Abstract
Separating breast meat with low water-holding capacity, conformation parameters (thickness, volume, bottom sarea, and perimeter), and color of chicken breast meat were measured by direct measurement and by imaging analysis with a digital camera. Samples were obtained from a production line. The L* value was used to separate the samples by three characteristics designating the quality of the meat: dark-colored samples (L*<50), normal-colored samples (50≤L*≤56), and light-colored samples (L*>56). Light-colored samples had higher moisture content, thawing loss, drip loss, and lower pH compared with those of normal- and dark-colored samples. Lower thickness was observed in the light-colored samples compared with those of normal- and dark-colored samples. Light- and normal-colored samples had a greater volume of meat than did the dark-colored samples. Imaging analysis showed that light-colored samples had a greater bottom area and perimeter compared with those of normal- and dark-colored samples. However, these conformation parameters showed low correlation with water-holding capacity, which was determined as thawing and drip loss of the samples. Therefore, the conformation parameters, determined by direct measurement or imaging analysis, could not be used to predict the water-holding capacity of breast meat. Nevertheless, waterholding capacity showed high correlation with the L* value of breast meat. Imaging analysis could be used to separate light-colored breast meat with mostly low water-holding capacity. The accuracy of determining the characteristics of light-, normal-, and dark-colored samples by imaging analysis was evaluated. The characteristics of light-colored samples were determined with higher accuracy by imaging analysis than were the characteristics of normal- and dark-colored samples. This result indicated that imaging analysis using a digital camera could be used to separate light-colored breast meat with mostly low water-holding capacity from normal- and dark-colored meat.
Keywords: broiler breast meat, conformation parameters, digital camera, imaging analysis, water-holding capacity
Introduction
Water-holding capacity is the ability of the protein in meat to hold water. In the poultry industry, water-holding capacity is an important factor that affects meat quality and yield (ElMasry et al., 2011). Generally, the methods for determining water-holding capacity, such as drip and cooking loss, destroy the meat sample during determination; thus, there is a need for a non-destructive method for assessing water-holding capacity (Qiao et al., 2007; Monroy et al., 2010; ElMasry et al., 2011). Imaging analysis, non-destructive method, is interesting for the assessment of meat quality in the recent years. Water-holding capacity is a physicochemical property of chicken breast meat; imaging analysis, using a digital camera, is presently used only for assessing the external appearance of the meat. Therefore, water-holding capacity of chicken breast meat determined by imaging analysis, seems to be impossible. However, these assumptions may be possible because one previous research reported that frozen breast meat with low water-holding capacity had more flat in shape during extended storage time (Lee et al., 2008). This change in the configuration of frozen breast meat is affected by water loss during storage and thawing. Fresh and chilled breast meat, with different water-holding capacity, may also manifest a difference in shape. However, whether breast meat with high, medium, or low waterholding capacity manifests differences in shape or conformation remains unknown. The color of breast meat has a high correlation with water-holding capacity. Light-colored breast meat has lower water-holding capacity than those of normal and dark-colored breast meats (Van Laack et al., 2000; Qiao et al., 2001; Petracci et al., 2004). Therefore, conformation parameters (thickness, volume, bottom area, and perimeter) and color might have correlation with water-holding capacity of breast meat. The conformation parameters and color of breast meat are characteristics of its external appearance that can be determined by non-destructive methods. AVernier caliper is used to determine the thickness of the meat, bead displacement is used to determine volume, a planimeter is used to measure the bottom area, and a HunterLab colorimeter is used to assess color. Nevertheless, direct measurements are inconvenient and time-consuming when used in the continuous processing of meat. Thus, imaging analysis with a digital camera may provide an alternative method for assessing the water-holding capacity by determining the conformation parameters and color, which have high correlation with the water-holding capacity of breast meat. Several high-performance techniques for determining the water-holding capacity of meat, such as the hyperspectral imaging technique (Qiao et al., 2007), near-infrared (NIR) hyperspectral imaging (ElMasry et al., 2011), and nuclear magnetic resonance (NMR) (Bertram et al., 2001), have been used. However, these techniques require costly equipment, whereas imaging analysis using a digital camera is inexpensive.
This study focused on the correlation between water-holding capacity and conformation parameters of breast meat assessed by direct measurement and imaging analysis using a digital camera. The conformation parameters, having a high correlation with the water-holding capacity of breast meat, could be adapted for imaging analysis using a digital camera; this analysis could be used to separate breast meat with low water-holding capacity during processing.
