Abstract
Objectives of the current work were to investigate the role of photoperiod and melatonin in the alteration of immune responses in a reptilian species. Animals were kept on a regimen of short or long days. Blood was obtained and leucocytes were isolated to study various innate immune responses. Lymphocytes were separated from blood by density gradient centrifugation and were used to study proliferation. Respiratory burst activity was measured through nitrobluetetrazolium reduction assay while nitric oxide production by leucocytes was assayed by nitrite assay. Lymphocytes were isolated and used to study proliferation with and without B and T cell mitogens. Photoperiodic manipulation acted differentially on leucocyte counts. Nitrite release was increased while superoxide production was decreased in cultures obtained from the snakes kept on the short day regimen. Significant enhancement of mitogen induced lymphocyte proliferation was observed in cultures from the animals kept in either long or short days compared to cultures from the animals kept in natural ambient day length. Use of in vitro melatonin showed that lymphocytes from the animals, kept in long days, were more reactive. Photoperiod induces changes in immune status which may permit adaptive functional responses in order to maintain seasonal energetic budgets of the animals. Physiological responses (like elevated immune status) are energetically expensive, therefore, animals have evolved a strategy to reduce immune functions at times when energy is invested in reproductive activities. Natrix piscator breeds from September to December and elevated pineal hormone in winter suppresses reproduction while immunity is stimulated.
Subject terms: Immunology, Zoology
Introduction
Seasonal adaptations by organisms tend to reflect interactions and coordinations between changing environmental conditions and individual internal rhythms. It is well known that immune function and reproduction are energetically costly physiological processes hence incompatible simultaneously1. Photoperiodic information, which is considered as the most effective initial predictive cue, tends to initiate and terminate seasonal adaptations2. Well documented fluctuations in disease and pathogen load are natural threats faced by wild populations3. However, rapid adaptations have been documented in wild populations in response to seasonal oscillations in climate and pathogen load4,5. The endocrine system has been shown to mediate communication between reproduction and immunity6. A variety of environmental factors, such as day length, social interaction, food availability, and temperature, may have significant influence on endocrine system that can change reproductive function and immunity7,8. For example, the effects of photoperiodic alteration on immune functions have been well studied in mammals8–11. The involvement of melatonin has also been implicated in immune functions via experimental alteration in photoperiod12–14. Changes in the immune responses that occur in response to maintaining animals in short days are likely to be directly or indirectly related to elevated melatonin secretion. Along these lines, enhancement of immune function in winter has been studied in a variety of rodent species15. Authors of various studies have found fairly robust effect of short-day increase in splenic mass in Peromyscus maniculatus16 and Mesocricetus auratus17,18. Among non-mammals, melatonin mediated changes in the immune functions have mainly been described in birds19. Some reports in birds demonstrate that photoperiod influences immune function20–22. Oxidative stress in leucocytes, measured by reactive oxygen species (ROS) level and lymphocyte proliferation was found to be seasonal23. In light of these studies, it may be that changes in the immune responses that occur in response to maintaining animals on short day regimens are likely to be directly or indirectly related to elevated melatonin secretion. To date, studies have shown that the efficacy of a given organism’s immune response can vary seasonally. However, as of yet our understanding of how photoperiod modulates immune functions remains limited. According to the energy trade-off hypothesis, elevated melatonin level in winter suppresses gonadal function while immunity is enhance24. Most research in this field is focused on mammals and birds, however, the study of unconventional animal models still represent a useful approach to obtain an insight of how photoperiod mediated immune function has changed during the course of vertebrate evolution. Limited works in lower vertebrates suggest that seasonality affects reproduction, food intake, locomotor activity, growth rate, and immune functions of teleosts25–27. Photoperiodic manipulation is utilized to affect sexual maturation and growth rate of many fishes28. Information regarding photoperiod induced alteration in immune function is currently unavailable in reptiles, despite the fact that developing a systemic approach to understand elements of immune function across vertebrate groups will ultimately help us to better understand the mammalian immune system. Information on this subject in reptiles is of particular significance from comparative point of view, as reptiles represent a pivotal phylogenic group, intermediate between heterotherms and homeotherms. In addition, certain characteristics make reptiles ideal for immunological research, such as optimal body temperature ranges that, when changed, may accelerate or slow down the immune response and the influence of season that may shut down or activate immune system. The checkered keelback snake (Natrix piscator) is an ovipararous reptile, endemic to Asia that breeds seasonally. In seasonal breeders the length of melatonin secretion at night appears to have a direct effect on the seasonal variation of physiological processes29. We have recently reported the variation in various immune functions due to changing environment in this ophidian species30 where daily and seasonal rhythms in immune responses were found. Along with circadian rhythms, we have reported significant circannual rhythm in seven out of nine tested immune parameters. In a previous study, we have also demonstrated that N. piscator exhibits seasonal variation in blood immunological traits31. Based on this research, we hypothesized that photoperiod plays an important role in the regulation of immune responses in this species. Specifically, we investigated the role of melatonin in modulation of lymphocyte proliferation as a method to deduce a relationship between the immune and endocrine systems. Understanding the immunity and its interaction with seasonality has provided important insights in the evolution of this physiological process.
Results
No significant change was observed in the number of monocyte due to photoperiodic manipulation (Fig. 1A). Total leucocytes decreased significantly (df = 14; F = 17.862 and p < 0.001) in snakes subjected to short days in comparison with snakes maintained in natural day length (12L:12D) or long days (Fig. 1C). Lymphocyte count also decreased (df = 14; F = 4.971 and p < 0.05) in the blood of animals kept in short days when compared with the animals kept in long days (Fig. 1B). Significant increase in the number of basophil (df = 14; F = 32.817 and p < 0.001) (Fig. 2B) was observed in the snakes kept in long days, when compared to the snakes maintained either in natural day length or in short days. Eosinophil count was also increased (df = 14; F = 38.924 and p < 0.001) in long day animals as compared to animals kept in natural day length (Fig. 2A).
Figure 1.
