Abstract
Sperm motility is an essential trait for successful fertilization in animals. In birds, ejaculated sperm migrate into sperm storage tubules before fertilization and are stored in a quiescent state. We previously reported that this type of sperm's flagellar quiescence was induced by lactic acid through flagellar dynein ATPase inactivation following cytoplasmic acidification (<pH 6.0). However, signal transduction in the sperm cells leading to motility inactivation is not well understood. The aim of the present study was to investigate the role of protein kinases in putative signal transduction in quail spermatozoa motility in vitro. Following incubation with bisindolylmaleimide II (BisII), a potent-competitive protein kinase C (PKC) inhibitor, sperm motility decreased in a dose related-manner. However, no such inhibitory effect was found in sperm exposed to bisindolylmaleimide V, H-89, or LY294002, a weak inhibitor of PKC, a potent inhibitor of protein kinase A (PKA) and a selective inhibitor of phosphatidylinositol 3-kinase, respectively. BisII-treated sperm exhibited no significant differences in pHi, [Ca2+]i, mitochondrial activity, intracellular cAMP or ATP concentration, as well as dynein ATPase activity, compared to the control sperm. However, when the phosphorylated substrate proteins by PKC were detected by Western blot analysis, the intensity of the band in sperm incubated in the presence of BisII decreased. Moreover, immunoreactive PKCι and µ isoforms in the sperm lysates were also detected. These results indicated that the PKC signaling pathway may be involved in sperm motility regulation, and protein phosphorylation by PKC may be required to maintain flagellar movement in the Japanese quail.
Keywords: Japanese quail, protein kinase C, protein phosphorylation, signal transduction, sperm motility
Introduction
Sperm motility is an essentialtrait for successful fertilization in animals whose spermatozoa have ATP-fueled flagella. Before ejaculation/release, spermatozoa are stored in a quiescent state in the male reproductive tract; however, upon ejaculation/release sperm motility is initiated by various external cues such as osmolality changes (Morisawa and Suzuki, 1980), changes in extracellular potassium concentration (Morisawa et al., 1983), sperm activating substances (Yoshida et al., 2002; Watanabe et al., 2010), or as a result of the dilution of a putative sperm inactivation factor present in seminalpl asma (Mochida et al., 1999).
In avian species, flagellar beating is not observed in sperm stored in the vas deferens; however, after dilution by a sperm extender, sperm motility is initiated (Supplementary Video 1). Once ejaculated, spermatozoa migrate to, and are stored in, the lumen of sperm storage tubules (SSTs), simple tubular invaginations located between the vagina and uterus (Bobr et al., 1964), where they can remain without loss of fertilizability for long periods of time (up to 15 weeks) at normal body temperatures (41°C) (Birkhead, 1993). In birds, it is of particular interest that spermatozoa are able to undergo motility alteration, from the quiescent to active state, within the female reproductive tract twice, after copulation and after their release from the SSTs (Bakst, 1987; Hiyama et al., 2013). It is known that sperm flagellar movements are generated by the sliding of tubulin and ATP-driven dynein motor protein in the axoneme (Summers and Gibbons, 1971), although the mechanism leading to this axonemal sliding is not well understood. In our previous study, we showed that low oxygen and high lactic acid concentrations are established in quail SSTs (Matsuzaki et al., 2015). Flagellar quiescence was induced by lactic acid through flagellar dynein ATPase inactivation following cytoplasmic acidification (<pH 6.0). This sperm quiescence occurs due to a narrow pH optima window (between 7.5 and 8.6) of dynein ATPase. However, the signal transduction in the cells leading to sperm quiescence is not well understood.
The aim of the present study was to investigate the role of protein kinases in putative signal transduction in quail spermatozoa motility. We employed 4 protein kinase inhibitors: bisindolylmaleimide II (BisII), a potent -competitive protein kinase C (PKC) inhibitor (Mahata et al., 2002); bisindolylmaleimide V (BisV), a weak inhibitor of PKC (Mahata et al., 2002); H-89, a potent, cell permeable inhibitor of protein kinase A (PKA) (Chijiwa et al., 1990) and LY294002, a selective inhibitor of phosphatidylinositol 3-kinase (PI3-kinase) (Vlahos et al., 1994).
