Abstract
The skull of six Japanese fowl breeds, namely, Chabo, Oh-Shamo, Onagadori, Shokoku, Tosajidori, and Totenko, were morphologically compared in this study. The morphological differences in the skull size and shape among the breeds were as follows. 1) Oh-Shamo possessed a wide bill, thick bill tip, small orbits and wide mandibular joint. The characteristics of the bill and mandible were interpreted as functional characteristics to endure the shock of pecking. We suggest that the small orbits and a wide frontal bone help in protection from pecking in games. 2) Chabo possessed a small skull. In terms of shape, this breed possessed relatively large orbits, a wide and high skull and a short bill. The wide and high skull and the short bill formed a circular-shaped face. We propose that these characteristics have led to its characterisation as ornament-type fowl. 3) Totenko, Shokoku, Onagadori and Oh-Shamo possess a long mandible. The long mandible led to an increase in the volume of the oral cavity. The wide resonance space is responsible for the low-frequency voice. The low-frequency crowing of Totenko, Shokoku, Oh-Shamo and Onagadori is a result of the enlarged resonance space created by the long mandible. The orbits of Totenko and Onagadori were larger than those of Shokoku and Oh-Shamo. We suggest that Shokoku possessed the small orbits as a fighting cock. Since Onagadori and Totenko had been bred as ornament-type fowl, they possessed larger orbits.
Keywords: Japanese fowl, morphological characteristic, shape, size, skull
Introduction
Domestic fowls have gained various morphological characteristic as not only as livestock animals but also as companion animals (Frahm and Rehkämper, 1998; Okamoto, 2001; Ichinoe and Kuwayama, 2007; Akishinonomiya and Komiya, 2009; Sheppy, 2011). In Japan, variegated breeds were developed and these were admired for their colour variation, voice and fighting abilities, in the mid-Edo Era (Oana, 1951; Okamoto, 2001; Ichinoe and Kuwayama, 2007; Akishinonomiya and Komiya, 2009). Fighting fowls are highly aggressive and were bred for gambling as a form of entertainment. Long-crowing fowls were selected on the basis of their ability to crow for a longer period of time. Ornament-type fowls acquired colourful feathers or long-tailed feathers for attracting people. Native fowls are called Jidori and their external characteristics are similar to those of the Red Jungle Fowl. These various types of fowls were established and developed before the Meiji period.
Japanese fowls vary not only in external characteristics but also in osteological characteristics. From the report of Hayashi et al. (1982), Japanese breeds were categorised into the big-, middle- and small-sized groups on the basis of the skull size, and the smallest breadth between orbits was shown as a remarkable differential characteristic. The size categories of skull were also supported by Samejima et al. (1988). Nishida et al. (1985) examined whole skeletons and indicated that Japanese breeds were separated into size-based groups and that morphological differences were confirmed in the tibiotarsus, tarsometatarsus and sternum.
The above-mentioned studies showed that Japanese fowls obviously differed in skull size, however, the morphological differences in the skull shape remain unclear. The skeleton morphologically reflects the result of artificial selection driven by assessing not only economical characters for agricultural productions but also by noneconomical characters for spiritual preferences such as a long voice, strong aggression and external appearances. Since Japanese fowls were selected for fighting fowls, long-crowing fowls and ornament-type fowls, we expected that the functional morphological skull characteristics peculiar to various breeds in their skull could be detected.
Materials and Methods
Specimens
To clarify the relationship between the skull shapes and breed types, the skulls of six breeds, namely, Oh-Shamo, Onagadori, Shokoku, Totenko, Tosajidori and Chabo, were osteometrically compared. The characters of each breed and the reason why we used these breeds are as follows; Oh-Shamo possess a large body and strong combative instinct as a fighting fowl, Chabo is equipped with a small body and colourful feathers as an ornament-type fowl, Totenko possesses the ability to crow for the longest period as a long-crowing and ornament-type fowl, Onagadori acquired longest tail feather as a long-tailed and ornament-type fowl, Shokoku possesses strong aggression, long voice and tail feather and it is considered as an ancestor of Totenko and Onagadori, and Tosajidori is one of the oldest breeds in Japan (Oana, 1951; Okamoto, 2001; Ichinoe and Kuwayama, 2007; Akishinonomiya and Komiya, 2009). It has also been estimated that the feather colour and body size of Tosajidori are similar to those of red jungle fowl. To compare the morphological differences, we used this breed as a control. We used the skulls of these breeds, which were provided by Hiroshima University and Tokyo University of Agriculture. The Nagoya University Museum permitted us to use the other specimens (Table 1). The adult skulls were used in this study. In the unidentified growth stage of specimens, we defined the growth stage by the degree of ossification of skull (Hogg, 1978). The specimens such as over one years old or completed ossification of skull, were defined as the adult (Table 1).