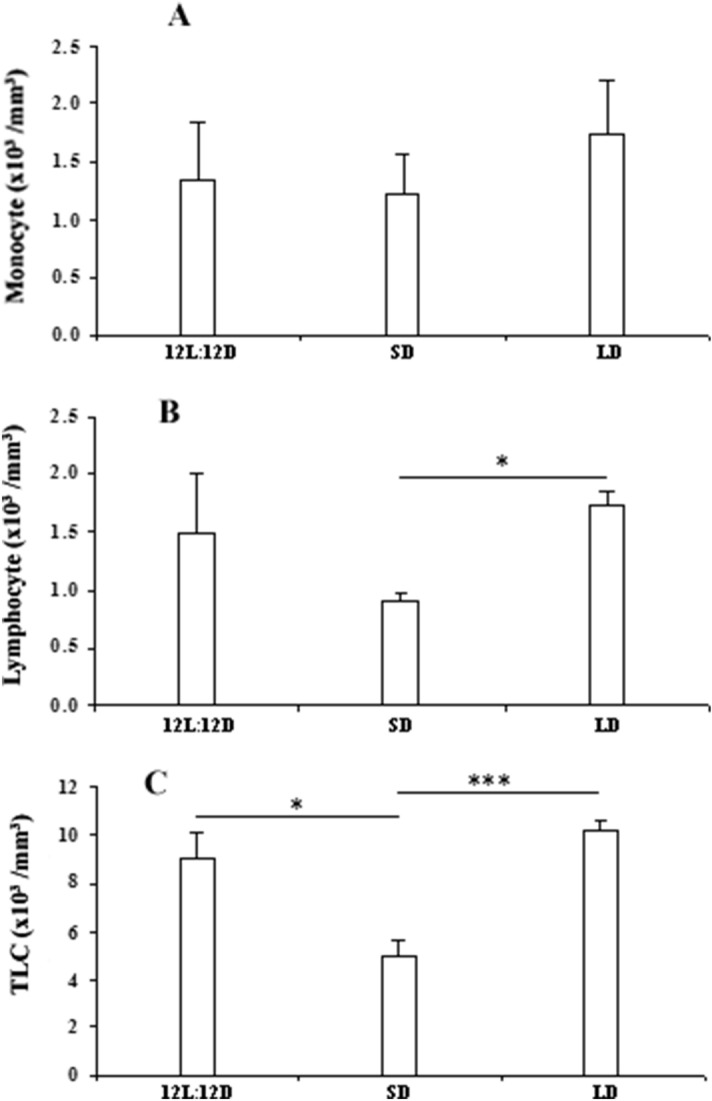
Effect of different photoperiodic regimens (12L:12D—light:dark 12:12; SD—short days, light:dark 8:16; LD—long days, light:dark 16:8) on total leucocytes count (TLC) (C), lymphocyte (B) and monocyte (A) count in the fresh-water snake, Natrix piscator. Data were analysed by One Way ANOVA: * (p < 0.05); *** (p < 0.001).
Figure 2.
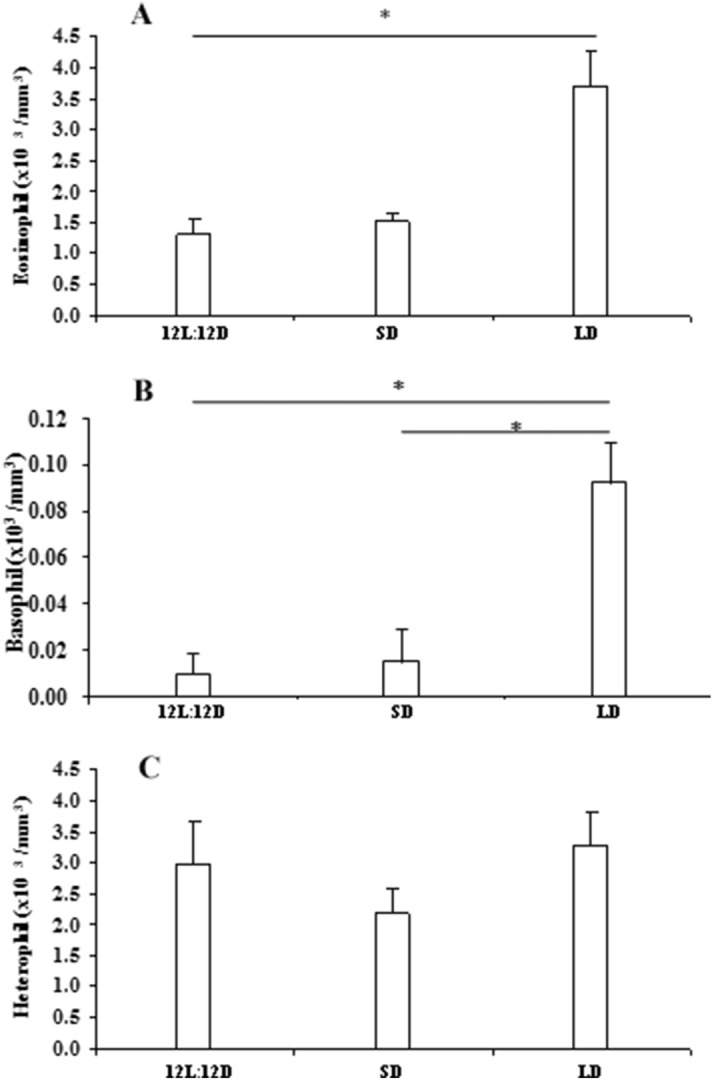
Effect of different photoperiodic regimens (12L:12D—light:dark 12:12; SD—short days, light:dark 8:16; LD—long days, light:dark 16:8) on eosinophil (A), basophil (B) and heterophil (C) count in the fresh-water snake, Natrix piscator. Data were analysed by One Way ANOVA: * (p < 0.05); *** (p < 0.001).
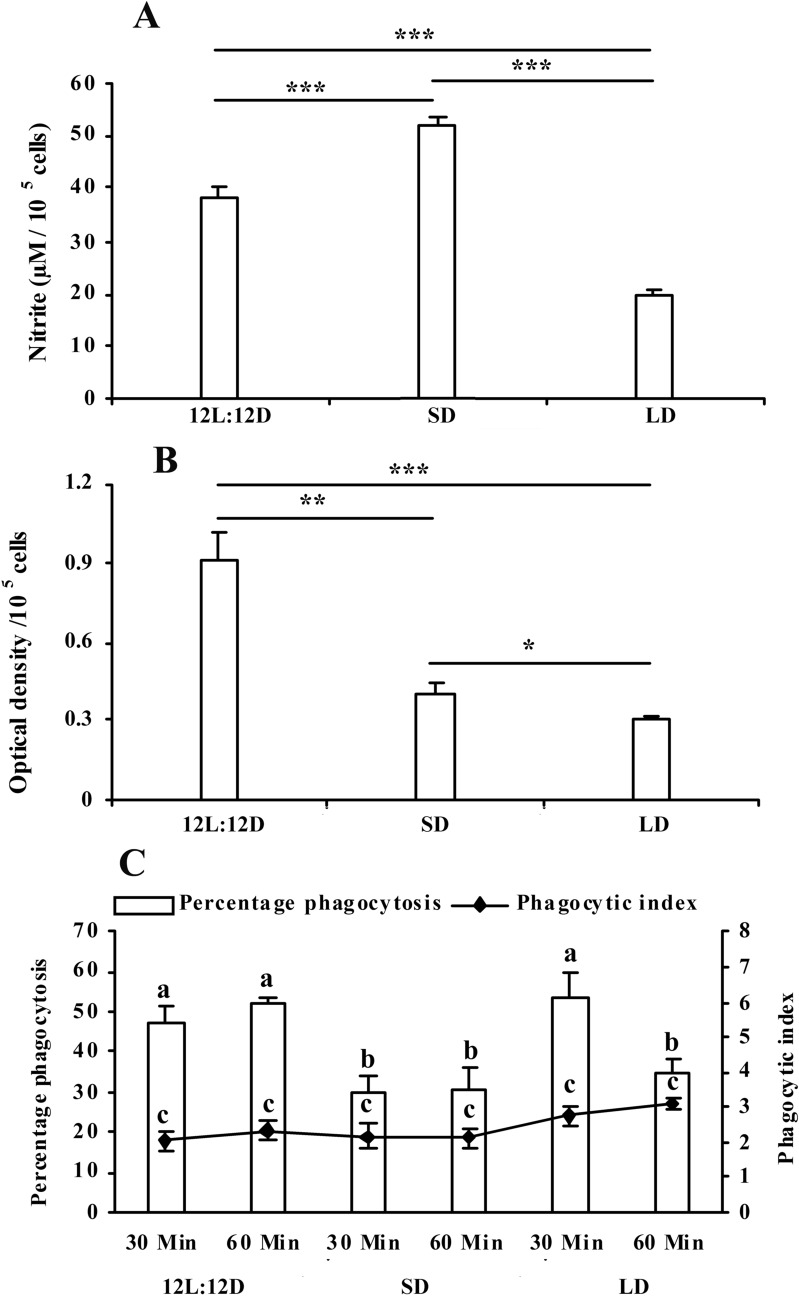
So far, phagocytosis by leucocytes is concerned, the number of cells performing phagocytosis decreased significantly (df = 27; F = 9.618 and p < 0.001) in the snakes kept in 08L:16D, however, there was no significant change in phagocytosis in animals kept in long days, when compared with control animals (Fig. 3C). Nitrite release decreased significantly (df = 38; F = 118.674 and p < 0.001) in leucocyte cultures, obtained from animals kept in long days. In contrast, nitrite release was significantly enhanced in cultures taken from snakes kept in short days, when compared with control animals (Fig. 3A). Super oxide production (NBT reduction) decreased in the leucocytes culture obtained either from short or long day animals, when compared with the control snakes (df = 26; F = 23.471 and p < 0.001) (Fig. 3B). Significant (df = 116; F = 21.695 and p < 0.001) enhancement of basal and Con A stimulated proliferation of lymphocyte was observed in cultures obtained from animals kept in short or long days (Fig. 4C). Somewhat similar trend was observed with PHA stimulated lymphocyte proliferation (df = 116; F = 19.492 and p < 0.001) where basal and PHA stimulated proliferations of lymphocyte were observed in cultures obtained from animals kept in short or long days (Fig. 4B). In vitro melatonin (500 pg ml-1) significantly (df = 77; F = 90.445 and p < 0.001) stimulated proliferation of the lymphocyte cultures obtained from animals kept in 16L:08D, as compared to that from animals kept in 08L:16D or 12L:12D (Fig. 4A).
Figure 3.
Effect of different photoperiodic regimens (12L:12D—light:dark 12:12; SD—short days, light:dark 8:16; LD—long days, light:dark 16:8) on leucocyte phagocytosis (C), superoxide production (B) and nitrite release (A) in the fresh-water snake, Natrix piscator. Data were analysed by One Way ANOVA and Post hoc comparisons were done utilizing Newman-Keul’s multiple-range test* (p < 0.05); *** (p < 0.001). Error bars bearing same superscript do not differ significantly.
Figure 4.
