE and LSE), eggshell weight (SW), eggshell thickness (STN, STB, and STE), eggshell strength (SS), and eggshell color (SCL, SCR, and SCY), albumen weight (AW), albumen height (AH), albumen size (LTA and STA), yolk weight (YW), yolk height (YH), yolk size (YL and YS), and yolk color (YCL, YCR, and YCY). Trait abbreviations were shown in main text of the section of egg-related traits.
X indicates the stage when the QTL was detected for egg-related traits. For AFE and external and internal egg traits, E=early stage (first 10 eggs), M=middle stage (during two weeks from 300 days of age), L=late stage (during two weeks from 400 days of age). For EPR, stage was shown in parentheses.
Epistatic QTL.
QTL at the suggestive level.
Significant QTL by a two-QTL model (Broman and Sen, 2009).
The degree of dominance calculated by the dominance effect of the QTL/the additive effect of the QTL. In the case of multiple QTLs detected in the region, the minimum and maximum values are indicated. The most likely mode of inheritance was estimated as overrecessive (d/a<−1.5), recessive (−1.5<d/a<−0.5), additive (−0.5<d/a<0.5), dominance (0.5<d/a< 1.5), and overdominance (1.5<d/a) at each locus.
indicates main-effect QTL on the Z chromosome and/or epistatic QTL detected.
[1] Goto et al., 2011, [2] Goto et al., 2014a, [3] Goto et al., 2014b.
As shown in Fig. 4, the complex genetic architecture of egg-related traits has been revealed using an experimental resource population based on the Oh-Shamo and White Leghorn breeds. Since the QTL regions found in the present study have relatively wide confidential intervals, it is likely difficult to identify the specific genes in each region. However, it seems advantageous that many loci showing variation in modes of inheritance were found. For understanding the genetic basis of quantitative traits, characterization of each kind of genetic effect described above is likely essential. Therefore, it is important to conduct not only studies at gene-level resolution such as GWAS (focusing on the additive effect) but also QTL mapping studies (focusing on additive, dominance, and epistatic-interaction effects). In addition, since phenotypic variation of quantitative traits differs with age, long-term studies focused on egg-related traits are required in future. Further efforts are necessary to understand the genotype-phenotype relationship in animals using several kinds of genetic resources and next-generation techniques such as whole genome sequencing.
Concluding Remarks
In this review article, we summarized the results of our genetic mapping studies for egg-related traits in chickens. Our original resource population created using an indigenous Japanese breed, Oh-Shamo, enabled us to reveal a large number of QTLs that contribute to phenotypic variations, and which have additive, dominance, and epistatic-interaction effects and age-specific natures. This holistic view of the genetic control of quantitative traits will prove useful for understanding the relationship between genotypes and phenotypes in livestock animals.
To date, layers and broilers created by commercial breeders have been studied intensively to understand the relationship between genotypes and phenotypes. Recent intensive artificial selection has in fact led to significant results for the production of eggs and meat, producing new lines that are strikingly different from other domestic chickens. Although these commercial resources are absolutely important, further chicken resources also offer a large potential (Tixier-Boichard et al., 2009). Classical breeds of chickens maintained worldwide show a wide variety of phenotypes (Roberts, 1997) as do many indigenous breeds of chickens in Japan (Tsudzuki, 2003). For example, it has been revealed that egg characteristics of the Japanese Extremely Long Tail (Onagadori) breed are apparently different from those of the White Leghorn breed (Goto et al., 2015b). This example is only the tip of an iceberg, demonstrating that unexploited genetic resources should be thoroughly investigated in the future. In addition, free-range scavenging village chickens (Dessie et al., 2011; Leroy et al. 2012; Desta et al., 2013) also offer large potential to understand the genetic control of phenotypic diversity. Since village chickens possess a richness of accumulated recombination and a number of adaptive characteristics for extreme environments such as high temperature, they provide a useful genetic resource for high-resolution genetic mapping (Wragg et al., 2012; Megens and Groenen, 2012).