Materials and Methods
Broiler chickens, aged 40 to 44 days, with live weight of approximately 2.16 to 2.56 kg, were obtained from B. Foods Product International Co., Ltd., Lopburi province. Boneless and skinless broiler breast meats (160–190 g) were randomized within 8±2 h postmortem from a production line. The samples were analyzed at the physical laboratory of B. Foods Product International Co., Ltd., Lopburi province.
Two hundred and forty chicken breast meats were divided into three groups by color, using the L* value as follows (Petracci et al., 2004): dark-colored samples (L*<50), normal-colored samples (50≤L*≤56), and light-colored samples (L*>56). The three groups of breast meats were analyzed for chemical composition, pH value, and water-holding capacity (determined as thawing and drip loss). Thereafter, each sample from each group was analyzed for conformation parameters by direct measurement and imaging analysis. In this research aimed to assess using imaging analysis to separate breast meats with low water-holding capacity and the accuracy of imaging analysis in determining the characteristics of chicken breast meat.
Chemical Composition
The methods for determining the chemical composition of breast meat samples, including moisture content (determined using an oven at 105°C), protein content (determined using the Kjeldahl method), fat content (determined using the Soxhlet solvent extraction), and ash content (determined using a furnace at 550°C) are outlined in the Official Methods of Analysis (AOAC, 1999).
pH
The pH of breast meat was determined using a pH meter (798 MPT Titrino, Metrohm Ltd., CH-9101 Herisan, Switzerland). The pH value of ground breast meat samples was assessed at the ratio of 1:5 (wt/vol) of meat to distilled water, respectively.
Determination of Water-holding Capacity
Drip Loss
A whole piece of breast meat was weighed, before being sealed in a Ziploc polyethylene plastic bag, and stored at 4°C for 24 h. After storage, the drip was drained off, and the sample was blotted with filter paper and weighed. Then, the percentage of drip loss was calculated based on the initial weight before storage (Honikel, 1998).
Thawing Loss
A whole piece of breast meat was weighed and stored at −20°C for 24 h in a Ziploc polyethylene plastic bag. After 24 h, the sample was thawed in a chilled room at 0 to 4°C until the core temperature of the sample reached 0 to 4°C. Then, the sample was weighed again after separating syneresis, and the difference in the initial and final weights was calculated for the percentage of thawing loss.
Conformation Parameters of Breast Meat
Direct Measurement Techniques
Thickness
The greatest thickness of a whole piece of breast meat was determined using the Vernier caliper and reported in centimeters.
Volume
The volume (mL) of a whole piece of breast meat was analyzed using the bead substitution method; this method was based on the seed displacement method, modified for using plastic beads (Artan et al., 2010). Beads, 2.5 mm in diameter, were placed into a plastic box having a known volume. The volume of beads, displaced from the plastic box by chicken breast meat, was used to calculate the volume of the whole piece of breast meat.
Bottom Area
A whole piece of breast meat was placed on a plastic sheet and the bottom area was traced onto the sheet. The bottom area (in cm2) of chicken breast meat was determined using a planimeter (Placom KP-92N, Japan).
Color Value
A whole piece of breast meat was evaluated for color using the HunterLab colorimeter (C04-1005-631 ColorFlex, Reston, VA, USA). The color of the breast meat was evaluated using the CIE color system, including L* (lightness), a* (greenness and redness), and b* (blueness and yellowness). The values of L*, a*, and b* were measured on the boned surface of the sample using the port size of 31.8 mm (45°/0°) and illuminant D65 was used as the light source.
Imaging Analysis Using a Digital Camera
A whole piece of chicken breast meat was imaged in a light box equipped with four fluorescent lamps. The Lamptan T8/10W Daylight D65 50-cm bulb was placed above the sample at an angle of 45°. The intensity of the light in the black box was set to 1021.20±1.16 lx, as determined using the light intensity meter (Digital Lux Meter, LX1010B, Weafo International Industrial Ltd., China). The Cyber-shot DSC-T99 digital camera (Sony, USA) was used to image the sample as shown in Fig. 1. The top and side views of the sample were imaged using the object distance of 30 cm. The camera parameters were set at follows: ISO (ISO 100), white balance (fluorescence white balance 3), macro (auto), and flash (off). The color of the conveyer was white. The L*, a*, and b* values of the conveyer, determined using imaging analysis, were 78.40, −8.14, and 8.11, respectively. The images of the samples were analyzed with respect to the thickness, perimeter, area of the bottom, and color using the ImageJ program (ImageJ freeware, the National Institutes of Health, USA). The original color image of the top view of the chicken breast was converted to 8-bit grayscale. Then, the measurement scale was set using the ImageJ program. The grayscale image was then converted to a black and white image using the threshold command. The black area of the image was adjusted and filled in. Then, the black area of the image was analyzed with respect to the perimeter and the area of the bottom, using the measurement functions in the ImageJ program. The thickness of the sample was measured using the side view image. Using the ImageJ program, a vertical line was drawn starting at the base of the sample and ending at its highest point, and then the height of the line was measured. The color of the image of breast meat was analyzed using the RGB color model provided in the ImageJ program (R: red color, G: green color, B: Blue color); the colors were converted into the L*, a*, and b* values using a color calculator (EasyRGB, 2015).