Materials and Methods
Animal and Tissue Preparation
Male Japanese quails, Coturnix japonica, 8–20 weeks of age (Motoki Corporation, Tokorozawa, Japan), were maintained individually under a photoperiod of 14 L:10D (lights went on at 05:00) with ad libitum access to water and a commercial diet (Motoki Corporation). Semen was obtained from male quails during mating prior to ejaculation in accordance with the procedure of Kuroki and Mori (1997). Semen obtained from two to three males was suspended in Hanks' balanced salt solution (HBSS). The concentrations of sperm were measured with a hemocytometer and sperm viability was assessed using a LIVE/DEAD sperm viability kit according to the manufacturer's instructions (Molecular Probes, Thermo Fisher Scientific K. K., Yokohana, Japan). Sperm were incubated at 39°C in all experiments. The experimental procedures for the care and use of animals were carried out in accordance with the approved guidelines of the Animal Care Committees of Shizuoka University (Approval number: 28–13).
Sperm Motility Analysis
Ejaculated sperm were incubated with HBSS containing various concentrations of BisII (0, 0.1, 0.3, 1, 3 or 10 µM), BisV (0, 0.1, 0.3, 1, 3 or 10 µM), H-89 (0, 0.01, 0.1, or 1 µM) or LY294002 (0, 1, 3 or 10 µM). Motility was evaluated by observing sperm in several areas of the petri dish directly using a stereomicroscope, and motility qualitative scores were assigned using an arbitrary grading system from 0 to 4, with a score of 0 indicating no movement, 1 indicating tail movements, but not sperm progression, 2 indicating that a large percentage of spermatozoa showed progressive, but not rectilinear, movement, 3 indicating that a large percentage of spermatozoa showed rectilinear, but not vigorous, movement and 4 indicating that a large percentage of spermatozoa showed vigorous rectilinear movement (Wheeler and Andrews, 1943). The sperm motility analysis was performed by one examiner in order to avoid changes due to subjectivity.
Measurement of Intracellular Ca2+ Concentration
A Ca2+ -sensitive indicator dye, Fluo-8H AM (AAT Bioquest, Inc., California, U. S. A.), was used to measure intracellular Ca2+ concentration ([Ca2+]i). The ejaculated sperm (2×107 cells /ml) were incubated with 1µM Fluo-8H AM for 10 min at 39°C. After incubation, the sperm suspension was added to sperm extender, supplemented with or without 1 µM BisII, and incubated for an additional 10 min. For positive control experiments, 1 µM A23187 (Sigma) was included in the incubation mixture. Sperm [Ca2+]i was determined by spectrofluorophotometry (RF-5300PC, Shimadzu Corporation, Kyoto, Japan).
Intracellular pH Measurement
For intracellular pH (pHi) measurement, the ejaculated sperm (2×107 cells /ml) were incubated with 1 µM BCECF-AM, a fluorescent pHi indicator (Molecular Probes), for 10 min at 39°C. After incubation, the sperm suspension was added to a sperm extender, supplemented with or without 1 µM BisII, and incubated for an additional 10 min. Sperm pHi was determined by spectrofluorophotometry (Shimadzu).
Evaluation of Mitochondrial Activity
The mitochondrial activity of the ejaculated spermatozoa was evaluated by staining with JC-1 (5,5′,6,6′-tetrachloro-1, 1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide, Molecular Probes). When the membrane potential of the inner mitochondrial membrane is high, indicating an active state, JC-1 emits orange fluorescence (590 nm), while green light (530 nm) is produced at a low membrane potential. The ejaculates were suspended in HBSS containing 1 µM BisII and 125nM JC-1 and incubated for 10 min. As a negative control, 10 µM carbonylcyanide m-chlorophenylhydrazone (CCCP, Sigma-Aldrich Japan K. K., Tokyo, Japan) was included in the incubation mixture. After 10 min, the fluorescent intensity (FI) ratio (590 nm / 530 nm) was determined by spectrofluorophotometry (Shimadzu).
