Abstract
The aim of the present study was to investigate the effects of oregano, attapulgite, benzoic acid and their combination on broiler performance, microflora composition of jejunum and cecum, intestinal architecture and breast and thigh meat composition. A total of 400 one-day-old broiler chicks were used in a 42-day trial. They were randomly distributed into five treatments with four replicates of twenty chickens per pen: Control group; Attapulgite group; Oregano essential oil group; Benzoic acid group; Mixed group. At the end of the trial, total counts of bacteria, Enterobacteriaceae, Lactobacilli, and Clostridium perfringens were enumerated by real time PCR at both jejunum and cecum. Intestinal morphology was carried out in duodenum, jejunum and ileum, for villus height and crypt depth. Cell proliferation was also evaluated in the small intestine and the cecum. The results showed that oregano and benzoic acid improved some growth performance parameters. The combined use of the examined substances increased enterobacteria counts in the jejunum, and cell proliferation in the duodenum and the jejunum. Benzoic acid improved intestinal wall morphology in the ileum. In conclusion, the combined dietary supplementation with oregano, attapulgite and benzoic acid can be a novel tool to beneficially modulate broiler chickens performance.
Keywords: attapulgite, benzoic acid, broiler chickens, intestinal microbiota, intestinal morphometry, oregano essential oil
Introduction
Antibiotics have long been used in poultry diets to reduce or prevent a number of diseases, such as post-hatching diarrhea, improve the growth and productivity of poultry flocks, and enhance the overall production yields of the poultry industry (Garcia et al., 2007). A variety off eed additives of different origin, such as organic acids, essential oils of aromatic plants and silicate minerals have often been proposed as the agents of first choice alternatives.
Essential oils are well known as inhibitors of microorganisms and are aromatic oily liquids obtained from plant material (Giannenas et al., 2013). One of the most commonly used spices in the food industry is oregano (Origanum vulgare) well known for its antioxidative and antimicrobial properties (Giannenas et al., 2005). The most representative compound in oregano essential oil is carvacrol. The mode of action of carvacrol appears to be associated with its hydroxyl group on the phenolic ring and its hydrophobic character; carvacrol interacts with the lipid bilayer of cytoplasmic membranes causing loss of integrity and leakage of cellular material such as ions, adenosine triphosphate and nucleic acid (Burt, 2004).
Silicate minerals such as attapulgite, zeolite, kaolinite and sepiolite were found to be effective as non-toxic, cheap, ecologically advantageous and affordable materials based on their high absorption capacity and ion-exchange properties (Zhou et al., 2014). For this reasons, such substances are widely used in many fields of animal nutrition, veterinary medicine, agriculture, environment protection, sanitation, and industry (Zhou et al., 2014).
Benzoic acid is a well-known conservative that has attracted wide research interest due to its antibacterial and antifungal properties (Jozefiak et al., 2006, 2010). However, dosages of this acid higher than 0.25% caused growth depression and poor feed conversion (Jozefiak et al., 2006; Jozefiak et al., 2010), whereas combinations with specific compounds of essential oils including carvacrol, thymol and eygenol cause positive effects on growth performance (Giannenas et al., 2014a, 2014b).
Mechanisms for the interactions of bioactive compounds from plants including Oregano spp., clay minerals or organic acids with the host organism may be related to extraintestinal effects, such as antioxidative protection, or intraintestinal effects that mostly concern the microflora and the gut integrity. Intestinal architecture, mucosa functionality and cell proliferation may be affected by several feed additives. The combined dietary supplementation of oregano, attapulgite and benzoic acid may be an effective novel approach to support both growth performance and health of broiler chickens.
The aim of the present study was to investigate the effects of oregano, attapulgite, benzoic acid and their combination on growth performance, microflora composition of jejunum and cecum, as well as on the intestinal architecture, such as villus height, crypt depth and intestinal cell proliferation. In addition, the effect of these natural feed additives on the chemical composition of breast and thigh chicken meat was also investigated.
Materials and Methods
Experimental Design
The trial protocol was approved by the Institutional Committee for Animal Use and Ethics of The Technological Institute of Epirus, Department of Animal Production. Throughout the trials, the birds were handled in compliance with local laws and regulations and in accordance to the principles and guidelines for poultry welfare (NRC, 1996).
Four hundred one-day-old broiler chickens (Ross-308) as hatched (mixed sex) were divided into 5 groups with 4 replicates (pens) of twenty chicks and reared for 42 days in a commercial farm in Arta (39°09′38″N; 20°59′07″E), Epirus, Greece.
Control group (CON) was fed diets in mash form, according on the bird age (Table 1) which contained no anticoccidal or antimicrobial growth promoters; the diet of the second group (ATT) was further supplemented with attapulgite (3 g/kg Ultrafed® powder containing 80% attapulgite; Geohellas SA); the diet of the third group (ORE) was supplemented with oregano essential oil (0.5 g/kg Ecodiar® powder containing 5% oregano essential oil; Ecofarm Hellas S.A.); the diet of the fourth group (BEN) was supplemented with benzoic acid (1 g/kg Vevovital® powdere containing 100% benzoic acid; DSM S.A.); the diet of the fifth group (MIX) was supplemented by a combination of attapulgite, oregano essential oil and benzoic acid (3 g/kg Ultrafeed®, 0.5 g/kg Ecodiar® and 1 g/kg Vevovital®). The major chemical compounds in attapulgite were: SiO2 56.00%, MgO 15.05 %, Fe2O3 10.60%, Al2O3 4.97%, CaO 0.33%, K2O 0.30%, Na2O 0.06% (Chalvatzi et al., 2014). The main constituents of the examined oregano essential oil are presented in Table 2 (the chemical composition was provided by the supplier).