Efforts are continuing to comprehensively describe the chicken genome (Schmid et al., 2015). Recently, population genomics studies, which search for genetic signatures of selection in the genome (Akey, 2009), have been conducted using high density SNPs genotyping and whole genome sequencing techniques in the livestock genome (e.g., Rubin et al., 2010; Elferink et al., 2012; Fan et al., 2013; Qanbari et al., 2015). This population genomics approach has allowed gene-level resolution mapping of loci that have been positively selected for, without any phenotypic information (Qanbari et al., 2014). In fact, this approach can reveal a number of interesting findings, including genes that affect adaptation to local environments such as high altitude (Wang et al., 2015). Most studies analyzed whether genetic signatures of selection were co-localized with QTLs listed in the Animal QTLdb (Hu et al., 2016), in order to validate the signals of positive selection (e.g., Zhang et al., 2012; Gholami et al., 2015). Since QTL mapping studies reveal more direct evidence of the relationships between certain phenotypes and DNA regions in resource populations, the integration of QTL information into the Animal QTLdb (Hu et al. 2016) will also be useful for researchers in the field. Using next-generation technologies and unexploited genetic resources, further efforts are required to uncover the genotype-phenotype relationship of livestock animals.
Acknowledgments
We thank the Japan Poultry Science Association for the award for promotion of scientific studies on Poultry Science for QTL mapping studies on chickens to TG. We really appreciate scientific colleagues related to the works. We thank Dr. L. M. Liao for English language editing.
References
- Abasht B, Sandford E, Arango J, Settar P, Fulton JE, O'Sullivan NP, Hassen A, Habier D, Fernando RL, Dekkers JCM, Lamont SJ. Extent and consistency of linkage disequilibrium and identification of DNA markers for production and egg quality traits in commercial layer chicken populations. BMC Genomics, 10: S2 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey JM. Constructing genomic maps of positive selection in humans: Where do we go from here? Genome Research, 19: 711-722. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics, 19: 889-890. 2003. [DOI] [PubMed] [Google Scholar]
- Broman KW, Sen S, Owens SE, Manichaikul A, Southard-Smith EM, Churchill GA. The X chromosome in quantitative trait locus mapping. Genetics, 174: 2151-2158. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman KW, Sen S. A guide to QTL mapping with R/qtl. Springer, New York: 2009. [Google Scholar]
- Chow CY. Bringing genetic background into focus. Nature Reviews Genetics, 17: 63-64. 2016. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics, 138: 963-971. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GA. Eric Lander and David Botstein on mapping quantitative traits. Genetics, 203: 1-3. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The variation of animals and plants under domestication. Murray, London: 1868. [PMC free article] [PubMed] [Google Scholar]
- Dessie T, Taye T, Dana N, Ayalew W, Hanotte O. Current state of knowledge on phenotypic characteristics of indigenous chickens in the tropics. World's Poultry Science Journal, 67, 507-516. 2011. [Google Scholar]
- Desta TT, Dessie T, Bettridge J, Lynch SE, Melese K, Collins M, Christley RM, Wigley P, Kaiser P, Terfa Z, Mwacharo JM, Hanotte O. Signature of artificial selection and ecological landscape on morphological structures of Ethiopian village chickens. Animal Genetic Resources, 52: 17-29. 2013. [Google Scholar]