Fig. 1.
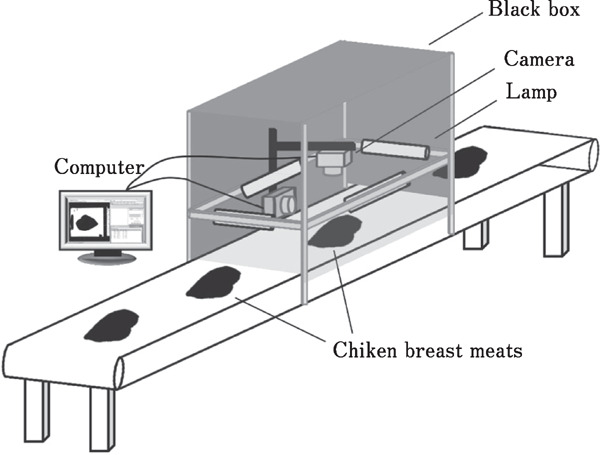
Imaging analysis using a digital camera
Accuracy of Determining Characteristics of Chicken Breast Meat
The 210 chicken breast meats were obtained from a processing line and separated using the L* value, which was determined using a HunterLab colorimeter, into three color characteristics; these included dark-colored samples (L*<50), normal-colored samples (50≤L*≤56), and light-colored samples (L*>56). The RGB color values of the samples were determined using imaging analysis. The R, G, and B values of the samples were then converted to L*, a*, and b* values using the EasyRGB color calculator. Thereafter, the accuracy of determining the characteristics of each sample, using imaging analysis, was evaluated by comparing the L* value obtained by the imaging analysis with that obtained using the HunterLab colorimeter; then, the percentages of correct and incorrect data were calculated.
Statistical Analysis
The completely randomized design (CRD) was applied with respect to the chemical composition, pH value, and water-holding capacity of the samples. The randomized complete block design (RCBD) was applied to color values and conformation parameters of the chicken breast meat samples. The data were analyzed using SPSS and one-way ANOVA. Significant differences between the means were analyzed by Duncan's multiple range tests. Correlation between the colors (L*, a*, and b*), conformation parameters, and water-holding capacity (determined as drip and thawing loss) were determined by linear and multiple regression using SPSS (SPSS program, SPSS Inc., Chicago, IL, USA).
Results and Discussion
Quality Characteristics of Differently Colored Chicken Breast Meats
The three color characteristics of chicken breast meats (dark-colored samples: L*<50; normal-colored samples: 50≤L*≤56; and light-colored samples: L*>56) were evaluated for chemical composition, pH, and water-holding capacity (determined as drip and thawing loss). The results are shown in Table 1; the light-colored samples had the highest (P<0.05) moisture content, thawing loss, drip loss, and lowest pH (P<0.05). This finding agrees with that of Qiao et al. (2001), who found that light-colored breast meat had the lowest pH, highest moisture, and lowest waterholding capacity compared with those of normal- and dark-colored breast meats. The high moisture content of the light-colored chicken breast meats may be caused by high water absorption during the water chilling of the carcass. Water chilling is a process for decreasing the temperature of a chicken carcass, using cold water, until the temperature of the carcass falls below 4°C process (ElMasry and Sun, 2010); this is performed before trimming and deboning a carcass. Barbut et al. (2005) reported that large intracellular gaps are observed in light-colored breast meat, which is characteristic of pale, soft, and exudative (PSE) meat. This could be contributed to more water being absorbed by the large intracellular gaps present in the light-colored samples than by the small intracellular gaps of normal- and dark-colored samples. The water absorbed and retained in the protein structure is termed free water. The fraction of free water in meat is mainly held by weak surface forces and is more easily released during processing than is bound and entrapped water (Huff-Lonergan and Lonergan, 2005). Moreover, breast meats having pale color and lower pH are similar in these characteristics to the PSE meats (Van Laack et al., 2000). Meat acquires PSE-like characteristics when the carcass is subjected to a rapid decrease in pH and high temperature during early postmortem. These factors cause protein denaturation, which can decrease the ability of the proteins to retain water, causing low water retention (Galobart and Moran, 2004). These factors contribute to the high thawing and drip loss in the light-colored chicken breast meats.