Table 1. Composition of diets in the trial.
| Control |
||||
|---|---|---|---|---|
| Composition, g kg−1 | ||||
| Ingredients | 1–12d | 13–24d | 25–36d | 37–42d |
| Maize | 600 | 618 | 625 | 632 |
| Soybean meal, 47.0 | 335 | 310 | 297 | 290 |
| Soybean oil | 25 | 25 | 25 | 25 |
| Coconut fat | — | 15 | 25 | 25 |
| Limestone | 14 | 11 | 10 | 10 |
| Dicalcium phosphate | 11 | 9 | 7 | 7 |
| L-Lysine, hydrochloride | 3.5 | 3.0 | 2.5 | 2.5 |
| DL-Methionine | 2.5 | 1.5 | 1.5 | 1.5 |
| Sodium bicarbonate | 2.5 | 1 | 1 | 1 |
| Salt | 2.5 | 2.5 | 2.0 | 2.0 |
| Vitamin, mineral and enzyme premix1 | 4 | 4 | 4 | 4 |
| Proximate analysis | ||||
| Crude protein, % | 22.0 | 21.0 | 20.0 | 20.0 |
| Ether extract, % | 6.2 | 6.6 | 6.8 | 6.8 |
| Crude fiber, % | 3.5 | 3.6 | 3.6 | 3.6 |
| Ash, % | 4.7 | 4.6 | 4.6 | 4.6 |
| Lysine, %* | 1.3 | 1.2 | 1.1 | 1.1 |
| Methionine + Cystine, %* | 1.0 | 0.96 | 0.94 | 0.94 |
| Metabolisable energy, kcal /kg* | 3100 | 3180 | 3220 | 3220 |
Supplying per kg feed: 12,000 IU vitamin A, 5,000 IU vitamin D3, 30 mg vitamin E, 3 mg vitamin K, 5 mg thiamin, 6 mg riboflavin, 6 mg pyridoxine, 0. 02 mg vitamin B12, 60 mg niacin, 15 mg pantothenic acid, 1.5 mg folic acid, 0.25 biotin, 10 mg vitamin C, 500 mg choline chloride, 100 mg Zn, 120 mg Mn, 20 mg Fe, 15 mg Cu, 0.2 mg Co, 1 mg I, 0.3 mg Se, and phytase in recommended quantities per kg of diet.
Calculated values
Table 2. Main compounds of the oregano essential oil.
| Compound | % |
|---|---|
| Carvacrol | 77.95 |
| p-cymene | 5.43 |
| γ-terpinene | 4.65 |
| Thymol | 3.02 |
| β-caryophyllene | 1.65 |
| β-myrcene | 1.22 |
| β-bisabolene | 1.13 |
| α-terpinene | 1.00 |
| α-thujene | 0.84 |
| α-pinene | 0.58 |
| terpinen-4-ol | 0.46 |
| β-phellandrene | 0.38 |
| carvacrol methyl ether | 0.31 |
| 1-octen-3-ol | 0.30 |
| Borneol | 0.28 |
| cis-sabinene hydrate | 0.20 |
| α-humulene | 0.15 |
| Camphene | 0.14 |
| α-phellandrene | 0.13 |
| β-pinene | 0.10 |
All groups were housed floor pens with rice hulls litter. The stocking density was found to be 16 birds per m2. During the trial, commercial breeding and management procedures were employed, natural and artificial light was provided on a basis of 23 h for the first 2 days, 16 hours from day 3 to day 14, 21 h from day 15 to the slaughter days, whereas ambient temperature and humidity were controlled. All birds were vaccinated against Marek disease after hatching; and against Newcastle disease, Infectious Bronchitis and Gumboro during the second week of their life. Feed and drinking water were offered to all birds ad libitum throughout the experiment. All birds were weighed at the time of their placing into the poultry house and at slaughter age. Feed consumption within each group was recorded during the experimental period and mortality was also daily recorded.
At the end of the trial all chickens were slaughtered under commercial conditions. From each replication (floor pen) 4 birds were randomly selected and further processed. During necropsy of the selected birds, the gastrointestinal tract was removed for further analysis. Also, from these birds the breasts and thighs were removed from the carcass, were weighted and then stored for chemical analysis.
Meat Chemical Analysis
The previously collected breast and thigh meat samples were were analyzed for moisture, crude protein and fat content, by near infra-red spectroscopy using a FoodScan™ Lab (FOSS, Denmark). From each sample the breast (Pectoralis major) or the thigh (Biceps femoris) meat was carefully separated from the skin and the bones, was minced (Cutter K35, Electrolux) and then 200 g of the minced meat was placed in the sample tray of the FoodScan.
Intestinal Microflora Determination
Bacterial isolates and culture conditions: Escherichia coli and Lactobacillus acidophilus isolated from poultry faeces and characterized by Vitek 2 (BioMerieux, Marcy l'Etoile, France) were used as reference strains to generate standard curves plotting Ct values against log cfu /ml E. coli was propagated on Luria Broth under aerobic conditions at 37°C. Lactobacillus acidophilus was grown on MRS medium agar anaerobically at 37°C. The total number of cf u of each culture was calculated by plating 100 µl of the appropriate 10-fold dilution series on LB and MRS plates for aerobic and anaerobic bacteria respectively (Rawsthorne and Phister, 2006; Skånseng et al., 2006; Fliegerova et al., 2014).
Digestal sample collection: From the collected gastrointestinal tracts of the birds, samples of 500 mg were obtained from two different sites (jejunum and cecum) and stored at −80°C until further analysis.
DNA extraction: DNA was extracted using NucleoSpin® soil kit (Macherey-Nagel, Duren, Germany) according to the manufacturer's instructions. Purity and concentration of the extracted DNA were measured using Nanodrop 2000 spectrophotometer (Nano-drop Technologies, Wilmington, DE, USA).
Oligonucleotide primers and probes: Primers and TaqMan probes were synthesised by VBC (VBC-Genomics, Bioscience Research GmbH, Vienna, Austria).
To quantify total bacteria, lactobacilli and enterobacteria, primers were adapted from Castillo et al. (2006). The primers and probe used in this study to quantify Clostridium perfringens were based on 16S rDNA gene (Accession Number NR 121697.1) and multiple sequence alignments from closely related species generated by Clustal Omega (2015). Clostridium perfringens primers and probe were designed with the help of the Genscript software (GenScript, 2015). Bacterial targets, primers and probes used in this study are listed in Table 3.