- Elferink MG, Megens HJ, Vereijken A, Hu X, Crooijmans RPMA, Groenen MAM. Signatures of selection in the genomes of commercial and non-commercial chicken breeds. PLoS One, 7: e32720 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan WL, Ng CS, Chen CF, Lu MYJ, Chen YH, Liu CJ, Wu SM, Chen CK, Chen JJ, Mao CT, Lai YT, Lo WS, Chang WH, Li WH. Genome-wide patterns of genetic variation in two domestic chickens. Genome Biology and Evolution, 5: 1376-1392. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholami M, Reimer C, Erbe M, Preisinger R, Weigend A, Weigend S, Servin B, Simianer H. Genome scan for selection in structured layer chicken populations exploiting linkage disequilibrium information. PLoS One, 10: e0130497 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goraga ZS, Nassar MK, Brockmann GA. Quantitative trait loci segregating in crosses between New Hampshire and White Leghorn chicken lines: I. egg production traits. Animal Genetics, 43: 183-189. 2012. [DOI] [PubMed] [Google Scholar]
- Goto T, Ishikawa A, Onitsuka S, Goto N, Fujikawa Y, Umino T, Nishibori M, Tsudzuki M. Mapping quantitative trait loci for egg production traits in an F2 intercross of Oh-Shamo and White Leghorn chickens. Animal Genetics, 42: 634-641. 2011. [DOI] [PubMed] [Google Scholar]
- Goto T, Ishikawa A, Goto N, Nishibori M, Umino T, Tsudzuki M. Mapping of main-effect and epistatic Quantitative trait loci for internal egg traits in an F2 resource population of chickens. Journal of Poultry Science, 51: 375-386. 2014. a. [Google Scholar]
- Goto T, Ishikawa A, Yoshida M, Goto N, Umino T, Nishibori M, Tsudzuki M. Quantitative trait loci mapping for external egg traits in F2 chickens. Journal of Poultry Science, 51: 118-129. 2014. b. [Google Scholar]
- Goto T, Matsumoto Y, Tanave A, Koide T. Genetic analyses for tame behavior in animals: exploration of genetic loci affecting animal domestication. Journal of Animal Genetics (in Japanese), 43: 3-11. 2015. a. [Google Scholar]
- Goto T, Shiraishi J-i, Bungo T, Tsudzuki M. Characteristics of egg-related traits in the Onagadori (Japanese Extremely Long Tail) breed of chickens. Journal of Poultry Science, 52: 81-87. 2015. b. [Google Scholar]
- Groenen MAM, Megens HJ, Zare Y, Warren WC, Hillier LW, Crooijmans RPMA, Vereijken A, Okimoto R, Muir WM, Cheng HH. The development and characterization of a 60K SNP chip for chicken. BMC Genomics, 12: 274 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley CS, Knott SA. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity, 69: 315-324. 1992. [DOI] [PubMed] [Google Scholar]
- Hansen C, Yi N, Zhang YM, Xu S, Gavora J, Cheng HH. Identification of QTL for production traits in chickens. Animal Biotechnology, 16: 67-79. 2005. [DOI] [PubMed] [Google Scholar]
- Honkatukia M, Tuiskula-Haavisto M, de Koning DJ, Virta A, Maki-Tanila A, Vilkki J. A region on chicken chromosome 2 affects both egg white thinning and egg weight. Genetics Selection Evolution, 37: 563-577. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkatukia M, Tuiskula-Haavisto M, Ahola V, Uimari P, Schmutz M, Preisinger R, Cavero D, Vennerström P, Arango J, O'Sullivan N, Fulton J, Vilkki J. Mapping of QTL affecting incidence of blood and meat inclusions in egg layers. BMC Genetics, 12: 55 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkatukia M, Tuiskula-Haavisto M, Arango J, Tabell J, Schmutz M, Preisinger R, Vilkki J. QTL mapping of egg albumen quality in egg layers. Genetics Selection Evolution, 45: 31 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZL, Park CA, Reecy JM. Developmental progress and current status of the Animal QTLdb. Nucleic Acids Research, 44: D827-D833. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Hatada S, Nagamine Y, Namikawa T. Further mapping of quantitative trait loci for postnatal growth in an intersubspecific backcross of wild Mus musculus castaneus and C57BL/6J mice. Genetical Research, 85: 127-137. 2005. [DOI] [PubMed] [Google Scholar]