Effect of different photoperiodic regimens (12L:12D—light:dark 12:12; SD—short days, light:dark 8:16; LD—long days, light:dark 16:8) on basal (A) and mitogens (Con A- (C) and PHA- (B)) induced lymphocyte proliferation in the fresh-water snake, Natrix piscator. Effect of in vitro melatonin (500 pg/mL, upper panel) on lymphocyte proliferation is also shown. The error bars bearing the same superscript do not differ significantly (Newman–Keul’s multiple-range test, p < 0.05).
Discussion
Photoperiod is the principal cue that animals use to determine seasonality. Species in temperate zone utilize the direction of change in photoperiod to start or stop important physiological processes such as reproduction. Even in the tropics, where animals remain active throughout the year, most animals reproduce seasonally. Restriction of reproduction to specific times of the year has adaptive significance as biotic and abiotic factors drive the seasonality in reproduction32. We posited that photoperiod modulates the immune responses differentially in order to correspond with the changing environment. Along with the suppression of reproductive function and growth, short photoperiod is also reported to influence immune functions. In non-tropical rodents, photoperiod modulates several aspects of immunity (33). In the present study, total leucocytes, lymphocyte, heterophil and monocyte counts decreased in the snakes kept under short day conditions, however, number of basophil and eosinophils increased in animals kept in long day conditions. Earlier works have shown that the number of total leucocyte, lymphocyte, specific T-cell, and Natural Killer Cell (NK Cell) are increased in short day exposed hamsters; but monocyte, neutrophil and B-cell numbers do not differ33–36. Short photoperiod stimulates NK Cell mediated cytotoxicity and number of circulating T- and B-cells37,38. Vaughan et al.17 and Drazen et al.39 have reported significant increase in total splenocyte number, and macrophage count in hamster exposed to short photoperiod; while no difference has been detected in the total or the differential leucocyte counts in laboratory rats, exposed to long or short photoperiod40. Kliger et al.19 have shown that exposure to continuous light increases heterophil number but decreases the number of lymphocytes in chickens. Differential changes in the number of leucocytes can be explained by two ways. Earlier works41 suggested that melatonin can change the number of blood cells or it can alter the intrinsic mitogenic activities of lymphocytes. The results of the current study show that both have happened. First, short photoperiod has altered the leucocyte populations and second, photoperiodic manipulation has differentially affected the mitogen stimulated lymphocyte proliferations. Thus, the enhanced immune response can be attributed to increase in cellular activity or simply to an increase in percentages of certain cell types.