Table 3. Primers and probes used in DNA intestinal flora determination.
| Target bacteria | Primers/Probes | Sequence (5′-3′) |
|---|---|---|
| Total bacteria | F-tot | GCAGGCCTAACACATGCAAGTC |
| R-tot | CTGCTGCCTCCCGTAGGAGT | |
| Tot bac probe | CGTATTACCGCGGCTGCTGGCAC | |
| Lactobacilli | F-lac | GCAGCAGTAGGGAATCTTCCA |
| R-lac | GCATTYCACCGCTACACATG | |
| Lactob probe | CTGATGGAGCAACGCCGCGTG | |
| Enterobacteria | F-ent | ATGGCTGTCGTCAGCTCGT |
| R-ent | CCTACTTCTTTTGCAACCCACTC | |
| Enterob probe | CGTAGTCCGGATTGGAGTCTGCA | |
| Clostridium perfrigens | ClosFor | AAGGCGACTCTCTGGACTGT |
| ClosRev | CCCGAAGGGATTTCCTCGATTA | |
| Clos probe | CCCGCACAAGTAGCGGAGCA |
Real time quantitative PCR: Real-time PCR was performed with the Chromo4 real-time PCR system (Bio-Rad, Hercules, California, USA). Each reaction included 10 µl of Fast Probe universal (Kapa Probe Fast universal qPCR Kit), 0.5 µl of each primer (10 mM), 0.5 µl of each probe and specific amount of DNA samples, at a final volume of 20 µl. The reaction conditions for amplification of DNA were 95°C for 5 min, 40 cycles of 95°C for 10 s, 62°C for 45 s. To determine the specificity of amplification, analysis of product melting curve was performed after the last cycle of each amplification. The cycle threshold was manually normalized to the background signal by the user (Reynisson et al., 2006; Saint-Cyr et al., 2014).
Intestinal Architecture Measurements
Morphometric analysis of the small intestine was evaluated according to Giannenas et al. (2011). From the removed gastrointestinal tract, the small intestine was divided into three parts: duodenum (from the gizzard outlet to the end of the pancreatic loop), jejunum (from the pancreatic loop to Meckel's diverticulum) and ileum (from Meckel's diverticulum to the ileo-caeco-colic junction). Segments one cm long were taken from the center of each part and fixed in 10% buffered formalin for morphometrical studies under light microscopy, with a Nikon microscope coupled with a Microcomp integrated digital imaging analysis system (Nikon Eclipse 200, Tokyo, Japan). Images were viewed (4×) to measure morphometric parameters of intestinal architecture. For this purpose, three favorably orientated sections cut perpendicularly from villus enterocytes to the muscularis mucosa were selected from each bird and measurements were carried as follows: villus height was estimated by measuring the vertical distance from the villus tip to villus-crypt junction level for 10 villi per section; crypt depth (the vertical distance from the villus-crypt junction to the lower limit of the crypt) was estimated for 10 corresponding crypts per section. Cecum morphology was also evaluated.
Intestinal Cell Proliferation
Samples from the duodenum, the jejunum, the ileum and the cecum were evaluated for cell proliferation according to the method described by Papaioannou et al. (2009). Fixed tissues were embedded in paraffin wax and serial sections (4–6 µm) were cut. For the immunohistochemical detection of the proliferation cell nuclear antigen (PCNA), the avidin-biotin immunoperoxidase method (ABC, Avidin Biotin Complex kit, peroxidase standard Vectastain, Vector Laboratories, Burlingame, CA, USA) was applied. Antigen retrieval was achieved through microwaving the section (Merck, Darmstadt, Germany) for 10 min at 700 Watt. Endogenous peroxidase was inhibited using 0.3% hydrogen hyperoxide in methanol for 30 min at room temperature. Normal horse serum (1:10 dilution) was used at room temperature for 30 min to block non specific reactions. Sections were incubated with the mouse anti-human monoclonal antibody clone PC10 (Oncogen Science, Cambride, USA) diluted 1:100, overnight at 4°C. This was followed by reaction with the horse anti-mouse biotinylated IgG (dilution 1:125) for 30 min at room temperature. All sections were incubated with avidin-biotin peroxidase complex (Vector) and rinsed with phosphate buffer solution.
Statistical Analysis
For the statistical evaluation of the experimental study results, data were subjected to analysis of variance (ANOVA), using the statistical package of IBM SPSS Statistics v. 21.0 Statistical Package (SPSS Inc., Chigaco, IL, USA). In every case the replication (floor pen) was used as the experimental unit. Tukey's multiple range test was used to distinguish the statistical difference among the mean value of each experimental group for each tested parameter. The level of significance was set at 5% (α=0.05). Values of p between 0.05 and 0.10 were considered as tendencies.
Results
The dietary supplementation of the examined substances affected the performance parameters of the broilers (Table 4). The body weight of the MIX group was significantly (P=0.007) higher at the end of the trial (day 42), compared to the control group. Also, the overall body weight gain of the MIX groups was significantly (P=0.007) higher compared to CON and ATT groups. The birds of groups ORE, BEN and MIX consumed significantly (P=0.001) less food and had significantly (P=0.001) better feed conversion ration compared to those of groups CON and ATT. In addition, the breast weight at slaughter was significantly (P=0.001) higher for the birds of groups ORE and MIX, compared to the other groups, while the breast weight for BEN group was also higher than those of groups CON and ATT. Moreover, the thigh weight at slaughter had a tendency (P=0.084) to be higher for group MIX, compared to group CON. No mortality was recorded in the experiment.
Table 4. Effect of dietary supplementation of attapulgite, oregano essential oil, benzoic acid, and their mixture on broiler performance parameters.
| CON2 | ATT2 | ORE2 | BEN2 | MIX2 | SEM3 | P-value4 | |
|---|---|---|---|---|---|---|---|
| Body Weight, 1 d | 43.3 | 43.3 | 43.3 | 43.3 | 43.3 | 0.04 | NS |
| Body Weight, 42 d | 2342.8a | 2356.5ab | 2506.3ab | 2381.3ab | 2530.0b | 16.87 | 0.007 |
| Body Weight gain | 2299.6a | 2313.2a | 2463.0ab | 2338.0ab | 2486.7b | 16.86 | 0.007 |
| Feed Intake | 4144.3a | 4094.4a | 3924.7b | 3839.0b | 3958.1b | 13.32 | 0.001 |
| FCR5 | 1.804a | 1.771a | 1.594b | 1.643b | 1.592b | 0.01 | 0.001 |
| Breast weight at 42 d | 784.8a | 792.9a | 831.4c | 812.1b | 840.2c | 1.87 | 0.001 |
| Thigh weight at 42 d | 465.5a | 466.7ab | 480.1ab | 473.8ab | 498.6b | 3.79 | 0.084 |
Results are given as means of groups (n=4 subgroups).