Table 1. Chemical composition, pH, and water-holding capacity (determined as thawing loss and drip loss) of broiler breast meats, evaluated using the three color characteristics.
| Chemical composition | Broiler breast meat |
||
|---|---|---|---|
| Dark (L*<50) | Normal (50≤L*≤56) | Light (L*>56) | |
| Moisture (%) | 74.26±0.12c | 76.15±0.03b | 76.88±0.06a |
| Protein (%) | 23.64±0.25a | 22.67±0.07b | 22.04±0.02b |
| Fat (%) | 2.70±0.17a | 1.32±0.10b | 1.27±0.08b |
| Ash (%) | 1.02±0.02a | 1.07±0.02a | 1.02±0.01a |
| pH | 5.73±0.00a | 5.62±0.01b | 5.49±0.00c |
| Thawing loss (%) | 2.57±0.18c | 4.46±0.05b | 5.70±1.20a |
| Drip loss (%) | 1.16±0.06c | 2.00±0.05b | 2.65±0.20a |
Means in the same row with different letters are significantly different (P<0.05).
The dark-colored samples were significantly higher in their protein content, which agrees with the results of Qiao et al. (2002). Differences in the color of chicken breast meats are attributed to different chemical compositions. Qiao et al. (2002) stated that a significant difference in the chemical composition of differently colored chicken breast meats may be caused by several factors, including processing conditions. Expectedly, the light-, normal-, and dark-colored chicken breast meats showed a significantly different waterholding capacity, as determined by different thawing loss and drip loss (P<0.05).
Colors of Breast Meat Determined by Direct Measurement and Imaging Analysis
The color characteristics of the three broiler breast meats were evaluated using direct measurement and imaging analysis. The results of the imaging analysis indicated that the light-colored samples had higher R, G, and L* values compared with those of the normal- and dark-colored broiler breast meats (Table 2). The colors of broiler breast meats, determined using imaging analysis, had higher L* and b* values, but a lower a* value, compared with those obtained using direct measurement. Previous studies have found that the L* and b* values of salmon (Yagiz et al., 2009), chicken, and pork (Girolami et al., 2013), measured using a machine vision system, demonstrated higher values compared with those obtained using a colorimeter. The color of the surface of the meat could be affected by the amount of overall light scattering and reflected electromagnetic wavelength, which was related to the amount of applied light that penetrates into the meat sample (Girolami et al., 2013). This indicated that the light in the colorimeter could penetrate into the meat sample deeper than the light in the machine vision system used in the imaging analysis (Girolami et al., 2013). This may be because the light source of the colorimeter is located closer to the sample that is the light source in the machine vision system. Normally, the outer layer of meat has a bright red color, caused by oxymyoglobin, whereas the lower layer (5 mm from the surface) is brown, caused by the oxidation of myoglobin to metmyoglobin under the conditions of low oxygen tension. The layers deeper than 5 mm from the surface are purple-red in color, which is caused by deoxymyoglobin (Girolami et al., 2013). Therefore, light reflection that occurs during imaging analysis results in a brighter color of the meat surface, indicated by the higher L* and b* values, than light reflection that occurs with using a colorimeter.
Table 2. Colors and water-holding capacity (determined as thawing loss and drip loss) of the three color characteristics of chicken breast meat.
| Parameters | Dark (L*<50) | Normal (50≤L*≤56) | Light (L*>56) |
|---|---|---|---|
| Thawing loss (%) | 2.48±0.23c | 4.51±0.21b | 5.76±0.28a |
| Drip loss (%) | 1.43±0.11c | 2.09±0.20b | 2.69±0.23a |
| Direct measurement data | |||
| L* | 48.35±0.25c | 54.34±0.06b | 58.21±0.29a |
| a* | 6.78±0.17a | 6.67±0.18a | 5.69±0.16b |
| b* | 11.81±0.29b | 16.03±0.21a | 16.51±0.24a |
| Imaging analysis data | |||
| R | 148.06±1.02c | 155.45±0.82b | 160.29±0.93a |
| G | 118.25±1.67c | 130.92±0.88b | 140.86±1.10a |
| B | 91.23±1.42c | 97.30±1.08b | 105.75±1.19a |
| L* | 51.92±0.57c | 56.18±0.32b | 59.47±0.40a |
| a* | 7.76±0.42a | 4.40±0.25b | 1.94±0.19c |
| b* | 19.32±0.35b | 21.68±0.32a | 21.44±0.36a |
Means in the same row with different letters are significantly different (P<0.05).