In contrast to our predictions, photoperiod did not alter all aspects of immune function as phagocytosis and super oxide production by leucocytes decreased in animals kept on either short or long day regimens when compared with animals kept in 12L:12D. Immunological non-responsiveness to photoperiod has also been documented in laboratory rats40 and in tropical rodent, Aztec mice42, as the leucocyte number, humoral immune response, cutaneous immune response as well as splenocyte proliferation responses are not different in rodents kept under short days when compared to rodents kept under long days. These studies have demonstrated that difference in the responses of species to photoperiod is an attribute of group and species difference. Short day induced decrease in superoxide production and phagocytosis can partly be explained by the observation of Turkowska et al.14. In their earlier experiments, they found that particular immune responses were differently modified by continuous lighting conditions and melatonin supplementation in birds43. Melatonin is not the sole factor responsible for the seasonality in immunity and hence short day induced enhancement of immunity is not always the case. On the other hand, nitrite release was increased in short day snakes, but decreased in snakes kept in long days. Similar observation was noted by Yellon et al.37 and Bilbo et al.44 where they reported decrease in oxidative burst activity, T-Cell dependent humoral immunity, phagocytosis, lymphocyte IL-1a production and in vitro lymphocyte proliferation in hamsters kept under short day conditions. However, Vaughan et al.17 and Drazen et al.39 have reported significant increase in splenic mass, total splenocyte number, macrophage count, and lymphocyte proliferation in hamster exposed to short photoperiod. Inconsistent results pertaining to the differential pattern of immune response has also been described by Brainard et al.45 and Zysling et al.8. They have shown that the number of splenic lymphocyte and splenic macrophage count are significantly elevated in short day housed Syrian hamsters when compared with animals kept in long days; whereas, humoral immunity, as assessed by serum antibody concentrations, is unaffected by changes in day lengths. In our study nitric oxide production was decreased significantly in the snakes housed in long days which might be due to reduced biosynthesis of melatonin in day light. In our previous study, we had found that melatonin, secreted predominantly during dark, plays a function in regulation of the immunity31. Here, we demonstrate that evening injection of melatonin causes an increase in leucocyte number in Natrix piscator. This observation was consistent with observations in mammal where Rai and Haldar46 reported low TLC (total leukocyte count) in pinealectomised Indian squirrels. In the present study, we found that T-cell mitogens (Con A and PHA) induced blood lymphocyte proliferation was enhanced in snakes kept either in short or long days. Differential regulation of immune function has also been reported in birds by Majewski et al.47 where ROS level and spelnocyte proliferation were higher in summer. These results are in disparity to the results described by Kliger et al.20 where they found non-significant change in lymphocyte proliferation due to different photoperiodic regimens in chickens. They further observed that there was no effect of different photoperiodic regimens on the lymphocytes proliferation from three-week old birds to B- and T-cell mitogens (pokeweed mitogen and Con A respectively) when the lymphocytes were cultured with melatonin. However, the authors again reported that when lymphocytes from six week old birds were cultured, mitogen stimulated lymphocytes, grown in uniform light, were significantly more reactive to pineal hormone when compared with chickens kept in either irregular or intermediate light. We also found that lymphocytes isolated from animals kept in long days were more reactive to pineal hormone stimulation than from animals kept either in 12L:12D or 16L:08D. Lighting conditions caused development of inflammation while winter like photoperiod caused virtually no inflammation in chickens48. Results of various studies in mammals have drawn two important evolutionary relationships between reproduction and immunity. First, long day induced typical immune responses coincide with short day pattern of gonadal maturation49,50. There is no known example of enhanced short day immune function and large functional gonads, so it remains unknown whether these two physiological processes are able to occur together. Second, photoperiod induced immunecompetence evolved independently of seasonal reproductive function.
Our study shows that photoperiodic manipulation influences the immune activity differentially which is of adaptive significance to the animal. This conclusion is consistent with the possible role of melatonin in the control of the diurnal and seasonal variability of bird immune defense and remains in line with the general agreement that particular immune parameters are differently regulated by melatonin13. Natrix piscator breeds seasonally and it has been suggested that in seasonally breeding animals, this seasonality permits individuals to sustain an enhanced immune status for a greater portion of the year. Since energy expenditure in obtaining optimal immune status and reproduction is highly unlikely51–53, natural selection has favoured energy expenditure in these processes at a specific time of the year1. Natrix piscator breeds from September to December when their gonads are maximal in size54 and as per previously reported hypothesis of energy trade-off between immunity and reproduction55, it is concluded that in wild animals elevated pineal hormone in winter suppresses reproduction while immunity is stimulated. Seasonal fluctuation in immune function is associated with the seasonal adjustment in diseases and death rates and a good correlation exists between them. Since photoperiod is primary cue to determine the season of the year, elevated status of immune function of seasonal breeders helps them fight with the seasonal challenges that will otherwise compromise immune responses and ultimately jeopardize the survival of species. It has been postulated that photoperiod induces changes in immune status which may permit adaptive functional responses in order to maintain seasonal energetic budgets of animals. Most physiological responses (like elevated immune status) are energetically expensive, therefore, animals have evolved a strategy to reduce immune function at certain times of the year when energy is diverted towards reproductive activities. However, when environmental conditions are not suitable for reproduction, investment in survival and defense (immunity) is favored.
Materials and methods
Animals
Male Natrix piscator (commonly called as fresh water snake), having weight 80–120 g, were collected during Feb and Mar from a local person who found the animals in small ponds and ditches in Varanasi (280 18′ N; 83001′ E), India. The snakes were kept in the laboratory (experiencing natural ambient photoperiod and temperature) and were acclimated for four weeks before the study was started. These snakes were kept in cages (made up of wood and wire net; size 50 × 30 × 30 cm). Each cage was made up of wooden floor having sides of wire net and a window on one side. Each cage was equipped with an earthen pot of 4 L capacity, filled with water and accommodated 4–5 snakes. The animals were given small fishes as food ab libitum. Each cage was regularly cleaned and earthen pot water was changed after feeding. The regulations of the committee for the purpose of control and supervision of experiment on animals (CPCSEA), Ministry of Statistics & Programme Implementation, Government of India, were followed in the care of the animals. The experimental protocols were approved by Udai Pratap Autonomous College Ethical Committee.
Chemicals
NBT (Nitroblue Tetrazolium salt) (Product no. N6876), MTT [3-(4, 5-dimethylthiozol-2-yl)-2, 5 diphenyltetrazolium bromide] (Product no. M2128), melatonin (Product no. M5250), and mitogens (Con A, Concanavalin A [Product no.L7647]; PHA, Phytohemagglutinin [Product no. 11082132001]) were obtained from Sigma Chemicals, Sigma-Aldrich, USA. Lymphocyte separation medium (HiSep) (Product no. LSM1084, LS003), culture medium (RPMI-1640) (Product no. AL060A), gentamycin (Product no. TC026), dimethylsulfoxide (DMSO) (Product no. MB058), fetal bovine serum (FBS) (Product no. RM1112), l-glutamine (Product no. TC243), and other chemicals were bought from Himedia Lab Pvt. Ltd., India. Culture medium was mixed with 10 µl ml−1 of 200 mM l-glutamine, 1 µl ml−1 Gentamycin, 10 µl ml−1 anti-anti (Gibco) and 5% FBS and was called as complete culture medium. All the chemicals purchased were of analytical grade.
Experiment
After acclimation of the animals in laboratory for 4 weeks, experiments were started. The animals were grouped into three having six animals in each group. Group 1 animals served as control and maintained in natural photoperiod of 12L:12D. Group 2 animals were maintained in long day (LD, light:dark 16:8, lights put off at 2,000 h). Group 3 animals were maintained in short day (SD, light:dark 8:16, lights put off at 1,600 h). Animal groups were treated with different photoperiodic regimen for 30 days. After the completion of the experiments, animals were mildly anaesthetized and about 2 ml of blood was isolated from each animal through cardiocentesis in heparinized tubes. It has been reported that ethylenediamine tetraacetic acid (EDTA) causes haemolysis of blood in reptiles, heparin is preferred as an anticoagulant56. The blood was utilized to study the NBT slide assay, total leucocyte count, differentiation leucocyte count, quantitative NBT reduction assay, leucocyte phagocytosis, lymphocyte proliferation, and nitrite assay. Lymphocytes from different experimental animals were also incubated with melatonin and mitogen induced proliferation was studied.