ATP and cAMP Assay
For measurements of intracellular ATP, the ejaculated sperm (2×107 cells /ml) were incubated with or without BisII (1 µM) for 10 min. After washing with sperm extender, the sperm pellet was dissolved in an ATP assay reagent (“Cellno” ATP Assay reagent, TOYO B-Net Co., Ltd., Tokyo, Japan) and the fluorescent signal measured using ImageQuant™ LAS 500 (GE Healthcare Japan Corporation, Hino, Japan). Intracellular cAMP was detected using a cAMP Biotrak enzyme immunoassay kit (GE Healthcare) according to the manufacturer's instructions. The optical density of the solution, at 492 nm, was measured using a microplate reader (Tecan Japan Co., Ltd., Kawasaki, Japan).
Measurement of ATPase Activity
Sperm ATPase activity was determined as described previously (Matsuzaki et al., 2015). Briefly, ejaculated sperm were washed with an assay buffer containing 120 mM KCl, 10 mM β-glycerophosphate, 1 mM dithiothreitol, 1.8 mM MgSO4 and 10 µM CCCP buffered at pH 7.0 with 10 mM HEPES, and the plasma membrane of the spermatozoa was removed by incubating with an assay buffer containing 0.1% Triton X-100 for 2 min. After washing with the assay buffer, the sperm were suspended in the assay buffer with or without BisII (1 µM). ATP (final concentration: 1 mM) was added to the incubation mixture and was incubated for 30 min at 39°C. The reaction was terminated by the addition of ice-cold trichloroacetic acid. The free phosphoric acid in the incubation mixture was colorized by the addition of ammonium molybdate and ferrous sulfate in the presence of sulfuric acid, and the opticaldensity of the solution, at 650 nm, was measured using a microplate reader (Tecan Japan).
Immunoblotting
Ejaculated sperm were collected as described above and were homogenized, sonicated, and the supernatant collected via centrifugation at 20,400×g for 10 min. Protein concentration was measured using a BCA protein assay kit (Pierce, Thermo Fisher Scientific). The extract (10 µg protein per lane) was resolved by SDS-PAGE (Laemmli, 1970) on 12% polyacrylamide gel and then transferred onto a PVDF membrane (Merck Japan, Tokyo, Japan). Following transfer and blocking for 30 min with 5% skimmed milk, the membrane was incubated for 1 hour with a rabbit anti-phospho-(Ser) PKC substrate polyclonal antibody (anti-phospho-PKC substrate antibody, Cell Signaling Technology Japan, K. K., Tokyo, Japan) and subsequently incubated for 30 min with goat anti-rabbit secondary antibodies conjugated with horse-radish peroxidase (MP Biomedicals, LLC-Cappel Products, California, U.S.A.). PKC isoforms in the sperm lysates were detected by anti-PKC isoform antibodies (anti-PKC α, β, γ, δ, ε, η, θ, ι, μ and ζ) included in the PKC sampler kit and according to the manufacturer's instructions (Nippon BD Co., Ltd., Tokyo, Japan).
Data Analysis
Data were analyzed for significant differences by Student's t-test. For percentage data, an arcsine square root transformation was performed and the transformed data compared by Student's t-test. The motility score comparisons between groups were performed by Mann-Whitney U test. Differences were considered statistically significant at P<0.05.