Table 1. Specimens used in this study.
| breed | sex | specimen No. | growth stage | donor | depository | breed | sex | specimen No. | growth stage | donor | depository |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Onagadori | female | UMUT-14014 | adult1 | TUA | UMUT | Totenko | female | UMUT-14029 | adult1 | TUA | UMUT |
| Onagadori | female | UMUT-14015 | adult1 | HU | UMUT | Totenko | female | UMUT-14030 | adult1 | TUA | UMUT |
| Onagadori | female | UMUT-14016 | adult1 | HU | UMUT | Totenko | male | UMUT-14031 | adult1 | TUA | UMUT |
| Onagadori | male | UMUT-14017 | adult1 | TUA | UMUT | Totenko | male | UMUT-14032 | adult1 | TUA | UMUT |
| Onagadori | male | NUM-ab1-1202 | adult2 | — | NUM | Totenko | male | UMUT-14033 | adult1 | TUA | UMUT |
| Oh-Shamo | female | UMUT-14018 | adult1 | TUA | UMUT | Totenko | male | UMUT-14034 | adult2 | HU | UMUT |
| Oh-Shamo | female | UMUT-14019 | adult1 | TUA | UMUT | Chabo | female | UMUT-14035 | adult1 | TUA | UMUT |
| Oh-Shamo | female | UMUT-14020 | adult1 | TUA | UMUT | Chabo | female | UMUT-14036 | adult1 | TUA | UMUT |
| Oh-Shamo | male | UMUT-14021 | adult1 | TUA | UMUT | Chabo | female | NUM-ab1-926 | adult2 | — | NUM |
| Oh-Shamo | male | NUM-ab1-1230 | adult2 | — | NUM | Chabo | male | UMUT-14037 | adult1 | TUA | UMUT |
| Oh-Shamo | male | UMUT-14022 | adult1 | TUA | UMUT | Chabo | male | UMUT-14039 | adult1 | TUA | UMUT |
| Oh-Shamo | unknown | UMUT-14023 | adult1 | TUA | UMUT | Chabo | male | UMUT-14040 | adult1 | TUA | UMUT |
| Oh-Shamo | unknown | UMUT-14024 | adult1 | TUA | UMUT | Chabo | male | UMUT-14041 | adult1 | TUA | UMUT |
| Shokoku | female | NUM-ab1-1256 | adult2 | — | NUM | Chabo | male | UMUT-14042 | adult1 | TUA | UMUT |
| Shokoku | female | NUM-ab1-1255 | adult2 | — | NUM | Chabo | male | NUM-abl1945 | adult2 | — | NUM |
| Shokoku | male | NUM-ab1-973 | adult2 | — | NUM | Chabo | male | NUM-ab1-922 | adult2 | — | NUM |
| Shokoku | male | NUM-ab1-1307 | adult2 | — | NUM | Chabo | unknown | UMUT-14043 | adult1 | TUA | UMUT |
| Shokoku | male | NUM-ab1-1347 | adult2 | — | NUM | Chabo | unknown | NUM-ab1-925 | adult2 | — | NUM |
| Shokoku | male | UMUT-14025 | adult1 | TUA | UMUT | ||||||
| Tosajidori | female | NUM-ab1-1336 | adult2 | — | NUM | ||||||
| Tosajidori | female | UMUT-14026 | adult1 | HU | UMUT | ||||||
| Tosajidori | male | UMUT-14027 | adult1 | TUA | UMUT | ||||||
| Tosajidori | male | NUM-ab1-1281 | adult2 | — | NUM | ||||||
| Tosajidori | unknown | UMUT-14028 | adult1 | TUA | UMUT |
UMUT indicates The University Museum, The University of Tokyo. NUM indicates Nagoya University Museum, Nagoya University. HU indicates Hiroshima University. TUA indicates Tokyo University of Agriculture. adult1 indicates over one years old. adult2 indicates completed ossification of skull.