Total leucocyte count (TLC)
Turk’s solution (0.2% gentian violet solution in 3% acetic acid) was used as diluting fluid for counting white blood cells57. Twenty microlitres of blood was diluted with 380 µl Turk’s diluting fluid. The diluted blood was spread on Haemocytometer and white blood cells from the four chambers were counted, and the number of leucocytes in /mm3 was calculated.
Differential leucocyte count (DLC)
A small drop of blood was taken on a clean dry slide and a uniform film was smeared. Slides were dried in air, fixed in methanol58,59 and stained in Giemsa and Leishman stain60,61. The slides were then washed in running water, dried, dehydrated, cleaned in xylene and mounted. The purpose of differential leucocyte count was to find out the number of individual leucocytes. The stained slides, as above, were observed under microscope (magnification 100X) from one end to other. Total hundred leucocytes were identified and counted from different areas of slide. Once the percentage of lymphocytes, monocytes, heterophils, basophils and eosinophils was obtained, their exact number was obtained in /mm3 blood from total leucocyte count.
Phagocytic assay
Yeast cells were utilized as target cells to study phagocytosis. Suspension of yeast cells was made by adding 20 mg of baker’s yeast (Saccharomyces cerevisae) in 0.2 M PBS (10 ml). The suspension was put at 80 °C for 15 min and was cooled. Yeast cells were then washed three times in PBS and were finally mixed in complete culture medium. Cells were counted using haemocytometer and density was adjusted to 1 × 108 cells ml−1. Twenty microlitres of blood was mixed with 20 µl of yeast cell suspension in a tube and was incubated for 30 and 60 min. After 30 and 60 min, smear was made on prewashed clean glass slides. The slides were air dried, fixed in methanol, stained with Giemsa, and were examined under microscope at 100 × magnification. From each slide 100 leucocytes showing phagocytosis were counted. The percentage phagocytosis was obtained by dividing the number of leucocytes showing phagocytosis by 100. The phagocytic index was obtained by counting the average number of yeast cells phagocytosed by single leucocyte.
Separation of leucocytes
Leucocytes were isolated from buffy coat (the layer of leucocytes between the plasma and RBCs) by slow rotation technique as described by Keller et al.62. Blood was centrifuged at 42 × g in a swinging bucket rotor for 25 min at 8 °C. The peripheral blood leucocytes (PBL) were isolated by smoothly spinning the buffy and transporting the cells into a new tube. After centrifugation at 200× g for 10 min, the plasma was discarded and the pellet was gently mixed in 1 ml of complete culture medium. For eliminating any residual plasma, the cells were centrifuged again at 200× g for 10 min at 8 °C, the supernatant was discarded, and the cells were resuspended in culture medium. The number of viable PBLs and purity were determined via trypan blue exclusion test using a hemocytometer and experiment was further processed only when viability and purity exceeded 90%. All the techniques and counts were performed by one person to ensure consistency.
NBT assay
Respiratory burst activity and superoxide anion production by phagocyte were examined through NBT assay63,64. NBT is a yellow colored, water soluble, and membrane permeable dye which is reduced to NBT-diformazan (Purple color) by superoxide. NBT assay is an important measure of respiratory burst activity and has been validated in lower vertebrates65. NBT assay was carried out following the method of Berger and Slapnickova66. Briefly, NBT solution (0.1%) was made in phosphate buffer saline (PBS) and was mixed at room temperature for 1 h. This solution was kept at 4 °C. Isolated leucocytes, as above, were counted and adjusted to 2 × 106 cells ml–1 in complete culture medium. Trypan blue exclusion test was used to determine cell viability, which was above 95%. Leucocyte suspension (50 µl) having 1 × 105 cells was mixed with 50 µl of culture medium containing NBT in culture plate from each animal in triplicates. Culture medium (100 ml) alone in triplicates served as blank. Cells were then incubated in 5% CO2 atmosphere at 25 °C for 2 h, centrifuged at 700× g in cooling centrifuge, washed thrice with PBS and fixed in methanol (70%). After fixation, 20 µl of triton X-100 (0.1%) was added in each culture well. Further, 120 µl KOH (2 M) and 140 µl DMSO were added to each well to dissolve the formazan crystals present inside the cells. Absorbance was measured with the help of ELISA plate reader at 620 nm.
Nitrite assay
Cytotoxicity involves various effector molecules and nitric oxide (NO) plays important roles in it. Phagocytes in reptiles have been reported to produce NO which acts as cytotoxic molecule and destroys pathogen67. NO is produced from l-arginine by enzyme nitric oxide synthase (NOS) and is very unstable compound. Nitric oxide is degraded to other nitrogen oxides such as nitrite (NO2–) and nitrate (NO3–) commonly known as reactive nitrogen intermediate (RNI)68. This method was thus assayed to measure the immune cytotoxicity. Nitrite content, produced by the cells in the supernatant, was measured following the method of Ding et al.69 with slight modification. Hundred microlitres of leucocytes (1 × 106 cells ml–1) were seeded to each well of culture plate. Cells were incubated at 25 °C in 5% CO2 atmosphere for 24 h, centrifuged at 200× g and supernatant was taken in another well. Equal volume of Griess reagent (1% sulfanilamide in 3 N HCl and 0.1% naphthylenediamine dihydrochloride in distilled water) and supernatant were added together and absorbance of the solution was read at 540 nm with the help of plate reader. Wells having culture medium alone without any cell served as blank. Following blank subtraction, triplicates were averaged.
Lymphocyte proliferation assay
Lymphocyte proliferation assay is an important marker of cell mediated immune response and its role in immunity has been reviewed in reptiles by Zimmermann et al.70. Proliferation of lymphocytes was assessed through colorimetric assay which utilizes tetrazolium salt (MTT) as described by Berridge et al.71. The method, utilizing MTT salt, is an advantageous alternative method measuring proliferation of lymphocytes72. Tetrazolium salts enter the mitochondria of metabolically active cells and mitochondrial dehydrogenase enzyme breaks thetetrazolium rings of MTT. Tetrazolium salts are reduced into dark blue formazan crystals which remain inside the cells. Formazan crystals are liberated after solubilisation of cells by the addition of a detergent. The amount of formazan product, as measured by the amount of absorbance 570 nm is directly related to the number of cultured cells73. Thus, MTT assay provides a measure of cell number during last hours of in vitro culture. Collection of colored formazan products in the cells is positively correlated with the incorporation of 3H-thymidine into DNA in the S-Phase of cell division during last hours of in vitro culture74. Lymphocytes were segregated from whole blood by density gradient centrifugation using lymphocyte separation gradient HiSep having density 1.077 g ml−1. Blood was carefully leveled over equal volume of HiSep in a test tube and centrifuged at 400× g for 30 min at 8 oC with brakes off. After centrifugation, ring of lymphocyte formed at the interface between medium and HiSep was carefully taken out with the help of Pasteur’s pipette, washed thrice with PBS, counted and viability was checked through trypan blue exclusion test. Viable cells which exceeded 95% were adjusted to 2 × 106 cells ml−1 in culture medium.