Results
To investigate the signal transduction pathway regulating the sperm motility in vitro, we incubated the ejaculated spermatozoa in the presence of various protein kinase inhibitors (Fig. 1). Following incubation with BisII, sperm motility decreased in a dose-dependent manner; however, no such inhibitory effects were found in sperm exposed to BisV, H-89 or LY294002 at a dose higher than the reported IC50 of each protein kinase inhibitor (Mahata et al., 2002; Chijiwa et al., 1990; Vlahos et al., 1994). In order to clarify the sperm motility arrest mechanism in response to BisII treatment, we next measured various sperm parameters after incubation of the sperm with 1 µM BisII. The results are presented in Fig. 2. Unexpectedly, pHi (panelA), [Ca2+]i (panel B), mitochondrial activity (panel C), intracellular cAMP (panel D), ATP concentration (panelE) and dynein ATPase activity (panelF) were not significantly affected by the addition of BisII. To elucidate further, we confirmed the phosphorylation status of the proteins in the sperm incubated with BisII by detection of sperm lysates with an anti-phospho-PKC substrate antibody (Fig. 3A), which interacts with the phosphorylated version of PKC substrate proteins. As the results show, immunoreactive bands in the control sperm were detected as smear bands migrating around 25–85 kDa (lane 0). Importantly, the intensity of these bands decreased in the presence of BisII. The decrease in the intensity of the bands at 85, 50, 28 and 27 kDa (indicated by asterisks) coincided with the sperm motility data (Fig. 1A). Moreover, the presence of the immunoreactive PKCι and µ isoforms in the sperm lysates were detected by Western blot analysis with anti-PKC isoform antibodies (Fig. 3B).
Fig. 1.
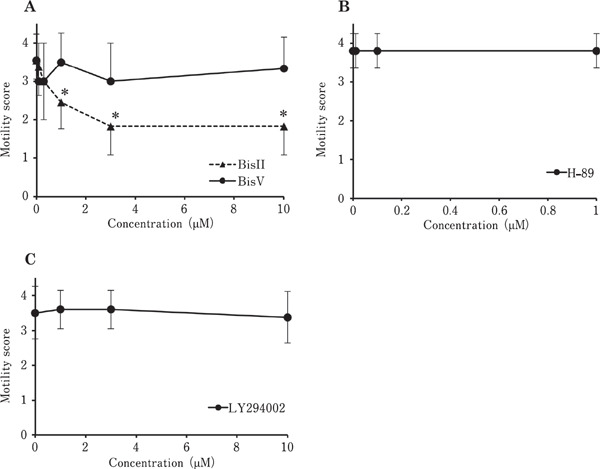
Effects of protein kinase inhibitor on sperm motility in vitro. Ejaculated sperm were suspended in media containing various concentrations of BisII or BisV (A), H-89 (B) or LY294002 (C) and sperm motility was evaluated on a scale of 0 to 5 at 30 min incubation. Values shown are means±SD from three independent experiments. *, P<0.05 vs. 0 µM.
Fig. 2.
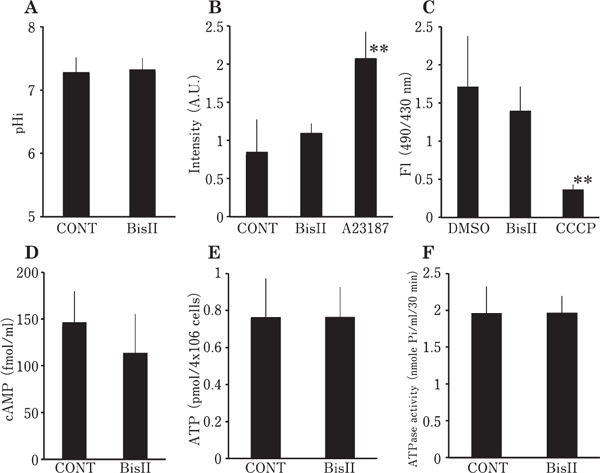
Measurement of various sperm parameters in the presence of BisII. Intracellular pH (pHi) (A), intracellular Ca2+ concentration (B) and mitochondrial activity (C) were measured by spectrofluorometry. cAMP (D), ATP (E) and ATPase activity (F) in the sperm lysates were assayed using a microplate reader. Values are means±SD of 3 independent experiments. No significant difference was found between CONT (vehicle alone) and BisII (1 µM) in all parameters tested. In panel B, calcium ionophore, A23187 (1 µM) was added as a positive control. In panel C, uncoupler CCCP (10 µM) was included as a negative control.