Groups: Control (CON), attapulgite (ATT), oregano essential oil (ORE), benzoic acid (BEN), and their mixture (MIX)
SEM: standard error of the mean.
NS: Not significant (P>0.10)
FCR: Feed conversion ratio
Values in the same row with no superscript in common differ significantly.
The effects dietary supplementation of attapulgite, oregano essential oil, benzoic acid, and their mixture on the chemical composition of broiler chicken breast and thigh meat are presented in Table 5. No significant (P>0.05) differences were found the examined parameters. The addition of attapulgite, oregano essential oil, benzoic acid or their combination did not affect the lipid or protein content of the broiler meat.
Table 5. Effect of dietary supplementation of attapulgite, oregano essential oil, benzoic acid, and their mixture on the chemical composition of broiler chicken breast and thigh meat1.
| Breast, % | CON2 | ATT2 | ORE2 | BEN2 | MIX2 | SEM3 | P-value4 |
|---|---|---|---|---|---|---|---|
| Moisture | 71.7 | 71.5 | 71.8 | 71.8 | 71.8 | 0.15 | NS |
| Crude protein | 21.9 | 21.6 | 21.9 | 21.8 | 21.9 | 0.11 | NS |
| Crude fat | 3.4 | 3.4 | 3.4 | 3.7 | 3.5 | 0.08 | NS |
| Thigh, % | |||||||
| Moisture | 70.2 | 69.9 | 70.0 | 70.4 | 70.1 | 0.09 | NS |
| Crude protein | 21.8 | 22.0 | 22.0 | 22.1 | 21.9 | 0.14 | NS |
| Crude fat | 7.9 | 8.1 | 7.9 | 8.0 | 8.0 | 0.13 | NS |
Results are given as means of groups (n=4 subgroups).
Groups: Control (CON), attapulgite (ATT), oregano essential oil (ORE), benzoic acid (BEN), and their mixture (MIX)
SEM: standard error of the mean.
NS: Not significant (P>0.05)
Table 6 presents the determined levels of total phenols in the feed and in the breast and thigh meat. Groups ORE and MIX had significantly (P=0.001) higher levels compared to the other groups. Total phenol feed values for these groups were on average triple compared to the control group value, whereas the meat values were about double again compared to the control group.
Table 6. Effect of dietary supplementation of attapulgite, oregano essential oil, benzoic acid, and their mixture on total phenols (mg/L gallic acid) in the feed and the breast and thigh meat1.
| CON2 | ATT2 | ORE2 | BEN2 | MIX2 | SEM3 | P-value | |
|---|---|---|---|---|---|---|---|
| Feed | 14.2a | 13.8a | 44.3b | 13.4a | 40.1b | 0.71 | 0.001 |
| Breast meat | 3.7a | 3.5a | 6.6b | 4.1a | 6.8b | 0.18 | 0.001 |
| Thigh meat | 4.2a | 4.1a | 8.2b | 4.6a | 7.9b | 0.06 | 0.001 |
Results are given as means of groups (n=4 subgroups).
Groups: Control (CON), attapulgite (ATT), oregano essential oil (ORE), benzoic acid (BEN), and their mixture (MIX)
SEM: standard error of the mean.
Values in the same row with no superscript in common differ significantly.
The composition of the microflora was determined at the day of slaughter (Table 7). Enterobacteria populations in the jejunum, were significantly higher (P=0.009) in the MIX group, compared to the control and group ATT, but the other examined populations did not differ significantly (P>0.05). In the cecum, no significant differences were noted (P>0.05).
Table 7. Effect of dietary supplementation of attapulgite, oregano essential oil, benzoic acid, and their mixture on cecum and jejunum microbial populations, measure by quantitative real time PCR (log cfu/g)1.
| Cecum | CON2 | ATT2 | ORE2 | BEN2 | MIX2 | SEM3 | P-value4 |
|---|---|---|---|---|---|---|---|
| Lactobacillus spp | 7.83 | 7.58 | 8.20 | 7.67 | 8.65 | 0.337 | NS |
| Clostridium perfringens | 4.85 | 4.33 | 4.22 | 4.67 | 4.18 | 0.217 | NS |
| Enterobacteria | 5.15 | 6.80 | 7.06 | 8.12 | 8.54 | 0.452 | NS |
| Total bacteria | 10.19 | 9.67 | 8.72 | 8.19 | 8.81 | 0.537 | NS |
| Jejunum | |||||||
| Lactobacillus spp | 8.05 | 8.01 | 6.80 | 6.70 | 8.35 | 0.380 | NS |
| Clostridium perfringens | 3.58 | 2.58 | 3.73 | 2.67 | 2.68 | 0.324 | NS |
| Enterobacteria | 5.05a | 5.80a | 7.49ab | 8.55ab | 9.61b | 0.376 | 0.009 |
| Total bacteria | 9.84 | 9.32 | 9.60 | 9.07 | 10.63 | 0.356 | NS |
Results are given as means of groups (n=4 subgroups).
Groups: Control (CON), attapulgite (ATT), oregano essential oil (ORE), benzoic acid (BEN), and their mixture (MIX)
SEM: standard error of the mean.
NS: Not significant (P>0.05)
Values in the same row with no superscript in common differ significantly.
The diet supplementation also affected the intestinal morphology (Table 8). Crypt depth was significantly lower (P<0.019) and villus height to crypt depth ration was significantly higher (P<0.020, respectively) for the BEN group, compared to the control, but did not differ compared to the other groups (P>0.05). No morphological lesions or any inflammatory responses were found in the examined intestinal parts of both cecum and small intestine.