Basal and mitogen (Con A and PHA) stimulated lymphocyte proliferation assays were performed. Mitogens were dissolved in 0.2 M PBS (pH 7.2) at a concentration of 1 mg ml−1 and further working concentrations were made in culture medium. Both the mitogens were used at concentration of 5 and 10 µg ml−1. Fifty microlitres of mitogen and 50 µl of cell suspension, having 2 × 106 cells ml–1,were added to the wells of culture plate. To study basal proliferation, 1 × 105cells were added into well of culture plate along with 50 µl of culture medium. Wells containing only 100 µl of culture medium, acted as blank. Further, to study the effect of in vitro melatonin, 50 µl cell suspension was added along with 50 µl of melatonin (500 pg ml–1, final concentration).
Plates were incubated in humidified 5% CO2 atmosphere for 48 h at 25 °C. Following incubation, 10 µl of MTT reagent (5 mg ml–1) was added to each well, and plates were again incubated for 4 h. Following incubation, plates were centrifuged at 200× g and the supernatant was aspirated. To solubilize the formazan crystals 100 µl of DMSO was added to each well and optical density was read at 570 nm with the help of ELISA plate reader.
Statistical analysis
All the measurements were taken in triplicate. Each experiment was performed three times with different animals (N = 5). Reproducibility was verified and then data of one of the experiments were analysed. Data are given as mean ± SEM. Various data of one independent experiment were analysed by generalized linear model procedure and by one-way Analysis of Variance (ANOVA) using SPSS 16.0 and Post hoc comparisons were carried out using Newman-Keul’s multiple-range test. Differences were considered significant when P < 0.05.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Informed consent
This article does not contain any studies with human participants hence informed consent is not applicable.
Author contributions
The authors’ responsibilities were as follows—M.K.T. and R.S. designed the research; M.K.T. and A.S. conducted the experimental works; M.K.T., A.S. and R.S. analysed the data; M.K.T. wrote the manuscript.
Funding
Corresponding author is obliged to Council of Scientific and Industrial Research, India for the financial support in the form of Junior and Senior Research Fellowship.
Data availability
All the necessary data pertaining to the findings of present study are available in the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weil, Z. M., Borniger, J. C., Cisse, Y. M., Abi Salloum, B. A. & Nelson, R. J. Neuroendocrine control of photoperiodic changes in immune function. Front. Neuroendocrinol. 37, 108–118 (2015). [DOI] [PMC free article] [PubMed]
- 2.Nelson RJ, Demas GE. Role of melatonin in mediating seasonal energetic and immunologic adaptations. Brain. Res. Bull. 1997;44:423–430. doi: 10.1016/s0361-9230(97)00222-0. [DOI] [PubMed] [Google Scholar]
- 3.Martinez ME. The calendar of epidemics: Seasonal cycles of infectious diseases. PLoS Pathol. 2018;14(11):e1007327. doi: 10.1371/journal.ppat.1007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gates, D. E., Valletta, J. J., Camille Bonneaud, C. & Recker, M. Quantitative host resistance drives the evolution of increased virulence in an emerging pathogen. J. Evol. Biol. 31, 1704–1714, 10.1111/jeb.13366 (2018). [DOI] [PubMed]
- 5.Hawley DM, Moyers SC, Caceres J, Youngbar C, Adelman JS. Characterization of unilateral conjunctival inoculation with Mycoplasma gallisepticum in house finches. Avian Pathol. 2018;47(5):526–530. doi: 10.1080/03079457.2018.1495312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson RJ, Demas GE. Seasonal changes in immune function. Q. Rev. Biol. 1996;71:511–548. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- 7.Klein SL, Nelson RJ. Influence of social factors on immune function and reproduction. Rev. Reprod. 1999;4:168–178. doi: 10.1530/ror.0.0040168. [DOI] [PubMed] [Google Scholar]
- 8.Zysling DA, Garst AD, Demas GE. Photoperiod and food restriction differentially affect reproductive and immune responses in Siberian hamsters Phodopus sungorus. Funct. Ecol. 2009;23:979–988. [Google Scholar]
- 9.Demas GE, Bartness TJ, Nelson RJ, Drazen DL. Photoperiod modulates the effects of norepinephrine on lymphocyte proliferation in Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R873–R879. doi: 10.1152/ajpregu.00209.2003. [DOI] [PubMed] [Google Scholar]
- 10.Bilbo SD, Nelson RJ. Photoperiod influences the effects of exercise and food restriction on an antigen specific immune response in Siberian hamsters. Endocrinology. 2004;145(2):556–564. doi: 10.1210/en.2003-1035. [DOI] [PubMed] [Google Scholar]
- 11.Prendergast BJ, Bilbo SD, Nelson RJ. Short day length enhance skin immune responses in gonadectomised Siberian Hamsters. J. Neuroendocrinol. 2005;17:18–21. doi: 10.1111/j.1365-2826.2005.01273.x. [DOI] [PubMed] [Google Scholar]
- 12.Carrillo-Vico A, et al. The modulatory role of melatonin on immune responsiveness. Curr. Opin. Investig. Drugs. 2006;7:423–431. [PubMed] [Google Scholar]
- 13.Carrillo-Vico A, Lardone PJ, Alvarez-Sanchez N, Rodriguez-Rodriguez A, Guerrero JM. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013;14:8638–8683. doi: 10.3390/ijms14048638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turkowska, E., Adamska, I., Niedziolka, S., Majewski, P. M. & Skwarlo-Sonta, K. Seasonality of inflammation in the chicken: Clock vs. melatonin control over the pro-inflammatory cytokine gene transcription. Biol. Rhythm Res.10.1080/09291016.2015.1073486 (2015).