Measurements
The details of measurements are shown in Table 2 and Fig. 1. With regard to size, we compared the measurement values in each breed. With regard to shape, we used measurement ratios which were obtained by dividing the measurement values by geometric means (GM) (Darroch and Mosimann, 1985). We calculated the GM, which consisted of the GLs, GBs and GLmp 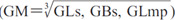 .
.
Table 2. The measurements used in this study.
| Abbreviation of measurements | Details | Abbreviation of measurements | Details |
|---|---|---|---|
| GLs | greatest length of skull; protuberantia occipitalis externa -apex praemaxillarisa,b,c | BLbt | basal length of the bill tip; most frontal point of the corpus premaxillare - most frontal point of the basal incisura nassled |
| GLi | greatest length of incisivum; apex praemaxillaris - most aboral point of the processus frontales of the incisivum in the median planea,b,c | CbL | condulobasal length; aboral border of the occipital condyle - apex praemaxiilarisa,b,c |
| BLb | basal length of bill; apex praemaxillaris -most caudal points of the processus maxiallis ossis premaxillarisd | GBsb | greatest breadth of the sphenoidal bone; between each most wide point on the limbus sphenoidalisc |
| GLbt | greatest length of bill tip; most frontal point of the premaxillaris - the most frontal point of the nasalsd | Bpr | breadth between the processus retriangularis; between each point of the processus retroangularisd |
| Hbt | height of bill tip; highest point above the most frontal point of the nasals - lowest point under the most frontal point of the nasalsd | GLm | greatest length of the mandible; apex to the most aboral point of the mandiblea,b,c |
| GLoc | greatest length of orbital cavity; most frontal point of the aucus zygomaticus - most aboral point of the processus frontalisd | Leaba | length between the aboral edge of articural bone to the apex; pars symphysialis - condylus musculris mandibularis caudalisa,b,c |
| GLmp | greatest length in the median plane; the basitemporale in the median plane - highest and median point of the braincasea,b,c | Lpaa | length between the processus angularis to the apex; apex to the processus angularisc |
| GBbt | greatest breadth of bill tip; most wide breadth in front of the most frontal points of the nasalsd | Ls | length of the symphesis; between frontal apex of mandible to aboral onea,b,c |
| Lnc | length of the neurocranium; processus frontalis of the paemaxilla - protuberantia occipitalis externaa,b,c | Lpapr | length between the processus angularis to processus retroangularis; pcrocessus angularis - processus retroangularisc |
| GBs | greatest breadth of skull; between each point of the processus postfrontalisa,b,c | GLabpa | greatest length between the edge of articular bone to the processus angularisc |
| GBnc | greatest breadth of the neurocranium; between each point of the os opistoideusc | Lpaps | length between the processus angularis to the aboral edge of the pars symphysialisd |
| SBnc | smallest breadth of the neurocranium; between each point of the os quadratumc | GBco | greatest breadth of the condylus occipitalisd |
| SBo | smallest breadth between the orbits; smallest breadth of the pars nasalis of the frontalea,b,c | GHfm | greatest height of the foramen magnumc |
| GBb | greatest breadth of the bill; between each point of the caudal edge of the processus maxillaris ossis premaxillarisd | GBfm | greatest breadth of the foramen magnumc |
Fig. 1.
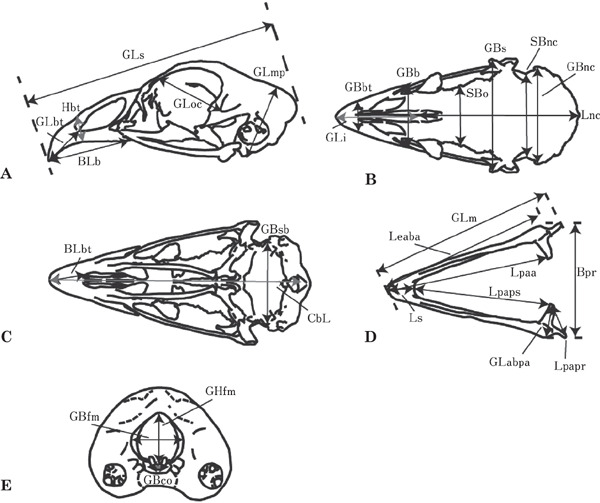
The measurements in cranium and mandible. (A) Cranium of lateral view from left side. (B) Cranium, from dorsal view. (C) Cranium from ventral view. (D) Mandible from dorsal view. (E) Cranium from caudal view. The abbreviated forms were remarked in Table 2.