Table 8. Effect of dietary supplementation of attapulgite, oregano essential oil, benzoic acid, and their mixture on intestinal morphology1.
| Duodenum | CON2 | ATT2 | ORE2 | BEN2 | MIX2 | SEM3 | P-value4 |
|---|---|---|---|---|---|---|---|
| Villus height (µm) | 1567.4 | 1541.7 | 1754.2 | 1796.7 | 1683.5 | 45.62 | NS |
| Crypt depth (µm) | 198.0 | 191.1 | 184.3 | 195.4 | 200.0 | 6.74 | NS |
| Villus height to crypt depth ratio | 8.0 | 8.1 | 10.1 | 8.2 | 8.8 | 0.32 | NS |
| Jejunum | |||||||
| Villus height (µm) | 1168.7 | 1327.7 | 1423.9 | 1389.4 | 1282.3 | 32.10 | NS |
| Crypt depth (µm) | 157.2 | 150.8 | 145.0 | 131.6 | 132.2 | 4.84 | NS |
| Villus height to crypt depth ratio | 7.6 | 8.9 | 10.1 | 10.6 | 9.8 | 0.32 | NS |
| Ileum | |||||||
| Villus height (µm) | 722.4 | 658.9 | 745.3 | 703.1 | 747.4 | 11.79 | NS |
| Crypt depth (µm) | 136.4a | 105.5ab | 106.9ab | 86.9b | 113.0ab | 3.93 | 0.019 |
| Villus height to crypt depth ratio | 5.5a | 6.3ab | 7.1ab | 8.1b | 6.7ab | 0.21 | 0.020 |
Results are given as means of groups (n=4 subgroups).
Groups: Control (CON), attapulgite (ATT), oregano essential oil (ORE), benzoic acid (BEN), and their mixture (MIX)
SEM: standard error of the mean.
NS: Not significant (P>0.05)
Values in the same row with no superscript in common differ significantly.
The effects dietary supplementation of attapulgite, oregano essential oil, benzoic acid, and their mixture on the cell proliferation of the duodenum, the jejunum and the ileum are given in Table 9. Cell proliferation for ORE and MIX groups was significantly higher compared to the other three groups in the duodenum (P=0.001) and the jejunum (P=0.012), while in the ileum the results were not significant (P>0.05). In addition, the cecum crypts of ORE groups showed strongly positive staining for PCNA (Fig. 1), whereas the staining was only mild for the CON group (Fig. 2).
Table 9. Effect of dietary supplementation of attapulgite, oregano essential oil, benzoic acid, and their mixture on cell proliferation in the small intestine (numbers of nuclei of enterocytes)1.
| CON2 | ATT2 | ORE2 | BEN2 | MIX2 | SEM3 | P-value4 | |
|---|---|---|---|---|---|---|---|
| Duodenum | 15.3a | 16.3a | 22.2b | 17.0a | 20.2b | 0.29 | 0.001 |
| Jejunum | 19.2a | 20.4ab | 23.9b | 20.5ab | 23.7b | 0.44 | 0.012 |
| Ileum | 21.9 | 22.7 | 24.7 | 23.3 | 24.8 | 0.35 | NS |
Results are given as means of groups (n=4 subgroups).
Groups: Control (CON), attapulgite (ATT), oregano essential oil (ORE), benzoic acid (BEN), and their mix- ture (MIX)
SEM: standard error of the mean.
NS: Not significant (P>0.05)
Values in the same row with no superscript in common differ significantly.
Fig. 1.
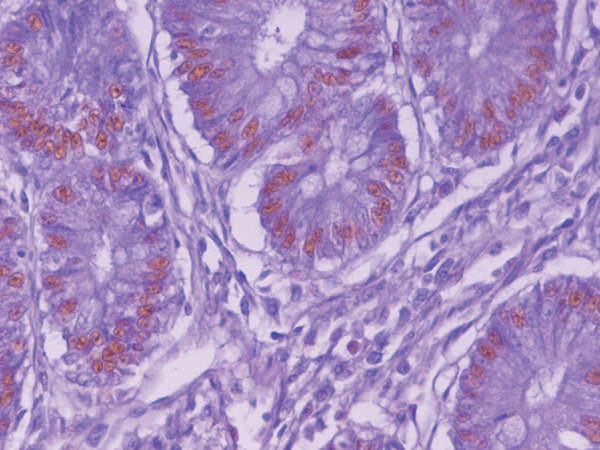
Cecum crypts of Oregano group: columnal lining cells of the crypts, strongly positive for PCNA. Paraffin-embedded, ABC method, DAB reaction and Mayer's haematoxylin counterstain, ×400 (size bar 1 µm).
Fig. 2.
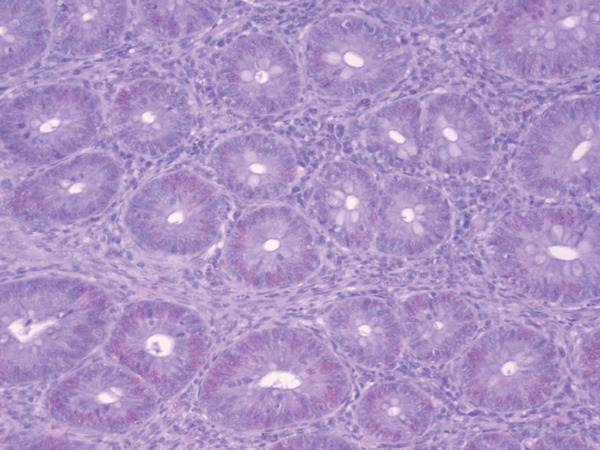
Cecum crypts of Control group: columnal lining cells of the crypts, mildly positive for PCNA Paraffin-embedded, ABC method, DAB reaction and Mayer's haematoxylin counterstain, ×400 (size bar 1 µm).