- Johnsson M, Jonsson KB, Andersson L, Jensen P, Wright D. Quantitative trait locus and genetical genomics analysis identifies putatively causal genes for fecundity and brooding in the chicken. G3: Genes, Genomes, Genetics, 6: 311-319. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney-Hunt JP, Vaughn TT, Pletscher LS, Peripato A, Routman E, Cothran K, Durand D, Norgard E, Perel C, Cheverud JM. Quantitative trait loci for body size components in mice. Mammalian Genome, 17: 526-537. 2006. [DOI] [PubMed] [Google Scholar]
- Kerje S, Carlborg O, Jacobsson L, Schütz K, Hartmann C, Jensen P, Andersson L. The two fold difference in adult size between the red junglefowl and White Leghorn chickens is largely explained by a limited number of QTLs. Animal Genetics, 34: 264-274. 2003. [DOI] [PubMed] [Google Scholar]
- Koide T, Goto T, Takano-Shimizu T. Genomic mixing to elucidate the genetic system of complex traits. Experimental Animal, 61: 503-509. 2012. [DOI] [PubMed] [Google Scholar]
- Kranis A, Ghryas AA, Boschiero C, Turner F, Yu L, Smith S, Talbot R, Pirani A, Brew F, Kaiser P, Hocking PM, Fife M, Salmon N, Fulton J, Strom TM, Haberer G, Weigend S, Preisinger R, Gholami M, Qanbari S, Simianer H, Watson KA, Woolliams JA, Burt DW. Development of a high density 600K SNP genotyping array for chicken. BMC Genomics, 14: 59 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Li D, Liu J, Chen S, Qu L, Zheng J, Xu G, Yang N. A genome-wide SNP scan reveals novel loci for egg production and quality traits in white leghorn and brown-egg dwarf layers. PLoS One, 6: e28600 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics, 121: 185-199. 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy G, Kayang BB, Youssao IAK, Yapi-Gnaoré CV, Osei-Amponsah R, Loukou NE, Fotsa JC, Benabdeljelil K, Bed'hom B, Tixier-Boichard M, Rognon X. Gene diversity, agroecological structure and introgression patterns among village chicken populations across North, West and Central Africa. BMC Genetics, 13: 34 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao R, Zhang X, Chen Q, Wang Z, Wang Q, Yang C, Pan Y. Genome-wide association study reveals novel variants for growth and egg traits in Dongxiang blue-shelled and White Leghorn chickens. Animal Genetics, 47: 588-596. 2016. [DOI] [PubMed] [Google Scholar]
- Manly KF, Cudmore RH, Jr, Meer JM. Map Manager QTX, cross-platform software for genetic mapping. Mammalian Genome, 12: 930-932. 2001. [DOI] [PubMed] [Google Scholar]
- Megens HJ, Groenen MAM. Domesticated species from a treasure-trove for molecular characterization of Mendelian traits by exploiting the specific genetic structure of these species in across-breed genome wide association studies. Heredity, 109: 1-3. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles C, Wayne M. Quantitative trait locus (QTL) analysis. Nature Education, 1: 208 2008. [Google Scholar]
- Osman SAM, Sekino M, Nishihata A, Kobayashi Y, Takenaka W, Kinoshita K, Kuwayama T, Nishibori M, Yamamoto Y, Tsudzuki M. The genetic variability and relationships of Japanese and foreign chickens assessed by microsatellite DNA profiling. Asian-Australasian Journal of Animal Sciences, 19: 1369-1378. 2006. [Google Scholar]
- Ozaki K, Ohnishi Y, Iida A, Sekine A, Yamada R, Tsunoda T, Sato H, Sato H, Hori M, Nakamura Y, Tanaka T. Functional SNPs in the lymphotoxin-alpha gene that are associated with susceptibility to myocardial infarction. Nature Genetics, 32: 650-654. 2002. [DOI] [PubMed] [Google Scholar]
- Podisi BK, Knott SA, Dunn IC, Law AS, Burt DW, Hocking PM. Overlap of quantitative trait loci for early growth rate, and for body weight and age at onset of sexual maturity in chickens. Reproduction, 141: 381-389. 2011. [DOI] [PubMed] [Google Scholar]
- Qanbari S, Pausch H, Jansen S, Somel M, Strom TM, Fries R, Nielsen R, Simianer H. Classic selective sweeps revealed by massive sequencing in cattle. PLoS Genetics, 10: e1004148 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qanbari S, Seidel M, Strom TM, Mayer KFX, Preisinger R, Simianer H. Parallel selection revealed by population sequencing in chicken. Genome Biology and Evolution, 7: 3299-3306. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance KA, Hill WG, Keightley PD. Mapping quantitative trait loci for body weight on the X chromosome in mice. I. Analysis of a reciprocal F2 population. Genetical Research, 70: 117-124. 1997. [DOI] [PubMed] [Google Scholar]