The GM was considered as an index of the skull size. To establish the measurements, we followed the procedure described by Driesch (1976), Hayashi et al. (1982), Samejima et al. (1988) and Yasuda (2002). The 28 selected measurements were determined using a caliper, and the values were rounded off to the nearest 0.05 mm (Table 2, Fig. 1).
Analyses
To clarify the morphological characters, we calculated the mean values and standard deviations (SD) of all measurements in each breed. Following this, we performed one-way analysis of variance (ANOVA) to identify differences among breeds. When ANOVA indicated significant morphological differences, it was followed by Tukey- Kramer method for multiple comparisons. Morphological differences were considered significant at a P value of <0.05. Statistical analyses were operated by R (R: A language and environment for statistical computing. URL http://www.R-project.org/). We utilised correlation analysis to examine the relationships between the skull size and shape.
Results
To detect the morphological differences among each breed, we did not separate the sex in each breed. Since the individuals of each breed were few, it was not appropriate to detect the sexual dimorphism. The morphological differences among breeds were confirmed by analysis of the size data from measurement values and the shape data from measurement ratios. The mean values and SDs in all measurement values and measurement ratios of each breed are shown in Tables 3 and 4. Each breed showed differences in morphological characteristics as follows.
Table 3. Mean values and standard deviations for skull measurement in various breeds.
| breed | measurements | GLs | GLi | BLb | GLbt | Hbt | GLoc | GLmp | GBbt | Lnc | GBs | GBnc | SBnc | SBo | GBb | BLbt | CbL | GBsb | Bpr | GLm | Leaba | Lpaa | Ls | Lpapr | GLabpa | Lpaps | GBco | GHfm | GBfm | GM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| comparing pair with significant difference | AEFGH IJLMO |
AEFGH IJLMO |
AEFGH ILMO |
ACEFG HILMO |
AFGHI | AFGHI | AEFGH ILMO |
AFGHI | ACEFG HILMO |
ACEFG HIJLMO |
ACEFG HIJLMO |
AFGHI LO |
AFGHI | AFGHI | AEFGH ILMO |
ACEFG HILMO |
ACEFG HIJLMO |
ACEFG HIJLMO |
AEFGH ILO |
ACEFG HIJLMO |
ACEFG HIJLMO |
ACEFG HIJLMO |
ACEFG HIMO |
ACEFG HIMO |
ACEFG HIJLMO |
AFGHI | EGIJL | ACEFG HILO |
ACEFG HIJLMO |
|
| Onagadori | mean value | 67.19 | 34.45 | 24.01 | 13.10 | 5.75 | 21.11 | 20.83 | 8.51 | 37.07 | 29.14 | 25.64 | 22.60 | 13.89 | 12.92 | 11.98 | 61.84 | 19.87 | 43.40 | 29.38 | 54.06 | 49.31 | 49.11 | 9.29 | 11.12 | 9.66 | 3.22 | 6.25 | 7.36 | 34.42 |
| standard deviation | 4.81 | 2.53 | 2.43 | 1.20 | 0.79 | 1.01 | 0.95 | 0.63 | 3.41 | 2.25 | 1.75 | 1.22 | 2.20 | 1.91 | 0.83 | 4.60 | 1.19 | 6.60 | 5.18 | 3.82 | 3.59 | 3.36 | 1.13 | 1.43 | 0.81 | 0.48 | 0.57 | 0.60 | 2.17 | |