Correlation between the colors, obtained using direct measurement and using imaging analysis, and water-holding capacity (determined as thawing loss and drip loss) of chicken breast meats are presented in Table 3. The L* value, obtained using direct measurement, had the highest correlation with thawing loss (r=0.72). Imaging analysis showed that the G value (r=0.63), a* value (r=0.63), and L* value (r=0.61) had a higher correlation with thawing loss than did other color parameters. Thawing loss had a higher correlation with the color values of the breast meat that did drip loss. This may have been caused by the higher value of water loss, which was obtained using the method for estimating thawing loss. Thawing loss is mostly used to determine water or weight loss in the frozen chicken meat during storage and product delivery from the industry processing facilities to dealers or retail customers; drip loss is the determination of water loss during storage and transportation of chilled chicken meat. In this study, drip loss of breast meat had a high correlation only with the L* value obtained using direct measurement (r=0.62). All color parameters, determined using imaging analysis, showed low correlation with drip loss. This may be because of the narrow range of drip loss observed between the groups of samples. These results indicated that the color values of chicken meat, determined by imaging analysis, could not be used to accurately predict the degree of thawing loss and drip loss. However, imaging analysis with a digital camera could be used to determine the characteristics of differently colored breast meats, as shown by the considerable differences in the characteristics of our chicken breast meats. Light-colored chicken breast meats have a low water-holding capacity, which could negatively affect the yield and quality of the meat during processing. Therefore, separating the light-colored breast meat, using imaging analysis, could improve the yield and quality of the meat.
Table 3. Correlation between the colors and water-holding capacity (determined as thawing loss and drip loss) of chicken breast meat.
| Parameters | Correlation coefficient (r) |
||
|---|---|---|---|
| Thawing loss | Drip loss | ||
| Direct measurement data | |||
| L* | 0.72 | 0.62 | |
| a* | 0.23 | 0.06 | |
| b* | 0.60 | 0.34 | |
| Imaging analysis data | |||
| R | 0.55 | 0.27 | |
| G | 0.63 | 0.38 | |
| B | 0.43 | 0.26 | |
| L* | 0.61 | 0.36 | |
| a* | 0.63 | 0.45 | |
| b* | 0.40 | 0.20 | |
The results in Table 2 show that there were various levels of water-holding capacity, determined as thawing loss and drip loss, between the groups of breast meat samples having different color characteristics. Therefore, all the groups of samples were used for assessing the correlation between the conformation parameters, and thawing and drip loss. In our further study, the parameters that had a high correlation with the water-holding capacity of chicken breast meat will be used to separate the breast meats with a low water-holding capacity using imaging analysis.
Conformation Parameters of Breast Meat Determined by Direct Measurement and Imaging Analysis
The three color characteristics of broiler breast meat, with different water-holding capacity, were evaluated with respect to their conformation parameters using direct measurement and imaging analysis. The results of the direct measurement showed that the light-colored meat had the least thickness (P<0.05) and greatest bottom area compared with those of normal- and dark-colored samples (Table 4). The light- and normal-colored samples had greater volume compared with that of the dark-colored samples. These results indicated that the light-colored samples were flatter in shape than were normal and dark-color samples. The results of the imaging analysis indicated that the light-colored samples had a greater perimeter compared with that of normal- and dark-colored samples. The light-colored samples had the least thickness, as determined by direct measurement, whereas no significant differences in thickness were obtained using imaging analysis. Therefore, the thickness determined by imaging analysis could not be applied for delineating the different characteristics of chicken breast meat. The conformation parameters of broiler breast meat, determined using imaging analysis, showed higher values for the bottom area and lower values for the thickness compared with the values obtained using direct measurement. The images obtained by the digital camera contained a shadow between the chicken breast and the conveyer belt; this complicated the measurement of the thickness and bottom area of the breast meat. The presence of the shadow may have contributed to the higher values for the bottom area, and lower ones for thickness, obtained using imaging analysis, compared with those obtained using direct measurement. However, the data obtained using direct measurement, and those obtained using imaging analysis, showed similar trends. The accuracy of determining the thickness and bottom area of the breast meat, using imaging analysis, could be improved by adjusting the position of the light until the image is clear, and by using a camera with a high resolution.