- Rubin CJ, Zody MC, Eriksson J, Meadows JRS, Sherwood E, Webster MT, Jiang L, Ingman M, Sharpe T, Ka S, Hallböök F, Besnier F, Carlborg O, Bed'hom B, Tixier-Boichard M, Jensen P, Siegel P, Lindblad-Toh K, Andersson L. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature, 464: 587-591. 2010. [DOI] [PubMed] [Google Scholar]
- Reynaud CA, Anquez V, Grimal H, Weill JC. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell, 48: 379-388. 1987. [DOI] [PubMed] [Google Scholar]
- Roberts JR. Factors affecting egg internal quality and egg shell quality in laying hens. Journal of Poultry Science, 41: 161-177. 2004. [Google Scholar]
- Roberts V. British Poultry Standards. 5th ed. Blackwell Science, Oxford: 1997. [Google Scholar]
- Sasaki O, Odawara S, Takahashi H, Nirasawa K, Oyamada Y, Yamamoto R, Ishii K, Nagamine Y, Takeda H, Kobayashi E, Furukawa T. Genetic mapping of quantitative trait loci affecting body weight, egg character and egg production in F2 intercross chickens. Animal Genetics, 35: 188-194. 2004. [DOI] [PubMed] [Google Scholar]
- Schmid M, Nanda I, Hoehn H, Schartl M, Haaf T, Buerstedde JM, Arakawa H, Caldwell RB, Weigend S, Burt DW, Smith J, Griffin DK, Masabanda JS, Groenen MAM, Crooijmans RPMA, Vignal A, Fillon V, Morisson M, Pitel F, Vignoles M, Garrigues A, Gellin J, Rodionov AV, Galkina SA, Lukina NA, Ben-Ari G, Blum S, Hillel J, Twito T, Lavi U, David L, Feldman MW, Delany ME, Conley CA, Fowler VM, Hedges SB, Godbout R, Katyal S, Smith C, Hudson Q, Sinclair A, Mizuno S. Second report on chicken genes and chromosomes 2005. Cytogenetic and Genome Research, 109: 415-479. 2005. [DOI] [PubMed] [Google Scholar]
- Schmid M, Smith J, Burt DW, Aken BL, Antin PB, Archibald AL, Ashwell C, Blackshear PJ, Boschiero C, Brown CT, Burgess SC, Cheng HH, Chow W, Coble D, Cooksey A, Crooijmans RPMA, Damas J, Davis RVN, de Koning DJ, Delany ME, Derrien T, Desta TT, Dunn IC, Dunn M, Ellegren H, Eory L, Erb I, Farre M, Fasold M, Fleming D, Flicek P, Fowler KE, Fresard L, Froman DP, Garceau V, Gardner PP, Gheyas AA, Griffin DK, Groenen MAM, Haaf T, Hanotte O, Hart A, Hasler J, Hedges SB, Hertel J, Howe K, Hubbard A, Hume DA, Kaiser P, Kedra D, Kemp SJ, Klopp C, Kniel KE, Kuo R, Lagarrigue S, Lamont SJ, Larkin DM, Lawal RA, Markland SM, McCarthy F, McCormack HA, McPherson M, Motegi A, Muljo SA, Munsterberg A, Nag R, Nanda I, Neuberger M, Nitsche A, Notredame C, Noyes H, O'Connor R, O'Hare EA, Oler AJ, Ommeh SC, Pais H, Persia M, Pitel F, Preeyanon L, Barja PP, Pritchett EM, Rhoads DD, Robinson CM, Romanov MN, Rothschild M, Roux P-F, Schmidt CJ, Schneider A-S, Schwartz MG, Searle SM, Skinner MA, Smith CA, Stadler PF, Steeves TE, Steinlein C, Sun L, Takata M, Ulitsky I, Wang Q, Wang Y, Warren WC, Wood JMD, Wragg D, Zhou H. Third repots on chicken genes and chromosomes 2015. Cytogenetic and Genome Research, 145: 78-179. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiweis MA, Hester PY, Settar P, Moody DE. Identification of quantitative trait loci associated with egg quality, egg production, and body weight in an F2 resource population of chickens. Animal Genetics, 37: 106-112. 2006. [DOI] [PubMed] [Google Scholar]
- Smith J, Burt DW. Parameters of the chicken genome (Gallus gallus). Animal Genetics, 29: 290-294. 1998. [DOI] [PubMed] [Google Scholar]
- Tixier-Boichard M, Bordas A, Rognon X. Characterisation and monitoring of poultry genetic resources. World's Poultry Science Journal, 65, 272-285. 2009. [Google Scholar]
- Tsudzuki M. Japanese native chickens. In The Relationship Between Indigenous Animals and Humans in APEC Region. (Chang HL, Huang YC. eds). pp. 91-116. CSAS, Tainan: 2003. [Google Scholar]