Discussion
In the past, nutritionists have focused their research on the effects of supplementing the diet of chickens with antibiotics. Today, the replacement of antibiotic growth promoters is a serious concern in poultry industry. Antibiotic growth promoters have been banned in the European Union and their use is also under scrutiny in the rest of the world. Many dietary additives are under examination as alternatives to antibiotic growth promoters. However, there is no clear answer as to which level and what source of natural feed additives supplementation is optimal for poultry. It is possible that no single dietary substitute exists that can fully replace the antibiotic growth promoters and that mixtures of beneficial substances have overall better results. Our study adds knowledge on the topic, since it examines oregano, attapulgite and benzoic acid that may benefit the intestinal microflora or functionality. Oregano and benzoic acid possess antimicrobial activity (Jozefiak et al., 2010; Hippenstiel et al., 2011). Dietary supplementation of attapulgite beneficially modulated cecal microbiota in laying pullets (Chalvatzi et al., 2016).
In our study, the use of oregano essential oil, benzoic acid and the combination of all examined substances improved production efficiency in broiler chickens, while they lowered the feed intake. Garcia et al. (2007) suggested that a combination of both organic acids and plant extracts in the diet of chickens could be of great interest in future studies as alternative growth promoters. Brenes and Roura (2010) and Hippensteil et al. (2011) reported that the essential oils of certain herbs, can show positive effects in performance, but show no consistency in feed intake when supplemented to diets between 12 and 300 ppm. In previous studies, the use of oregano (herb or its major compounds) alone or in combination with benzoic acid were examined as candidate replacers of antibiotic growth promoters with controvertial results (Langhout, 2000; Symeon et al., 2009; Symeon et al., 2010; Giannenas et al., 2013, 2014a, 2014b, 2016b; Skoufos et al., 2016).
Dietary supplementation with oregano essential oil, attapulgite, benzoic acid or their mixture did not alter the protein and fat content of breast and thigh meat of broiler chickens, although higher levels of phenolic compounds were noticed in the groups fed oregano essential oil that could increase its antioxidant status. In a similar study, Giannenas et al. (2016a) found that a dietary mixture of oregano and sage essential oils increased the total phenolic content of chicken meat and its antioxidant status, in both raw and cooked meat during storage under refrigeration.
Although, chicken cecal microbiota has been widely investigated due to the significant role of the cecum in heath, performance, and disease (Clench and Mathias, 1995; Stanley et al., 2015). It has been suggested that the microbial diversity of chicken cecum has been altered with the common use of antibiotics in feed worldwide and that it has lost much of its natural chicken specialised microbiota and with it microbial potential and metabolic capabilities (Stanley et al., 2015).
Our results did not show significant effects on the proliferation of C. perfringens in the broiler intestine. In a previous study by Mitsch et al. (2004) it was reported that different compositions of essential oil blends may have different efficiencies in this respect. Further studies on the effect of oregano essential oil or benzoic acid on C. perfringens toxins, along with weight gain, and feed efficiency are necessary.
Plant essential oils are well known to exert antibacterial, antifungal and antiviral activity in in vitro experiments (Windisch et al., 2008). It is generally accepted that essential oils are slightly more active against Gram-positive than Gram-negative bacteria (Brenes and Roura, 2010). Comparable in vivo studies also found inhibiting effects of essential oils against pathogens such as C. perfringens, E. coli or Eimeria species (Bozkurt et al., 2013). The control of pathogenic load may also contribute to healthy microbial metabolites, improved intestinal integrity and protection against enteric disease (Bozkurt et al., 2013). Attention should also be paid to the potential negative effects induced by essential oils on healthy intestinal bacteria. Horosova et al. (2006) reported that oregano essential oil exhibited a strong bactericidal effect against Lactobacilli isolated from fecal samples of chickens fed diets with oregano. In contrast, Giannenas et al. (2014a; 2014b) found a promoting effect on intestinal Lactobacilli by a blend that contained aromatic compounds mainly carvacrol and a mixture of this product with benzoic acid. In an in vivo anti-bacterial study (Thapa et al., 2012) it was concluded that beneficial commensal Faecalibacterium prausnitzii was sensitive to essential oil at similar or even lower concentrations than the pathogens. In addition, Cross et al. (2007) and Muhl and Liebert (2007) reported that essential oils had no effect on the microbial population and composition in the digestive tract or fecal excretions of broilers.
In this study, the combined dietary supplementation of oregano, attapulgite and benzoic acid increased enterobactera populations in the jejunum. In general, changes in diet, aging, medication, stress or infection may lead to an increase in anaerobs and E. coli in the small intestine and to an increase of Enterobacteriaceae in the colon, concomitantly with a decrease of bifidobacteria (Holzapfel et al., 1998). In our study intestinal samples were collected at 42 days of age, however no clinical signs of stress or disease were seen in any groups.
Studies evaluating the effect of attapulgite on gastrointestinal tract microflora or intestinal architecture are scarce. Although, it has been suggested that other silica substances such as clinoptilolite, could also exert a growth promoting effect on broiler chicken tissues after dietary supplementation (Wu et al., 2013), no published data were found for attapulgite.
The gastrointestinal tract is not only recognized as an organ of digestion and absorption but also assumes an important immunological role because of its participation in the defense against external pathogens. The intestine is exposed to a wide variety of antigens from feedstuffs, resident bacteria, and invader microorganisms, and they need to be limited by the mucosal barrier that provides an immunological defense against harmful antigens. The intestinal villus can be regarded as the capacity of the bird to absorb nutrients from the feed. Longer villi are typically associated with excellent gut health and high absorptive efficiency (Garcia et al., 2007), whereas shorter villi and deeper crypts have been associated with the presence of toxins or higher tissue turnover (Miles et al., 2006).
Cell proliferation in the intestine, as expressed by PCNA, can be also regarded as the ability of the intestine to cope with pathogens and support the gut health. Proliferative activity of enterocytes is the sign of a healthy tissue turnover and maintenance (Garcia et al., 2007). In chickens, enterocyte proliferation is mostly localized in the crypt region and the site of enterocyte proliferation is not precisely localized (Uni et al., 1998). In the present study, we detected very potent immunoreactivity for PCNA in all samples examined, especially in oregano and mix groups compared to control group and the groups that received benzoic acid or attapulgite. In addition, in the present study it is possible that the increased cell proliferation in the jejunum could be linked with the increased Enterobacteriaceae counts in the same intestinal part.