Table 4. Conformation parameters of the three color characteristics of chicken breast meat.
| Parameters | Dark L*<50 | Normal (50≤L*≤56) | Light L*>56 |
|---|---|---|---|
| Direct measurement data | |||
| Thickness (cm) | 3.56±0.06a | 3.56±0.05a | 3.41±0.04b |
| Bottom area (cm2) | 85.78±0.52a | 85.68±0.94a | 86.23±0.92a |
| Volume (mL) | 180.90±3.74b | 203.80±4.27a | 204.53±4.62a |
| Imaging analysis data | |||
| Thickness (cm) | 3.09±0.07a | 3.18±0.05a | 3.12±0.05a |
| Bottom area (cm2) | 92.83±0.84ab | 91.73±0.96b | 94.60±0.96a |
| Perimeter (cm) | 44.07±0.30b | 43.95±0.30b | 45.58±0.39a |
Means in the same row with different letters are significantly different (P<0.05).
Although the correlation between the thickness, volume, bottom area, and water-holding capacity of breast meat remains elusive, Lee et al. (2008) reports that the thickness and water-holding capacity of breast meat are decreased by frozen storage. In our study, the chicken breast meats with different water-holding capacity showed differences in the values of thickness and volume (obtained using direct measurement), and those of the bottom area and perimeter (obtained using imaging analysis).
Linear and multiple regression between conformation parameters (thickness, volume, bottom area, and perimeter) and water-holding capacity (determined as thawing loss and drip loss) were evaluated. The conformation parameters, determined by direct measurement and imaging analysis, showed a low correlation with the thawing and drip loss of the breast meat (Table 5). This result indicated that drip loss and thawing loss could not be accurately predicted from the conformation parameters of breast meat obtained using direct measurement or imaging analysis.
Table 5. Correlation between conformation parameters and water-holding capacity (determined as thawing loss and drip loss) of chicken breast meat.
| Parameters | Correlation coefficient (r) |
|
|---|---|---|
| Thawing loss | Drip loss | |
| Direct measurement data | ||
| Linear regression | ||
| Thickness | 0.04 | 0.14 |
| Bottom area | 0.10 | 0.10 |
| Volume | 0.32 | 0.03 |
| Multiple regression | ||
| Thickness & volume | 0.18 | 0.16 |
| Imaging analysis data | ||
| Linear regression | ||
| Thickness | 0.30 | 0.10 |
| Bottom area | 0.04 | 0.11 |
| Perimeter | 0.20 | 0.22 |
| Multiple regression | ||
| Bottom area & perimeter | 0.21 | 0.26 |
Separating the Light-colored Breast Meat with Low Waterholding Capacity Using Imaging Analysis
The L* value, determined by direct measurement and imaging analysis, showed a greater correlation with thawing and drip loss than did the conformation parameters. Thawing loss and drip loss increased, as indicated by the increasing L* value of the breast meat. Drip loss in the meat industry is generally in the range of 1.5–3%. Broiler breast meat with a low water-holding capacity and light color may demonstrate drip loss that is higher than 3%, which substantially affects the yield of the final product. As shown in Fig. 2, the colorimeter could be used to separate the light-colored breast meat which might be low water-holding capacity. These data showed that 45% of light-colored breast meat demonstrated a high thawing loss of >6% and drip loss of >3%. The L* value of the breast meat, obtained using imaging analysis, was higher than that obtained using a colorimeter. This discrepancy affected some of the normal and dark-colored breast meats, which were incorrectly classified as light- and normal-colored samples, respectively. The L* values of the light-colored breast meat samples, obtained using both imaging analysis and a colorimeter, were higher value than 56. This result indicated that the L* value, obtained using imaging analysis, could be used to separate the light-colored breast meat. However, using a digital camera with a high performance and resolution will increase the accuracy of determining the L* value.
Fig. 2.
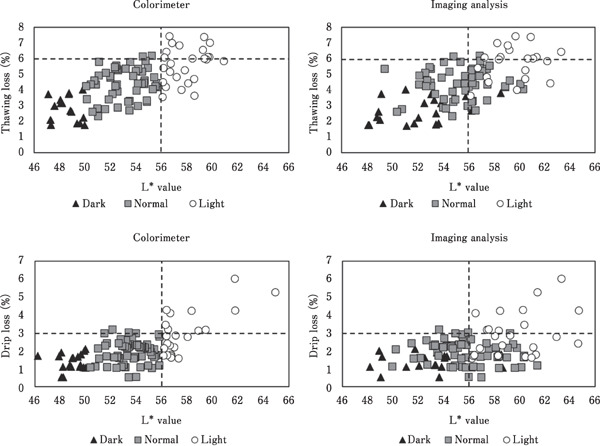
Separating the light-colored breast meat, with a high thawing and drip loss, by its L* value obtained using a colorimeter and imaging analysis.