| Oh-Shamo | mean value | 85.86 | 45.37 | 30.86 | 16.53 | 8.46 | 24.49 | 25.40 | 11.77 | 46.31 | 37.83 | 32.48 | 28.37 | 19.80 | 19.43 | 14.85 | 78.29 | 25.03 | 52.60 | 39.33 | 70.00 | 63.06 | 62.74 | 10.49 | 15.24 | 13.69 | 4.54 | 6.46 | 8.31 | 43.52 |
| standard deviation | 6.39 | 4.50 | 2.61 | 1.25 | 0.92 | 1.88 | 1.37 | 2.10 | 2.82 | 1.84 | 2.02 | 1.42 | 1.43 | 1.49 | 0.97 | 5.84 | 1.50 | 4.40 | 3.30 | 5.70 | 5.40 | 4.97 | 0.78 | 1.32 | 0.97 | 0.31 | 0.63 | 0.53 | 2.36 | |
| Shokoku | mean value | 67.27 | 35.96 | 23.87 | 12.54 | 5.56 | 18.91 | 20.31 | 8.70 | 36.14 | 29.20 | 25.89 | 22.98 | 13.70 | 13.73 | 11.18 | 60.73 | 20.28 | 40.33 | 28.80 | 53.25 | 48.43 | 48.11 | 8.27 | 10.57 | 9.48 | 3.42 | 6.48 | 6.89 | 34.17 |
| standard deviation | 5.10 | 3.31 | 2.40 | 1.54 | 0.89 | 2.60 | 1.46 | 1.33 | 2.67 | 2.44 | 1.52 | 1.58 | 2.01 | 2.46 | 1.63 | 4.72 | 1.01 | 2.98 | 2.68 | 4.20 | 3.89 | 3.48 | 0.50 | 1.20 | 1.01 | 0.37 | 0.71 | 0.56 | 2.62 | |
| Tosajidori | mean value | 58.26 | 28.78 | 20.38 | 10.67 | 4.87 | 18.32 | 18.76 | 7.45 | 32.32 | 25.39 | 22.66 | 20.88 | 12.22 | 12.25 | 10.03 | 52.72 | 17.18 | 33.52 | 24.95 | 45.27 | 40.94 | 40.57 | 7.46 | 8.97 | 7.96 | 2.93 | 5.28 | 6.24 | 30.26 |
| standard deviation | 2.77 | 0.99 | 1.16 | 0.69 | 0.77 | 0.45 | 0.47 | 0.32 | 1.19 | 1.51 | 1.15 | 0.75 | 1.46 | 0.82 | 0.65 | 3.28 | 0.79 | 1.20 | 0.97 | 1.49 | 1.52 | 1.68 | 0.50 | 0.46 | 0.60 | 0.34 | 0.26 | 0.33 | 0.99 | |
| Totenko | mean value | 67.73 | 34.98 | 25.07 | 13.29 | 5.77 | 20.91 | 21.16 | 9.03 | 37.10 | 30.13 | 25.65 | 22.73 | 14.58 | 13.86 | 12.20 | 62.08 | 20.39 | 41.58 | 29.52 | 55.13 | 50.03 | 49.63 | 8.48 | 10.72 | 9.81 | 3.34 | 5.71 | 7.05 | 35.08 |
| standard deviation | 5.66 | 3.45 | 2.08 | 1.14 | 1.04 | 1.21 | 1.32 | 0.71 | 2.89 | 2.13 | 2.11 | 1.87 | 1.42 | 2.09 | 0.89 | 5.34 | 2.01 | 4.16 | 2.35 | 4.13 | 3.90 | 4.55 | 0.73 | 0.54 | 0.85 | 0.28 | 0.85 | 0.54 | 2.45 | |
| Chabo | mean value | 57.67 | 28.82 | 20.26 | 10.40 | 5.08 | 19.06 | 18.54 | 7.96 | 32.11 | 25.58 | 22.46 | 20.69 | 12.53 | 12.70 | 9.60 | 51.60 | 16.85 | 33.08 | 24.58 | 44.99 | 40.50 | 40.07 | 7.22 | 9.31 | 8.18 | 3.01 | 5.28 | 6.00 | 30.14 |
| standard deviation | 3.99 | 2.06 | 1.31 | 0.79 | 0.56 | 0.83 | 0.76 | 0.59 | 1.30 | 1.15 | 1.00 | 0.76 | 2.05 | 1.17 | 0.93 | 2.94 | 0.92 | 1.35 | 1.63 | 1.78 | 1.58 | 1.66 | 0.50 | 0.45 | 0.46 | 0.32 | 0.44 | 0.38 | 1.35 | |
Each alphabet indicates comparing pair with significant difference as follows; A Onagadori - Oh-Shamo, B Onagadori - Shokoku, C Onagadori - Tosajidori, D Onagadori - Totenko, E Onagadori - Chabo, F Oh-Shamo - Shokoku, G Oh-Shamo - Tosajidori, H Oh-Shamo - Totenko, I Oh-Shamo - Chabo, J Shokoku - Tosajidori, K Shokoku - Totenko, L Shokoku - Chabo, M Tosajidori - Totenko, N Tosajidori - Chabo and O Totenko - Chabo.