Fig. 3.
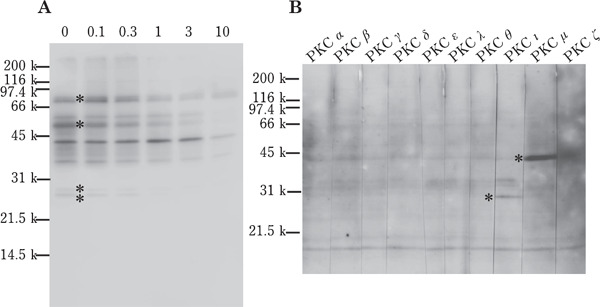
Western blot analysis of sperm lysates. (A) Ejaculated sperm were incubated with BisII (0, 0.1, 0.3, 1, 3 or 10 µM) for 30 min and the sperm lysates were separated by SDS-PAGE followed by Western blot analysis using anti-phospho-PKC substrate antibody. The intensity of the bands indicated by asterisk in lane 0 was decreased by the addition of 1 µM BisII. (B) The lysates of fresh ejaculates were separated by SDS-PAGE and detected with anti-PKC antibodies. The immunoreactive band in lane PKCι and PKCµ was indicated by asterisk. A representative image of repeated experiments is shown.
Discussion
A significant finding of the present study is that BisII, a selective inhibitor for PKC, induced sperm motility inactivation in the Japanese quail. It is reported in the spermatozoa from many animals, including mammals and marine invertebrates, that cAMP-dependent PKA-mediated signal transduction contributes to the initiation and maintenance of flagellar beating (Si and Okuno, 1995; Nomura et al., 2000). However, in our results, H-89, a selective inhibitor for PKA, had no inhibitory effects on quailsperm motility (Fig. 1B). In addition, supplementation of dibutyryl cyclic AMP, a cAMP analogue in the sperm extender, had no effect on sperm motility (data not shown). Moreover, the current study showed that the intracellular cAMP concentration did not change in the extender supplemented with 1 µM BisII, where sperm motility was significantly inhibited (Fig. 2D). Many types of kinases and phosphatases are implicated in sperm motility initiation and maintenance in various species. Indeed, in sea urchin sperm, both cAMP-dependent and cAMP-independent phosphorylation of proteins, including PKC substrates, contribute to flagellar movement (Brokaw, 1987; White et al., 2007). Therefore, it seems that PKA-mediated cell signaling may not make a major contribution to sperm motility regulation in Japanese quail.
We employed a specific PI3-kinase inhibitor, LY294002, for analysis of the signaling pathway in sperm motility because Ashizawa et al. (2009) previously reported that chicken sperm motility was suppressed by this inhibitor. However, in our test, no inhibitory effect was found within the wide dose ranges over the reported IC50 value (Fig. 1C) (Vlahos et al., 1994). Although the details of this discrepancy between chickens and quails are not known, the previous report focused on the unique phenomenon of reversible temperature-dependent immobilization in chicken sperm, in which sperm become immotile at 40°C, although motility is restored by decreasing the temperature (i.e. 30°C) in a simple salt solution (ie, 150 mM NaCl with 20 mM N-Tris (hydroxymethyl) methyl-2-aminoethanesulfonic acid) (Ashizawa et al., 1994). We did not examine whether this unique trait functions in quail spermatozoa, although Wishart and Wilson (1999) reported no such temperature-dependent immobilization in the Japanese quail. From these reports, we assume that the intracellular signal transduction leading to the maintenance of sperm motility in quails and chickens may differ. Indeed, evidence has shown that PKC activation may contribute to a decrease in the flagellar movement of fowlspermatozoa (Ashizawa et al., 1994), which is contrary to our observations in the Japanese quail.