Breast meat samples sorted by L* value using CIE Hunter-Lab colorimeter into three quality characteristics (light-, normal-, and dark-colored breast meats) were determined using imaging analysis. Then, the accuracy of determining the quality characteristics of chicken breast meat using imaging analysis technique was evaluated. The characteristics of light-colored breast meat could be determined with high accuracy compared with those of other samples. The characteristics of light-colored samples were determined correctly in 100% of samples in lot No. 1 and lot No. 2 (Table 6). The normal- and dark-colored samples showed higher percentages of incorrect determination because of their brighter color, indicated by the higher L* and b* values; the L* and b* values were derived previously by comparing the values obtained using imaging analysis with those obtained using a colorimeter. Therefore, the percentage of normal- and dark-colored samples, incorrectly identified as light- and normal-colored samples, respectively, was high. These results indicated that using imaging analysis with a low-resolution digital camera was ineffective at correctly defining the characteristics of normal- and dark-colored meat samples. However, this result indicated that imaging analysis was effective at separating light-colored breast meat from normal- and dark-colored breast meats. The research was elucidated by high percentage in correction of determination. During production, storage, and transportation, the meat industry has to evaluate the drip and thawing loss of chilled and frozen chicken meats in advance. Such data are used for predicting the loss in the weight of the meat, and for determining the correct weight of packed meat, which is necessary for control the packed weight as committed in customer compliance. However, the conventional methods for the determination of drip and thawing loss do not provide results in real time during production, leading to overweight or underweight packaging of chicken meat. Light-colored breast meat caused variation in weight, resulting in overweight or underweight packaging if incorrect data were used for estimating drip and thawing loss. Therefore, separating light-colored breast meat by the L* value, obtained using imaging analysis, could help to properly manage the packed weight and quality control in the meat industry. In a processing facility, imaging analysis could also be used to continuously determine sample characteristics in real-time without destroying the samples; this could be used to separate light-colored breast meat. The separated light-colored breast meat could be used as raw material for other applications, such as marinated chicken meat, in which its water-holding capacity could be improved. These approaches could increase product yield and reduce the cost of determining the water-holding capacity of breast meat. However, the accuracy of determining the characteristics of breast meat by imaging analysis could be improved by using a digital camera with high performance and resolution.
Table 6. Accuracy of determining the characteristics of chicken breast meats using imaging analysis.
| Samples (n=210) | Accuracy of determining the characteristics of samples using imaging analysis |
||
|---|---|---|---|
| Correct (%) | Incorrect (%) | Incorrect identification of meat characteristics (%) | |
| Lot No.1 (n=105) | |||
| Light (L*>56) | 100 | 0 | Light 97, Dark 3 Light 14, Normal 86 |
| Normal (50≤L*≤56) | 57 | 43 | |
| Dark (L*<50) | 36 | 64 | |
| Total | 59 | 41 | |
| Lot No.2 (n=105) | |||
| Light (L*>56) | 100 | 0 | Light 97, Dark 3 Light 21, Normal 79 |
| Normal (50≤L*≤56) | 57 | 43 | |
| Dark (L*<50) | 30 | 70 | |
| Total | 61 | 39 | |
Conclusions
The colors of broiler breast meat could be used to estimate the differences in the water-holding capacity (determined as thawing loss and drip loss) of the meat. Light-colored samples had a lower water-holding capacity and pH compared with those of normal- and dark-colored samples. These characteristics of light-colored samples were similar to PSE meat. Although the shapes of the light-colored samples were flatter than those of normal- and dark-colored samples, conformation parameters had a low correlation with waterholding capacity. The color characteristics, especially the L* value, showed a higher correlation with the water-holding capacity of breast meat. Direct measurement using a colorimeter, and imaging analysis, delineated by the cut-off value of L*, could be used to separate light-colored breast meat from normal- and dark-colored breast meats. Moreover, imaging analysis was used with high accuracy for determining the characteristics of light-colored samples. However, a high-resolution digital camera should be used to improve the accuracy of imaging analysis, especially when using imaging analysis for determining correlation. Imaging analysis using a digital camera could potentially be used for separating light-colored meat, with mostly low waterholding capacity, from normal-colored meat. The data in this study could be used to develop imaging analysis with a video camera, which could be used in meat-processing facilities. Therefore, suitable conditions for using a video camera, and the effect of conveyer speed, should be further assessed for separating light-colored breast meat.