- 15.Vriend J, Lauber JK. Effects of light intensity, wavelength and quanta on gonads and spleen of the deer mouse. Nature. 1973;244:37–38. doi: 10.1038/244037a0. [DOI] [PubMed] [Google Scholar]
- 16.Brainard GC, Knobler RL, Podolin PL, Lavasa M, Lublin FD. Neuroimmunology: Modulation of the hamster immune system by photoperiod. Life Sci. 1985;40:1219–1326. doi: 10.1016/0024-3205(87)90589-3. [DOI] [PubMed] [Google Scholar]
- 17.Vaughan MK, Hubbard GB, Champney GM, Little JC, Reiter RJ. Splenic hypertrophy and extramedullary hematopoesis induced in male Syrian hamsters by short photoperiod or melatonin injection and reversed by melatonin pellet or pinealectomy. Am. J. Anat. 1987;179:131–136. doi: 10.1002/aja.1001790205. [DOI] [PubMed] [Google Scholar]
- 18.Skwarlo-Sonta K, Majewski P, Markowska M, Oblap R, Olszanska B. Bidirectional communication between the pineal gland and the immune system. Can. J. Physiol. Pharmacol. 2003;81:342–349. doi: 10.1139/y03-026. [DOI] [PubMed] [Google Scholar]
- 19.Kliger CA, Gehad AF, Hulet RM, Roush WB, Lillehoj HS, Mashaly MM. Effect of photoperiod and melatonin on lymphocyte activities in male broiler chicken. Poul. Sci. 1999;79:18–25. doi: 10.1093/ps/79.1.18. [DOI] [PubMed] [Google Scholar]
- 20.Brennan CP, Hendricks GL, El-Sheikh TM, Mashaly MM. Melatonin and the enhancement of immune responses in immature male chickens. Poult Sci. 2002;81(3):371–375. doi: 10.1093/ps/81.3.371. [DOI] [PubMed] [Google Scholar]
- 21.Schultz EM, Hahn TP, Klasing KC. Photoperiod but not food restriction modulates innate immunity in an opportunistic breeder, Loxia curvirostra. J. Exp. Biol. 2017;15:722–730. doi: 10.1242/jeb.149898. [DOI] [PubMed] [Google Scholar]
- 22.Majewski P, Adamska I, Pawlak J, Baranska A, Skwarlo-Sonta K. Seasonality of pineal gland activity and immune functions in chickens. J. Pineal Res. 2005;39(1):66–72. doi: 10.1111/j.1600-079X.2005.00214.x. [DOI] [PubMed] [Google Scholar]
- 23.Sudhakumari CC, Haldar C, Senthilkumaran B. Seasonal changes in adrenal and gonadal activity in the quail, Perdicula asiatica: Involvement of the pineal gland. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2001;128(4):793–804. doi: 10.1016/s1096-4959(01)00302-5. [DOI] [PubMed] [Google Scholar]
- 24.Bromage N, Porter M, Randall C. The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture. 2001;197:63–98. [Google Scholar]
- 25.Bowden, T. J., Thompson, K. D., Morgan, A. L., Gratacap, R. M. L. & Nikoskelainen, S. Seasonal variation and the immune response: A fish perspective. Fish Shellfish Immunol. 22, 695e706 (2007). [DOI] [PubMed]
- 26.Morgan, A. L., Thompson, K. D., Porter, M. J. R., Burrells, C, & Bromage, N. R. Effect of seasonality on the immune response of rainbow trout. in European Association of Fish Pathologists Conference, 2003 (Malta, 2003).
- 27.Leonardi, M. O. &Klempau, A. E. Artificial photoperiod influence on the immune system of juvenile rainbow trout (Oncorhynchus mykiss) in the southern hemisphere. Aquaculture.221, 581e91 (2003).
- 28.Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J. Pineal. Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 29.Tripathi MK, Singh R, Pati AK. Daily and seasonal rhythms in immune responses of splenocytes in the freshwater snake, Natrix piscator. PLoS ONE. 2015;10(2):e0116588. doi: 10.1371/journal.pone.0116588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh A, Tripathi MK, Singh R. Melatonin modulates leucocytes immune responses in freshwater snakes, Natrix piscator. J. Herpetol. 2016;50(1):130–137. [Google Scholar]
- 31.Bilbo, S. D., Dhabhar, F. S., Vishwanathan, K., Alison, S., Yellon, S. M. & Nelson, R. J. Short day lengths augment stress-induced leucocyte trafficking and stress-induced enhancement of skin immune function. J. Proc. Natl. Acad. Sci. 99(6), 4067–4072 (2002). [DOI] [PMC free article] [PubMed]
- 32.Brown GP, Shine R. Why do most tropical animals reproduce seasonally? Testing hypotheses on an Australian snake. Ecology. 2006;87(1):133–143. doi: 10.1890/04-1882. [DOI] [PubMed] [Google Scholar]
- 33.Bilbo SD, Dhabhar FS, Vishwanathan K, Saul A, Nelson RJ. Photoperiod affects the expression of sex and species differences in leucocyte number and leucocyte trafficking in congeneric hamsters. J. Psychoneuroendocrinol. 2003;28:1027–1043. doi: 10.1016/s0306-4530(02)00122-1. [DOI] [PubMed] [Google Scholar]
- 34.Prendergast BJ, Edward KEW, Yellon SM, Nelson RJ. Photorefractoriness of immune function in male Siberian hamsters (Phodopus sungorus) J. Neuroendocrinol. 2002;14:318–329. doi: 10.1046/j.1365-2826.2002.00781.x. [DOI] [PubMed] [Google Scholar]
- 35.Prendergast BJ, Bilbo SD, Nelson RJ. Photoperiod controls the induction, retention, and retrieval of antigen-specific immunological memory. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R54–R60. doi: 10.1152/ajpregu.00381.2003. [DOI] [PubMed] [Google Scholar]
- 36.Prendergast BJ, Bilbo SD, Dhabhar FS, Nelson RJ. Effects of Photoperiod history on immune responses to intermediate day lengths in Siberian hamsters (Phodopus sungorus) J. Neuroimmunol. 2004;149:31–39. doi: 10.1016/j.jneuroim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Yellon SM, Teasley LA, Fagoaga OR, Nguyen HC, Truong HN, Nehlsen-Cannarella L. Role of photoperiod and the pineal gland in T cell-dependent humoral immune reactivity in the Siberian hamster. J. Pineal. Res. 1999;27:243–248. doi: 10.1111/j.1600-079x.1999.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 38.Prendergast BJ, Hotchkiss AK, Nelson RJ. Photoperiodic regulation of circulating leucocytes in juvenile Siberian hamsters: Mediation by melatonin and testosterone. J. Biol. Rhythms. 2003;18(6):473–480. doi: 10.1177/0748730403258486. [DOI] [PubMed] [Google Scholar]
- 39.Drazen DL, Jasnow AM, Nelson RJ, Demas GE. Exposure to short days, but not short term melatonin, enhances humoral immunity of male Syrian hamsters (Mesocricetus auratus) J. Pineal. Res. 2002;33:118–124. doi: 10.1034/j.1600-079x.2002.02915.x. [DOI] [PubMed] [Google Scholar]
- 40.Gatien ML, Hotchkiss AK, Neigh GN, Dhabhar FS, Nelson RJ. Immune and stress responses in C57BL\6 and C3H\HeN mouse strains following photoperiod manipulation. J. Neuroendocrinol. Lett. 2004;25(4):267–272. [PubMed] [Google Scholar]
- 41.Champney TH, Prado J, Youngblood T, Appel K, McMurray DN. Immune responsiveness of splenocytes after chronic daily melatonin administration in male syrian hamsters. Immunol. Lett. 1997;58:95–100. doi: 10.1016/s0165-2478(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 42.Demas GE, Nelson RJ. Lack of immunological responsiveness to photoperiod in a tropical rodent, Peromyscus aztecushylocetes. J. Comp. Physiol. 2003;173:171–176. doi: 10.1007/s00360-002-0325-5. [DOI] [PubMed] [Google Scholar]
- 43.Turkowska E, Rai S, Majewski PM, Skwarlo-Sonta K. Diurnal and seasonal changes in IL-6 and IL-18 gene expression in blood leukocytes of male chickens with experimental peritonitis: The impact of lighting conditions and melatonin. J. Anim. Feed Sci. 2013;22:149–157. [Google Scholar]
- 44.Bilbo, S. D., Drazen, D. L., Quan, N. He. L. & Nelson, R. J. Short day lengths attenuate the symptoms of infection in Siberian hamsters. J. Proc. R. Soc. Lond.269, 447–454 (2002). [DOI] [PMC free article] [PubMed]
- 45.Brainard GC, Watson-Whitmeyer M, Knobler RL, Lubin FD. Neuroendocrine regulation of immune parameters: Photoperiod control of spleen in the Syrian Hamsters. Ann. N. Y. Acad. Sci. 1988;540:704–706. doi: 10.1111/j.1749-6632.1988.tb27219.x. [DOI] [PubMed] [Google Scholar]
- 46.Rai S, Haldar C. Pineal control of immune status and hematological changes in blood and bone marrow of male squirrels (Funambulus pennanti) during their reproductively inactive phase. Comp. Biochem. Physiol. 2003;136:319–328. doi: 10.1016/j.cca.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Majewski P, Markowska M, Pawlak J, Piesiewicz A, Turkowska E, Skwarlo-Sonta K. Pineal gland and melatonin: Impact on the seasonality of immune defence in mammals and birds. Adv. Neuroimmune Biol. 2012;3:95–108. [Google Scholar]
- 48.Skwarlo-Sonta, K., Majewski, P., Pawlak, J. & Markowska, M. Photoperiodic vs. non-photoperiodic animals—Circadian and seasonal changes in immunity. in (Pandi-Perumal, S.R. & Cardinali D. P. eds.) Melatonin: From Molecules to Therapy 247–260 (Nova Science Publishers Inc, New York, 2007).