Table 4. Mean values and standard deviations for skull measurement ratio in various breeds.
| breed | measurements | GLs | GLi | BLb | GLbt | Hbt | GLoc | GLmp | GBbt | Lnc | GBs | GBnc | SBnc | SBo | GBb | BLbt | CbL | GBsb | Bpr | GLm | Leaba | Lpaa | Ls | Lpapr | GLabpa | Lpaps | GBco | GHfm | GBfm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| comparing pair with significant difference | — | gijl | — | eio | fghi | abijl | agijl | — | — | — | — | cegimo | — | a | — | ei | l | cei | i | egilo | cegilo | cegilo | ae | afghi | afghi | — | afi | a | |
| Onagadori | mean value | 1.95 | 1.00 | 0.70 | 0.38 | 0.17 | 0.61 | 0.61 | 0.25 | 1.08 | 0.85 | 0.75 | 0.66 | 0.40 | 0.37 | 0.35 | 1.80 | 0.58 | 1.26 | 0.85 | 1.57 | 1.43 | 1.43 | 0.27 | 0.32 | 0.28 | 0.09 | 0.18 | 0.21 |
| standard deviation | 0.03 | 0.02 | 0.04 | 0.03 | 0.01 | 0.02 | 0.01 | 0.01 | 0.05 | 0.02 | 0.02 | 0.02 | 0.05 | 0.03 | 0.02 | 0.03 | 0.01 | 0.18 | 0.12 | 0.04 | 0.03 | 0.03 | 0.03 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | |
| Oh-Shamo | mean value | 1.97 | 1.04 | 0.71 | 0.38 | 0.19 | 0.56 | 0.58 | 0.27 | 1.06 | 0.87 | 0.75 | 0.65 | 0.46 | 0.45 | 0.34 | 1.80 | 0.58 | 1.21 | 0.90 | 1.61 | 1.45 | 1.44 | 0.24 | 0.35 | 0.31 | 0.10 | 0.15 | 0.19 |
| standard deviation | 0.06 | 0.06 | 0.03 | 0.01 | 0.02 | 0.01 | 0.01 | 0.05 | 0.02 | 0.02 | 0.01 | 0.01 | 0.03 | 0.03 | 0.01 | 0.06 | 0.02 | 0.04 | 0.04 | 0.06 | 0.06 | 0.05 | 0.01 | 0.02 | 0.01 | 0.00 | 0.02 | 0.01 | |
| Shokoku | mean value | 1.97 | 1.05 | 0.70 | 0.37 | 0.16 | 0.55 | 0.59 | 0.25 | 1.06 | 0.85 | 0.76 | 0.67 | 0.40 | 0.40 | 0.33 | 1.78 | 0.59 | 1.18 | 0.84 | 1.56 | 1.42 | 1.41 | 0.24 | 0.31 | 0.28 | 0.10 | 0.19 | 0.20 |
| standard deviation | 0.02 | 0.04 | 0.04 | 0.03 | 0.02 | 0.04 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.04 | 0.05 | 0.03 | 0.03 | 0.02 | 0.02 | 0.03 | 0.02 | 0.03 | 0.02 | 0.01 | 0.01 | 0.01 | 0.00 | 0.02 | 0.01 | |
| Tosajidori | mean value | 1.92 | 0.95 | 0.67 | 0.35 | 0.16 | 0.61 | 0.62 | 0.25 | 1.07 | 0.84 | 0.75 | 0.69 | 0.40 | 0.40 | 0.33 | 1.74 | 0.57 | 1.11 | 0.82 | 1.50 | 1.35 | 1.34 | 0.25 | 0.30 | 0.26 | 0.10 | 0.17 | 0.21 |
| standard deviation | 0.05 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.03 | 0.02 | 0.01 | 0.04 | 0.02 | 0.01 | 0.07 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |
| Totenko | mean value | 1.93 | 1.00 | 0.71 | 0.38 | 0.16 | 0.60 | 0.60 | 0.26 | 1.06 | 0.86 | 0.73 | 0.65 | 0.42 | 0.39 | 0.35 | 1.77 | 0.58 | 1.18 | 0.84 | 1.57 | 1.43 | 1.41 | 0.24 | 0.31 | 0.28 | 0.10 | 0.16 | 0.20 |
| standard deviation | 0.04 | 0.06 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.05 | 0.02 | 0.04 | 0.02 | 0.06 | 0.03 | 0.04 | 0.03 | 0.06 | 0.01 | 0.02 | 0.01 | 0.01 | 0.03 | 0.01 | |
| Chabo | mean value | 1.91 | 0.96 | 0.67 | 0.35 | 0.17 | 0.63 | 0.62 | 0.26 | 1.07 | 0.85 | 0.75 | 0.69 | 0.42 | 0.42 | 0.32 | 1.71 | 0.56 | 1.10 | 0.82 | 1.49 | 1.34 | 1.33 | 0.24 | 0.31 | 0.27 | 0.10 | 0.18 | 0.20 |
| standard deviation | 0.06 | 0.05 | 0.04 | 0.02 | 0.01 | 0.04 | 0.01 | 0.02 | 0.03 | 0.02 | 0.01 | 0.02 | 0.06 | 0.03 | 0.03 | 0.05 | 0.02 | 0.04 | 0.04 | 0.05 | 0.05 | 0.05 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |
Each alphabet indicates comparing pair with significant difference as follows; a Onagadori - Oh-Shamo, b Onagadori - Shokoku, c Onagadori - Tosajidori, d Onagadori - Totenko, e Onagadori - Chabo, f Oh-Shamo - Shokoku, g Oh-Shamo - Tosajidori, h Oh-Shamo - Totenko, i Oh-Shamo - Chabo, j Shokoku - Tosajidori, k Shokoku - Totenko, l Shokoku - Chabo, m Tosajidori - Totenko, n Tosajidori - Chabo and o Totenko - Chabo. - signified that every pair shows no significant difference.