As previously explained, factors that are present in the digesta can lead relatively quickly to changes in the intestinal mucosa due to the close proximity of the mucosal surface to the intestinal content. Activity of nutrient assimilation is in close connection with the proliferative activity of enterocytes enhancing healthy tissue turnover and maintenance (Garcia et al., 2007). The significant increase of enterocyte proliferation along with the duodenal villi during the finisher period and tendency for increased proliferative activity in other samplings found in our trial demonstrate the benefit properties of oregano essential oil on the proliferative ability of intestinal epithelial cells. Also, benzoic acid supplementation decreased crypt depth in the ileum and increased cell proliferation in the duodenum.
Conclusions
The digestive tract of the chicken plays a major role in its health. The findings presented in the current study provide ample evidence that the combination of oregano essential oil with benzoic acid and attapulgite exerts a positive effect on broiler growth performance and carcass yield, without any detrimental changes in meat quality. At the same time, oregano improved the intestinal cell proliferation in both duodenum and jejunum, while benzoic acid positively affected crypt depth in the ileum. Moreover, no major changes were noted in the broiler intestinal microbiota balance. Combinations of essential oils with other substances such as organic acids or clay minerals may offer an effective tool to improve the growth performance and intestinal health in broiler chickens.
Acknowledgments
This work was supported by the project “GreenPoultry”, funded by the national action “COOPERATION 2011 -Partnerships of Production and Research Institutions”, of the NSRF 2007-2013 Operational Programme “Competitiveness and Entrepreneurship”, General Secretariat for Research and Technology, Ministry of Education and Religious Affairs.
References
- Bozkurt M, Giannenas I, Kucukyilmaz K, Christaki E, Florou-Paneri P. An update on approaches to controlling coccidia in poultry using botanical extracts. British Poultry Science, 54: 713-727, 2013. [DOI] [PubMed] [Google Scholar]
- Brenes A, Roura E. Essential oils in poultry nutrition: Main effects and modes of action. Animal Feed Science and Technology, 158: 1-14, 2010. [Google Scholar]
- Burt S. Essential oils: Their antibacterial properties and potential applications in foods. International Journal of Food Microbiology, 94: 223-253, 2004. [DOI] [PubMed] [Google Scholar]
- Castillo M, Martin-Orue SM, Manzanilla EG, Badiola I, Martin M, Gasa J. Quantification of total bacteria, enterobacteria and lactobacilli populations in pig digesta by real-time PCR. Veterinary Microbiology, 114: 165-170, 2006. [DOI] [PubMed] [Google Scholar]
- Chalvatzi S, Arsenos G, Tserveni-Goussi A, Fortomaris P. Tolerance and efficacy study of palygorskite incorporation in the diet of laying hens. Applied Clay Science, 101: 643-647, 2014. [Google Scholar]
- Chalvatzi S, Kalamaki MS, Arsenos G, Fortomaris P. Dietary supplementation with the clay mineral palygorskite affects performance and beneficially modulates caecal microbiota in laying pullets. Journal of Applied Microbiology, 120: 1033-1040, 2016. [DOI] [PubMed] [Google Scholar]
- Clench MH, Mathias JR. The avian cecum: a review. Wilson Bulletin, 107: 93-121, 1995. [Google Scholar]
- Clustal Omega. Multiple Sequence Alignment. http://www.ebi.ac.uk/Tools/msa/clustalo Accessed in 2015.
- Cross DE, McDevitt RM, Hillman K, Acamovic T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. British Poultry Science, 48: 496-506, 2007. [DOI] [PubMed] [Google Scholar]
- Fliegerova K, Tapio I, Bonin A, Mrazek J, Callegari ML, Bani P, Bayat A, Vilkki J, Kopečný J, Shingfield KJ. Effect of DNA extraction and sample preservation method on rumen bacterial population. Anaerobe, 29: 80-84, 2014. [DOI] [PubMed] [Google Scholar]
- Garcia V, Catala-Gregori P, Hernandez F, Megias MD, Madrid J. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology and meat yield of broilers. Journal of Applied Poultry Research, 16: 555-562, 2007. [Google Scholar]
- GenScript. GenScript Real-time PCR (TaqMan) Primer Design. https://www.genscript.com/ssl-bin/app/primer Accessed in 2015.