In our recent research, we found that lactic acid in the SST lumen immobilized the resident sperm through cytoplasmic acidification that led to the inactivation of dynein ATPase (Matsuzaki et al., 2015). In contrast, BisII-mediated sperm motility inactivation is not related to pHi (Fig. 2A) or ATPase activity changes (Fig. 2F), indicating that the signal transduction between BisII and lactic acid-mediated sperm quiescence differ. To identify the cellular events caused by the BisII treatment, we measured several parameters in the cells and none of the the parameters, including [Ca2+]i (Fig. 2B), cAMP (Fig. 2D), and ATP (Fig. 2E) concentrations and mitochondrial activity (Fig. 2C), were affected, suggesting that BisII-mediated motility inactivation is not linked to these parameters. The results were unexpected because one of these parameters generally correlates to sperm motility in many species. For example, sperm -attracting and -activating factor (SAAF) in Ciona induces Ca2+ influx, membrane hyperpolarization and activation of adenylyl cyclase to produce cAMP for sperm motility activation (Nomura et al., 2004). A transient increase in intracellular cAMP and subsequent cAMP-dependent phosphorylation of dynein subunits were observed in response to the addition of SAAF (Shiba and Inaba, 2014; Nomura et al., 2000). Thus, cAMP is one of the important factors in SAAF-induced sperm motility activation in Ciona. In sea urchin sperm, mitochondrialrespiration is a good indicator of motility (Tombes and Shapiro, 1985). We also reported that heat shock protein 70 mediated sperm motility activation is associated with increases in [Ca2+]i and intracellular ATP in the Japanese quail(Hiyama et al., 2013). Although none of these parameters tested were altered under the conditions employed here, the decrease in phosphorylation of several PKC substrate proteins in the presence of BisII (Fig. 3A) should be noted. It was reported that PKC-mediated phosphorylation of 200, 100, 65 and 28 kDa proteins enhances sperm motility in sea urchin (White et al., 2007), and several PKC target proteins are reported to be involved in sperm physiological responses such as the induction of acrosomal exocytosis (Ashizawa et al., 2006; Harayama and Miyake, 2006). Although we did not identify the nature of these phosphoproteins, the phosphorylation pattern coincided closely with the motility data (Fig. 1A and Fig. 3A), indicating that these phosphoproteins may be required for the maintenance of sperm motility in Japanese quail. The identification of the nature of these phosphoproteins awaits discovery in future studies.
It was found by Western blotting, using anti-PKC isoform antibodies, that immunoreactive PKCι and µ exist in the lysates of quail spermatozoa (Fig. 3B). Three classifications for PKC isoforms have been suggested based on the mode of activation by second messenger requirements; a conventional PKC (α, β and γ isoforms) that requires both Ca2+ and diacylglycerol (DG), a novel PKC (δ, ε, η and θ isoforms) that only needs DG and an atypical PKC (ι and ζ), which uses neither Ca2+ nor DG for activation (Mellor and Parker, 1998). PKCµ was originally identified in human cells and was categorized as atypical PKC; however, in later studies, it was referred to as protein kinase D due to its unique structure (Wang, 2006). Although direct experimental evidence is not available, our preliminary experiments showing the inefficacy of phorbolester, which mimics the action of DG for the restoration of BisII-induced sperm inactivation, support our hypothesis that atypical PKC (i. e. PKCι and µ) may be involved in the cell signaling of sperm motility (Supplementary Video 2). In addition, the finding that the BisII-induced sperm motility inactivation did not coincide with [Ca2+]i also supports this idea (Fig. 2B).
In conclusion, this study provides evidence that the PKC signaling pathway may be involved in sperm motility regulation and that protein phosphorylation by PKC may be required for the maintenance of flagellar movement in the Japanese quail. As BisII-mediated sperm motility inactivation differs from that of lactic acid-induced sperm inactivation in the SSTs, further investigation is required to understand the physiological importance of PKC-mediated sperm motility regulation in the Japanese quail.
Acknowledgments
MM is supported by fellowships from the Japan Society for Promotion of Science (DC2). This work was supported by Grant-in-Aid for Challenging Exploratory Research (16K15022 to TS), Japanese Association for Marine Biology (JAMBIO) (No. 24-64 and No. 25-57 to TS), Toukai Foundation for Technology (No. 7 to TS) and Kieikai Research Foundation (to TS).