Oh-Shamo
The highest measurement values were observed in all measurements (Table 3). The GHfm did not show significant size differences among Onagadori, Shokoku and Totenko (Table 3). In terms of the shape, Oh-Shamo possessed wider SBo, GBb and GLabpa, lowest height of GLmp and GHfm, thickest Hbt and smaller GLoc. In the Hbt, GLmp, GLabpa and GHfm, we also confirmed the significant differences (Table 4).
Shokoku
The size of Shokoku comparatively ranged between that of Oh-Shamo and Tosajidori (Table 3). The middle values of all measurements, except for GLoc and GBb, were similar to those of Onagadori and Totenko. No definite difference was observed among each breed, except for Oh-Shamo, however, GLoc was relatively smaller (Table 3). According to the ANOVA results, with regard to shape, GLoc was significantly smaller than that of the other breeds, except for Oh-Syamo and Totenko (Table 4). Thus, Shokoku possessed longer GLi, wider GBnc and GBsb, and narrower GBb (Table 4).
Onagadori
All the measurements with regard to size did not show any significant differences in Shokoku and Totenko. All the measurement values were similar to those of Shokoku and Totenko, however, the Lpapr was larger than that of Shokoku and Totenko (Table 3). In terms of measurement ratios, Lpapr and GBfm indicated higher ratios than those of the other breeds (Table 4).
Totenko
Significant size differences were detected in Oh-Shamo, Chabo and Tosajidori in size. Their measurement values were smaller than those of Oh-Shamo and larger than those of Chabo (Table 3). All measurement values were similar to those of Shokoku and Onagadori (Table 3). With regard to shape, the mean ratio of GBnc was lower than that of the other breeds, however, it was not significantly difference (Table 4).
Tosajidori
The size of Tosajidori was similar to that of Chabo. They had smaller values than those of the other breeds in each measurement (Table 3). The measurements with the significant differences between each breed without Chabo were Bpr, GBnc, GBs, GBsb, Leaba, Lpaa, Lpaps and Ls (Table 3). Although similar results were also obtained with regard to shape, the mean ratios of BLb, GBb, GBbt, GBco, GBs, GLabpa, GLi, GLoc, Hbt and Lpapr were lower than those of Chabo (Table 4).
Chabo
All measurement values were smaller than those of each breed, except for Tosajidori (Table 3). Comparison of the mean values of each shape measurement showed longer GLoc and wider GBs, GBb and GBco than those of Tosajidori (Table 4). In terms of the significant coefficient, they indicated lower ratios of Leaba and Lpaa than those of all breeds, except for Tosajidori (Table 4).
Discussion
The Functional Morphology of the Skull of Oh-Shamo
This study clarified that Oh-Shamo is equipped with a wider bill, thicker bill tip, smaller orbits and wider mandibular joint and frontal bone than the other breeds (Tables 3 and 4). Bock (1966) indicated that an impact force is received by the quadrate in birds. He also suggested that a long and wide bill is adapted to endure the shock of drill in woodpeckers (Bock, 1999). Oh-Shamo frequently uses its bill for attacking in games. When it attacks with its bill, the bill needs to endure the impact. The wide bill shown in GBb and Hbt and wide mandibular joint seen in GLabpa and Lpapr were interpreted as the functional characteristics for enduring the shock when pecking during a fight. Bock (1966) remarked that the impact force acts especially on the bill tip. The decurved bill tip was interpreted as a suitable characteristic for enduring the impact force (Bock, 1966). We did not measure the bill tip curvature, however, a higher ratio of Hbt was observed in Oh-Shamo (Table 4). The thicker bill tip is also a functional characteristic for enduring the shock.