- Giannenas I, Florou-Paneri P, Botsoglou NA, Christaki E, Spais AB. Effect of supplementing feed with oregano and/or a-tocopheryl acetate on growth of broiler chickens and oxidative stability of meat. Journal of Animal and Feed Sciences, 14: 521-535, 2005. [Google Scholar]
- Giannenas I, Tsalie E, Chronis E, Mavridis S, Kyriazakis I. Consumption of Agaricus bisporus mushroom affects the performance, intestinal microbiota composition and morphology, and antioxidant status of turkey poults. Animal Feed Science and Technology, 165: 218-229, 2011. [Google Scholar]
- Giannenas I, Bonos E, Christaki E, Florou - Paneri P. Essential oils and their applications in animal nutrition. Medicinal & Aromatic Plants, 2: 1-12, 2013. [Google Scholar]
- Giannenas I, Papaneophytou C, Tsalie E, Mavridis S, Triantafillou E, Kontopidis G, Tontis D. Effect of benzoic acid and essential oil compounds on performance of turkeys, intestinal microbiota, intestinal morphology and antioxidant status. Asian-Australasian Journal of Animal Sciences, 27: 225-236, 2014. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannenas IA, Papaneophytou CP, Tsalie E, Triantafillou E, Tontis D, Kontopidis GA. The effects of benzoic acid and essential oil compounds in combination with protease on the performance of chickens. Journal of Animal and Feed Sciences, 23: 73-81, 2014. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannenas I, Karamoutsios A, Bartzanas T, Tsinas A, Tzora A, Skoufos I. Oregano and sage essential oils improve antioxidant status of raw and cooked breast and thigh chicken meat. Agro Food Industry Hi-Tech, 27: 10-14, 2016. a. [Google Scholar]
- Giannenas I, Tzora A, Sarakatsianos I, Karamoutsios A, Skoufos S. The effectiveness of the use of oregano and laurel essential oils in chickens' feeding. Annals of Animal Science. DOI 10.1515/aoas 2016. b. [DOI] [Google Scholar]
- Hippenstiel F, Abdel-Wareth AAA, Kehraus S, Sudekum KH. Effects of selected herbs and essential oils, and their active components on feed intake and performance of broilers - a review. Archiv fur Geflugelkunde, 75: 226-234, 2011. [Google Scholar]
- Holzapfel WH, Haberer P, Snel J, Schillinger U, Huis in't Veld JHJ. Overvew of gut flora and probiotics. International Journal of Food Microbiology, 41: 85-101, 1998. [DOI] [PubMed] [Google Scholar]
- Horošová K, Bujňáková D, Kmet' V. Effect of oregano essential oil on chicken Lactobacilli and E. coli. Folia Microbiologica, 51: 278-280, 2006. [DOI] [PubMed] [Google Scholar]
- Jozefiak D, Kaczmarek S, Bochenek M, Rutkowski A. A note on effects of benzoic acid supplementation on the performance and microbiota populations of broiler chickens. Journal of Animal and Feed Sciences, 16: 252-256, 2006. [Google Scholar]
- Jozefiak D, Kaczmarek S, Rutkowski A. The effects of benzoic acid supplementation on the performance of broiler chickens. Journal of Animal Physiology and Animal Nutrition, 94: 29-34, 2010. [DOI] [PubMed] [Google Scholar]
- Langhout P. New additives for broiler chickens. World Poultry, 16: 22-27, 2000. [Google Scholar]
- Miles RD, Butcher GB, Henry PR, Littell RC. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poultry Science, 85: 476-485, 2006. [DOI] [PubMed] [Google Scholar]
- Mitsch P, Zitterl-Eglseer K, Kohler B, Gabler C, Losa R, Zimpernik I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poultry Science, 83: 669-675, 2004. [DOI] [PubMed] [Google Scholar]
- Muhl A, Liebert F. Growth and parameters of microflora in intestinal and faecal samples of piglets due to application of a phytogenic feed additive. Journal of Animal Physiology and Animal Nutrition, 91: 411-418, 2007. [DOI] [PubMed] [Google Scholar]
- NRC. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC: 1996. [Google Scholar]
- Papaioannou N, Psalla D, Zavlaris M, Loukopoulos P, Tziris N, Vlemmas I. Immunohistochemical expression of dog TERT in canine testicular tumours in relation to PCNA, ki67 and p53 expression. Veterinary Research Communications, 33: 905-919, 2009. [DOI] [PubMed] [Google Scholar]
- Rawsthorne H, Phister TG. A real-time PCR assay for the enumeration and detection of Zygosaccharomyces bailii from wine and fruit juices. International Journal of Food Microbiology, 112: 1-7, 2006. [DOI] [PubMed] [Google Scholar]
- Reynisson E, Josefsen MH, Krause M, Hoorfar J. Evaluation of probe chemistries and platforms to improve the detection limit of real-time PCR. Journal of Microbiological Methods, 66: 206-216, 2006. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr MJ, Perrin-Guyomard A, Houee P, Vasseur MV, Laurentie M. Use of accuracy profile procedure to validate a real-time PCR method to quantify bacteria in feces. Journal of AOAC International, 97: 573-579, 2014. [DOI] [PubMed] [Google Scholar]
- Skånseng B, Kaldhusdal M, Rudi K. Comparison of chicken gut colonisation by the pathogens Campylobacter jejuni and Clostridium perfringens by real-time quantitative PCR. Molecular and Cellular Probes, 20: 269-279, 2006. [DOI] [PubMed] [Google Scholar]
- Skoufos I, Giannenas I, Tontis D, Bartzanas T, Kittas C, Panagakis P, Tzora A. Effects of oregano essential oil and attapulgite on growth performance, intestinal microbiota and morphometry in broilers. South African Journal of Animal Science, 46: 77-88, 2016. [Google Scholar]
- Stanley D, Geier MS, Chen H, Hughes RJ, Moore RJ. Comparison off ecal and cecal microbiotas reveals qualitative similarities but quantitative differences. BMC Microbiology, 15: 51, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symeon GK, Zintilas C, Ayoutanti A, Bizelis LA, Deligeorgis SG. Effect of dietary oregano essential oil supplementation for an extensive fattening period on growth performance and breast meat quality of female medium-growing broilers. Canadian Journal of Animal Science, 89: 331-334, 2009. [Google Scholar]
- Symeon GK, Zintilas C, Demiris N, Bizelis LA, Deligeorgis SG. Effects of oregano essential oil dietary supplementation on the feeding and drinking behaviour as well as the activity of broilers. International Journal of Poultry Science, 9: 401-405, 2010. [Google Scholar]
- Thapa D, Losa R, Zweifel B, John Wallace R. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology (United Kingdom), 158: 2870-2877, 2012. [DOI] [PubMed] [Google Scholar]
- Uni Z, Platin R, Sklan D. Cell proliferation in chicken intestinal epithelium occurs both in the crypt and along the villus. Journal of Comparative Physiology B, 168: 241-247, 1998. [DOI] [PubMed] [Google Scholar]
- Windisch W, Schedle K, Plitzner C, Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. Journal of Animal Science, 86: E140-148, 2008. [DOI] [PubMed] [Google Scholar]
- Wu QJ, Wang LC, Zhou YM, Zhang JF, Wang T. Effects of clinoptilolite and modified clinoptilolite on the growth performance, intestinal microflora, and gut parameters of broilers. Poultry Science, 92: 684-692, 2013. [DOI] [PubMed] [Google Scholar]
- Zhou P, Tan YQ, Zhang L, Zhou YM, Gao F, Zhou GH. Effects of dietary supplementation with the combination of zeolite and attapulgite on growth performance, nutrient digestibility, secretion of digestive enzymes and intestinal health in broiler chickens. Asian-Australasian Journal of Animal Sciences, 27: 1311-1318, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