Supplementary Information (SI)
https://www.jstage.jst.go.jp/article/jpsa/54/1/54_0160079/_article/supplement/
Supplementary Video 1: Motility initiation of the stored sperm in the vas deferens. The spermatozoa were collected from the vas deferens and placed on a glass slide. Sperm movement was recorded under a stereomicroscope and HBSS was dropped on the sperm mass (at 6 sec). Note that sperm motility was initiated immediately after the addition of HBSS.
Supplementary Video 2: Inefficacy of phorbol ester on sperm motility restoration in the presence of BisII. The ejaculated sperm were incubated with 1 µM BisII and 10 µM phorbol-12-myristate-13-acetate dissolved in HBSS perfused from a glass pipette connected to a micro injector. Sperm movement was recorded under a stereomicroscope. Note that no sperm motility restoration was observed.
References
- Ashizawa K, Katayama S, Kobayashi T, Tsuzuki Y. Possible role of protein kinase C in regulation of flagellar motility and intracellular free Ca2+ concentration of fowlspermatozoa. Journal of Reproduction and Fertility, 101: 511-517. 1994. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Wishart GJ, Katayama S, Takano D, Ranasinghe AR, Narumi K, Tsuzuki Y. Regulation of acrosome reaction of fowlspermatozoa: evidence for the involvement of protein kinase C and protein phosphatase-type 1 and/or -type 2A. Reproduction, 131: 1017-1024. 2006. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Omura Y, Katayama S, Tatemoto H, Narumi K, Tsuzuki Y. Intracellular signal transduction pathways in the regulation of fowl sperm motility: evidence for the involvement of phosphatidylinositol 3-kinase (PI3-K) cascade. Molecular Reproduction and Development, 76: 603-610. 2009. [DOI] [PubMed] [Google Scholar]
- Bakst MR. Anatomicalbasis of sperm-storage in the avian oviduct. Scanning Microscopy, 1: 1257-1266. 1987. [PubMed] [Google Scholar]
- Birkhead TR. Sexualsel ection and the temporalseparation of reproductive events: sperm storage data from reptiles, birds and mammals. Biological Journal of the Linnean Society, 50: 295-311. 1993. [Google Scholar]
- Bobr LW, Lorenz FW, Ogasawara FX. Distribution of spermatozoa in the oviduct and fertility in domestic birds. I. Residence sites of spermatozoa in fowloviducts. Journalof Reproduction and Fertility, 8: 39-47. 1964. [DOI] [PubMed] [Google Scholar]
- Brokaw CJ. Regulation of sperm flagellar motility by calcium and cAMP-dependent phosphorylation. Journal of Cellular Biochemistry, 35: 175-184. 1987. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino) ethyl] -5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. The Journal of Biological Chemistry, 265: 5267-5272. 1990. [PubMed] [Google Scholar]
- Harayama H, Miyake M. A cyclic adenosine 3′, 5′-monophosphate-dependent protein kinase C activation is involved in the hyperactivation of boar spermatozoa. Molecular of Reproduction and Development, 73: 1169-1178. 2006. [DOI] [PubMed] [Google Scholar]
- Hiyama G, Matsuzaki M, Mizushima S, Dohra H, Ikegami K, Yoshimura T, Shiba K, Inaba K, Sasanami T. Sperm activation by heat shock protein 70 supports the migration of sperm released from sperm storage tubules in Japanese quail (Coturnix japonica). Reproduction, 147: 167-178. 2013. [DOI] [PubMed] [Google Scholar]
- Kuroki M. and Mori M. Binding of spermatozoa to the perivitelline layer in the presence of a protease inhibitor. Poultry Science, 76: 748-752. 1997. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680-685. 1970. [DOI] [PubMed] [Google Scholar]