- Tsudzuki M, Onitsuka S, Akiyama R, Iwamizu M, Goto N, Nishibori M, Takahashi H, Ishikawa A. Identification of quantitative trait loci affecting shank length, body weight and carcass weight from the Japanese cockfighting chicken breed, Oh-Shamo (Japanese Large Game). Cytogenetic and Genome Research, 117: 288-295. 2007. [DOI] [PubMed] [Google Scholar]
- Tuiskula-Haavisto M, Honkatukia M, Vilkki J, de Koning DJ, Schulman NF, Maki-Tanila A. Mapping of quantitative trait loci affecting quality and production traits in egg layers. Poultry Science, 81: 919-927. 2002. [DOI] [PubMed] [Google Scholar]
- Tuiskula-Haavisto M, de Koning DJ, Honkatukia M, Schulman NF, Maki-Tanila A, Vilkki J. Quantitative trait loci with parent-of-origin effects in chicken. Genetical Research, 84: 57-66. 2004. [DOI] [PubMed] [Google Scholar]
- Tuiskula-Haavisto M, Honkatukia M, Preisinger R, Schmutz M, de Koning DJ, Wei WH, Vilkki J. Quantitative trait loci affecting eggshell traits in an F2 population. Animal Genetics, 42: 293-299. 2011. [DOI] [PubMed] [Google Scholar]
- Wang MS, Li Y, Peng MS, Zhong L, Wang ZJ, Li QY, Tu XL, Dong Y, Zhu CL, Wang L, Yang MM, Wu SF, Miao YW, Liu JP, Irwin DM, Wang W, Wu DD, Zhang YP. Genomic analyses reveal potential independent adaptation to high altitude in Tibetan chickens. Molecular Biology and Evolution, 32: 1880-1889. 2015. [DOI] [PubMed] [Google Scholar]
- Wardecka B, Olszewski R, Jaszczak K, Zieba G, Pierzchala M. Preliminary mapping of QTLs affecting egg quality on chromosomes 1–5 in chickens. Czech Journal of Animal Science, 48: 97-105. 2003. [Google Scholar]
- Wolc A, Arango J, Settar P, Fulton JE, O'Sullivan NP, Preisinger R, Habier D, Fernando R, Garrick DJ, Hill WG, Dekkers JCM. Genome-wide association analysis and genetic architecture of egg weight and egg uniformity in layer chickens. Animal Genetics, 43, 87-96. 2012. [DOI] [PubMed] [Google Scholar]
- Wolc A, Arango J, Jankowski T, Dunn I, Settar P, Fulton JE, O'Sullivan NP, Preisinger R, Fernando RL, Garrick DJ, Dekkers JC. Genome-wide association study for egg production and quality in layer chickens. Journal of Animal Breeding and Genetics, 131: 173-182. 2014. [DOI] [PubMed] [Google Scholar]
- Wragg D, Mwacharo JM, Alcalde JA, Hocking PM, Hanotte O. Analysis of genome-wide structure, diversity and fine mapping of Mendelian traits in traditional and village chickens. Heredity, 109: 6-18. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D, Kerje S, Lundström K, Babol J, Schütz K, Jensen P, Andersson L. Quantitative trait loci analysis of egg and meat production traits in a red junglefowl x White Leghorn cross. Animal Genetics, 37: 529-534. 2006. [DOI] [PubMed] [Google Scholar]
- Yalcin B, Flint J. Association studies in outbred mice in a new era of full-genome sequencing. Mammalian Genome, 23: 719-726. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G, Shen M, Yuan J, Sun C, Duan Z, Qu L, Dou T, Ma M, Lu J, Guo J, Chen S, Qu L, Wang K, Yang N. Genome-wide association study dissects genetic architecture underlying longitudinal egg weights in chickens. BMC Genomics, 16: 746 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Ishikawa A, Goto T, Goto N, Nishibori M, Tsudzuki M. QTL mapping for meat colortr aits using the F2 intercross between the Oh-Shamo (Japanese Large Game) and White Leghorn chickens. Journal of Poultry Science, 50: 198-205. 2013. [Google Scholar]
- Zhang GX, Fan QC, Wang JY, Zhang T, Xue Q, Shi HQ. Genome-wide association study on reproductive traits in Jinghai Yellow Chicken. Animal Reproduction Science, 163: 30-34. 2015. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu X, Wang Z, Zhang Y, Wang S, Wang N, Ma L, Leng L, Wang S, Wang Q, Wang Y, Tang Z, Li N, Da Y, Li H. Selection signature analysis implicates the PC1/PCSK1 region for chicken abdominal fat content. PLoS One, 7: e40736 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