- 49.Weil ZM, Pyter LM, Martin LB, Nelson RJ. Perinatal photoperiod organizes adult immune responses in Siberian hamsters (Phodopus sungorus) Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1714–R1719. doi: 10.1152/ajpregu.00869.2005. [DOI] [PubMed] [Google Scholar]
- 50.Weil ZM, Workman JL, Nelson RJ. Housing condition alters immunological and reproductive responses to day length in Siberian hamsters (Phodopus sungorus) Horm. Behav. 2007;52:261–266. doi: 10.1016/j.yhbeh.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanssen SA, Hasselquist D, Folstad I, Erikstad KE. Cost of reproduction in a long-lived bird: Incubation effort reduces immune function and future reproduction. Proc. Biol. Sci. 2005;272:1039–1046. doi: 10.1098/rspb.2005.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin LB, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: A link between direct and indirect costs? Proc. Biol. Sci. 2003;270:153–158. doi: 10.1098/rspb.2002.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Speakman JR. The physiological costs of reproduction in small mammals. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:375–398. doi: 10.1098/rstb.2007.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haldar C, Pandey R. Effect of pinealectomy on testicular cycle of Indian checkered water snake Natrix piscator. Gen. Comp. Endocrinol. 1989;76:214–222. doi: 10.1016/0016-6480(89)90152-4. [DOI] [PubMed] [Google Scholar]
- 55.Martin LB, Weil ZM, Nelson RJ. Seasonal changes in vertebrate immune activity: Mediation by physiological trade-offs. Philos. Trans. R Soc. Lond. B Biol. Sci. 2008;363(1490):321–339. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tavares-Dias M, Oliveira-Junior AA, Marcon JL. Methodological limitations of counting total leukocytes and thrombocytes in reptiles (Amazon turtle, Podocnemis expansa): An analysis and discussion. Acta Amazon. 2008;38(2):351–356. [Google Scholar]
- 57.Tosunoglu M, et al. Some hematologic parameters of Elaphe sauromates (PALLAS, 1811) Herpetozoa. 2010;23:79–83. [Google Scholar]
- 58.Kumarasinghe MP, Constantine SR, Hemamali RL. Methanol as an alternative fixative for cytological smears. Malays. J. Pathol. 1997;19(2):137–140. [PubMed] [Google Scholar]
- 59.Kumar K, Bhattacharyya S, Sarfraz A. Evaluation of two new fixatives for peripheral malaria smear. Int. J. Contemp. Med. Res. 2018;5(1):1–2. [Google Scholar]
- 60.Arikan H, Cicek K. Haematology of amphibians and reptiles: A review. North-West J. Z. 2014;10(1):190–209. [Google Scholar]
- 61.Kularatne, S. A. M. Ranasinghe, J. G. S. & Rajapakse, P. V. J. Hematological and plasma biochemical parameters in a wild population of Naja naja (Linnaeus, 1758) in Sri Lanka. J. Venom. Anim. Toxins Incl. Trop. Dis.23(8),10.1186/s40409-017-0098-7 (2017). [DOI] [PMC free article] [PubMed]
- 62.Keller JM, McClellan-Green PD, Lee AM, Arendt MD. Mitogen-induced lymphocyte proliferation in loggerhead sea turtles: Comparison of methods and effects of gender, plasma testosterone concentration, and body condition on immunity. Vet. Immunol. Immunopathol. 2005;103:269–281. doi: 10.1016/j.vetimm.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 63.Sakai M, Kobayashi M, Kawauchi H. In vitro activation of fish phagocytic cells by GH, prolactin and somatolactin. J. Endocrinol. 1996;151(1):113–118. doi: 10.1677/joe.0.1510113. [DOI] [PubMed] [Google Scholar]
- 64.Khan UW, Rai U. Role of gonadotropin and Leydig cell-secreted factors in the control of testicular macrophage activities in the wall lizard Hemidactylus flaviviridis. Dev. Comp. Immunol. 2008;32:348–355. doi: 10.1016/j.dci.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Vera-Jimenez, N. I., Pietretti, D., Wiegertjes, G. F. & Nielsen, M. E. Comparative study of b-glucan induced respiratory burst measured by nitrobluetetrazolium assay and real-time luminol-enhanced chemiluminescence assay in common carp (Cyprinus carpio L.). Fish Shellfish Immunol.34, 1216e1222 (2013). [DOI] [PubMed]
- 66.Berger J, Slapnickova M. Circadian structure of rat neutrophil phagocytosis. Comp. Clin. Pathol. 2003;12:84–89. [Google Scholar]
- 67.Mondal, S. & Rai, U. In vitro effect of temperature on phagocytic and cytotoxic activities of splenic phagocytes of the wall lizard, Hemidactylus flaviviridis. Comp. Biochem. Physiol.129(a), 391–398 (2001). [DOI] [PubMed]
- 68.Jorens PG, Matthys KE, Bult H. Modulation of nitric oxide synthase activity in macrophages. Mediators Inflamm. 1995;4:75–89. doi: 10.1155/S0962935195000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediated and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J. Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 70.Zimmerman LM, Vogel LA, Bowden RM. Understanding the vertebrate immune system: Insights from the reptilian perspective. J. Exp. Biol. 2010;213:661–671. doi: 10.1242/jeb.038315. [DOI] [PubMed] [Google Scholar]
- 71.Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005;11:127–152. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]
- 72.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 73.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 74.Gienei, R. S., Li, Y. & Hay Glass, K. T. Comparison of [3H] thymidine incorporation with MTT- and MTS-based bioassays for human and murine IL-2 and IL-4 analysis. Tetrazolium assays provide markedly enhanced sensitivity. J. Immunol. Methods.187, 85–93 (1995). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the necessary data pertaining to the findings of present study are available in the manuscript.