- Mahata M, Mahapatra NR, O'Connor DT, Mahata SK. Chromaffin cell catecholamine secretion: bisindolylmaleimide compounds exhibit noveland potent antagonist effects at the nicotinic cholinergic receptor in pheochromocytoma cells. Molecular pharmacology, 61: 1340-1347. 2002. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Mizushima S, Hiyama G, Hirohashi N, Shiba K, Inaba K, Suzuki T, Dohra H, Ohnishi T, Sato Y, Kohsaka T, Ichikawa Y, Atsumi Y, Yoshimura T, Sasanami T. Lactic acid is a sperm motility inactivation factor in the sperm storage tubules. Scientific Reports, 5: 17643 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochemical Journal, 32: 281-292. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida K, Kondo T, Matsubara T, Adachi S, Yamauchi K. A high molecular weight glycoprotein in seminal plasma is a sperm immobilizing factor in the teleost Nile tilapia, Oreochromis niloticus. Development Growth and Differentiation, 41: 619-627. 1999. [DOI] [PubMed] [Google Scholar]
- Morisawa M, Suzuki K. Osmolality and potassium ion: their roles in initiation of sperm motility in teleosts. Science, 210: 1145-1147. 1980. [DOI] [PubMed] [Google Scholar]
- Morisawa M, Suzuki K, Morisawa S. Effects of potassium and osmolality on spermatozoan motility of salmonid fishes. Journalof ExperimentalBiol ogy, 107: 105-113. 1983. [DOI] [PubMed] [Google Scholar]
- Nomura M, Inaba K, Morisawa M. Cyclic AMP- and calmodul-independent phosphorylation of 21 and 26 kda proteins in axoneme is a prerequisite for SAAF-induced motile activation in ascidian spermatozoa. Dvelopment Growth and Differentiation, 42: 129-138. 2000. [DOI] [PubMed] [Google Scholar]
- Nomura M, Yoshida M, Morisawa M. Calmodulin/calmodul-independent protein kinase II mediates SAAF-induced motility activation of ascidian sperm. Cell Motility and Cytoskeleton, 59: 28-37. 2004. [DOI] [PubMed] [Google Scholar]
- Shiba K, Inaba K. Distinct roles of soluble and transmembrane adenylyl cyclases in the regulation of flagellar motility in Ciona sperm. International Journal of Molecular Sciences, 15: 13192-13208. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y, Okuno M. Activation of mammalian sperm motility by regulation of microtubule sliding via cyclic adenosine 5′-monophosphate-dependent phosphorylation. Biology of Reproduction, 53: 1081-1087. 1995. [DOI] [PubMed] [Google Scholar]
- Summers KE, Gibbons IR. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proceedings of the National Academy of Science of the United States of America, 68: 3092-3096. 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombes RM, Shapiro BM. Metabolite channeling: a phosphoryl-creatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell, 41: 325-334. 1985. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). The Journal of Biological Chemistry, 269: 5241-5248. 1994. [PubMed] [Google Scholar]
- Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends in Pharmacological Sciences, 27: 317-323. 2006. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kubo H, Takeshima S, Nakagawa M, Ohta M, Kamimura S, Takayama-Watanabe E, Watanabe A, Onitake K. Identification of the sperm motility-initiating substance in the newt, Cynops pyrrhogaster, and its possible relationship with the acrosome reaction during internal fertilization. The International Journal of Developmental Biology, 54: 591-597. 2010. [DOI] [PubMed] [Google Scholar]
- Wheeler NC, Andrews FN. The influence of season on semen production in the domestic fowl. Poultry Science, 22: 361-367. 1943. [Google Scholar]
- White D, de Lamirande E, Gagnon C. Protein kinase C is an important signaling mediator associated with motility of intact sea urchin spermatozoa. Journal of Experimental Biology, 210: 4053-4064. 2007. [DOI] [PubMed] [Google Scholar]
- Wishart GJ, Wilson YI. Temperature-dependent inhibition of motility in spermatozoa from different avian species. Animal Reproduction Science, 57: 229-235. 1999. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Murata M, Inaba K, Morisawa M. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proceedings of the National Academy of Science of the United States of America, 99: 14831-14836. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