Acknowledgments
This research was supported by the grant provided by the Prince of Songkla University, the Thailand Research Fund via the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0130/2553), and B. Foods Product International co., Ltd.
References
- AOAC. Official Methods of Analysis. 16th ed. Association of Agricultural Chemists; Washington. D.C. 1999. [Google Scholar]
- Artan MY, Karim R, Chern BH, Ariffin AA, Man YC, Chin NL. The influence of different formulations of palm oil-palm stearin-based shortening on the quality of white bread. Middle-East Journal of Scientific Research, 5: 469-476. 2010. [Google Scholar]
- Barbut S, Zhang L, Marcone M. Effects of pale, normal, and dark chicken breast meat on microstructure, extractable proteins, and cooking of marinated fillets. Poultry Science, 84: 797-802. 2005. [DOI] [PubMed] [Google Scholar]
- Bertram HC, Andersen HJ, Karlsson AH. Comparative study of low-field NMR relaxation measurements and two traditional methods in the determination of water holding capacity of pork. Meat Science, 57: 125-132. 2001. [DOI] [PubMed] [Google Scholar]
- EasyRGB. Easyrgb Web. http: //www.easyrgb.com Accessed on May 15, 2015.
- ElMasry G, Sun D-W. Meat Quality Assessment Using a Hyperspectral Imaging System. In Hyperspectral Imaging for Food Quality Analysis and Control; Elsevier Inc., San Diego, California, USA: 2010. [Google Scholar]
- ElMasry G, Sun D-W, Allen P. Non-destructive determination of water-holding capacity in fresh beef by using NIR hyperspectral imaging. Food Research International, 44: 2624-2633. 2011. [Google Scholar]
- Galobart J, Moran ET. Freeze-thaw and cooking effects on broiler breast fillets with extreme initial L* values. Poultry Science, 83: 2093-2097. 2004. [DOI] [PubMed] [Google Scholar]
- Girolami A, Napolitano F, Faraone D, Braghieri A. Measurement of meat color using a computer vision system. Meat Science, 93: 111-118. 2013. [DOI] [PubMed] [Google Scholar]
- Honikel KO. Reference methods for the assessment of physical characteristics of meat. Meat Science, 49: 447-457. 1998. [DOI] [PubMed] [Google Scholar]
- Huff-Lonergan E, Lonergan SM. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Science, 71: 194-204. 2005. [DOI] [PubMed] [Google Scholar]
- Lee YS, Saha A, Xiong R, Owens CM, Meullenet JF. Changes in broiler breast fillet tenderness, water-holding capacity, and color attributes during long-term frozen storage. Journal of Food Science, 73: E162-E168. 2008. [DOI] [PubMed] [Google Scholar]
- Monroy M, Prasher S, Ngadi MO, Wang N, Karimi Y. Pork meat quality classification using Visible/Near-Infrared spectroscopic data. Biosystems Engineering, 107: 271-276. 2010. [Google Scholar]
- Petracci M, Betti M, Bianchi M, Cavani C. Color variation and characterization of broiler breast meat during processing in Italy. Poultry Science, 83: 2086-2092. 2004. [DOI] [PubMed] [Google Scholar]
- Qiao M, Fletcher DL, Smith DP, Northcutt JK. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poultry Science, 80: 676-680. 2001. [DOI] [PubMed] [Google Scholar]
- Qiao M, Fletcher DL, Northcutt JK, Smith DP. The relationship between raw broiler breast meat colour and composition. Poultry Science, 81: 422-427. 2002. [DOI] [PubMed] [Google Scholar]
- Qiao J, Wang N, Ngadi MO, Gunenc A, Monroy M, Gariépy C, Prasher SO. Prediction of drip-loss, pH, and color for pork using a hyperspectral imaging technique. Meat Science, 76: 1-8. 2007. [DOI] [PubMed] [Google Scholar]
- Van Laack RLJM, Liu C-H, Smith MO, Loveday HD. Characteristics of pale, soft, exudative broiler breast meat. Poultry Science, 79: 1057-1061. 2000. [DOI] [PubMed] [Google Scholar]
- Yagiz Y, Balaban MO, Kristinsson HG, Welt BA, Marshall MR. Comparison of Minolta colorimeter and machine vision system in measuring colour of irradiated Atlantic salmon. Journal of the Science of Food and Agriculture, 89: 728-730. 2009. [Google Scholar]