Dundes (1994) mentioned that when fighting cocks injured their eyes in combat, their eyelid were sewed up and continued the battle. The eye is considered as the weak point in fight. We suggest that the smaller orbits decrease the exposed area of eye and the wider frontal bone contribute to protection from pecking by covering the eye.
The Motivation of the Selection in Ornament-type Fowls
All measurements of Chabo and Tosajidori were smaller than those of the other breeds (Table 3). With regard to shape, they possess a wider and higher skull, shorter bill and larger orbits than the other breeds (Table 4). With regard to mean measurement ratios, Chabo was equipped with a wider skull and larger orbits than Tosajidori (Table 4). In the dorsal and caudal views, a wide skull shows a circle-like silhouette. The higher skull and shorter bill indicate the circular-like outline in lateral view. Chabo and Tosajidori possessed similar GM, however, Chabo was equipped with a wider skull than Tosajidori in terms of measurement ratios. In summary, Chabo have a small-sized skull and the circular-like silhouette of the skull showed larger orbits.
Ahead shape such as a circular face attracts humans (Alley, 1981). Hildebrandt and Fitzgerald (1979) reported that people are attracted to big eyes in humans. Japanese find small objects to be cute (Nittono, 2009). We propose that people also preferred Chabo, which has a circular small skull and big orbits, as an ornament-type breed. In Chabo, poultry breeders put high value on external characteristics of the head such as an attractive face.
Characteristics such as big eyes and a round face are one of the “baby schemas” (Lorenz, 1943). “Baby schemas” induce motivation and behaviour for approach and caregiving (Glocker et al., 2009). Dogs and cats, which possess infant characters, are preferred (Archer and Monton, 2011; Little, 2012). The observed characteristics such as larger orbits and a circular-like silhouette in the present study are estimated to be the functional morphological characteristics for acquiring caregiving in ornament-type fowls.
The Morphological Characters of the Bill and Orbit among the Oh-Shamo, Onagadori, Shokoku and Totenko
Kuwayama et al. (1996) studied the duration and the pitch of crowing and reported that Totenko, Shokoku, Onagadori and Oh-Shamo produce a low voice. In this study, we noticed that Totenko, Shokoku, Onagadori and Oh-Shamo possess a larger mandible than Chabo and Tosajidori (Tables 3 and 4). The large mandible led to an increase in the oral cavity space. As the resonance volume increases, the frequency of sound becomes lower (Stevens, 1998). Palacios and Tubaro (2000) indicated that the longer beak contribute to the lower acoustic frequencies of the song in wood*creepers. We suppose that the presence of a large mandible increases the resonance volume. The wide resonance volume contributes to a low voice in these breeds.
Totenko and Onagadori possess larger orbits than Shokoku and Oh-Shamo (Table 4). Akisinonomiya and Komiya (2009) described that Shokoku is characterized by long crowing abilities, a strong aggressive instinct and a long tail feather and has been bred as a fighting cock. Totenko and Onagadori have been bred as ornament-type fowls and not as fighting cocks. Since Shokoku has been bred as a fighting cock, it has retained small orbits such as that observed in Oh-Shamo. Onagadori and Totenko have larger orbits since they have been bred as ornament-type fowls.
The Relationships of Size between Orbit and Foramen Magnum
We observed a smaller orbit and foramen magnum in Oh-Shamo (Table 4). The size of the foramen magnum and brain shows a positive correlation in birds (Mlikovský, 2003). In addition, the brain size is also positively correlated with the eye size in birds (Burton, 2008). Since Oh-Shamo possesses smaller orbits and foramen magnum, we estimate that its brain size is presumably small. GLmp and SBnc are interpreted to be indices of the brain size. The ratio of GLmp and SBnc was undoubtedly smaller than that of the other breeds (Table 4). Observed characteristics such as larger orbits and larger SBnc and GLmp in Chabo (Table 4) suggest that its brain may be larger, however, its foramen magnum was not larger than that of the other breeds except for Oh-Shamo (Table 4). The foramen magnum is surrounded by basioccipital, exoccipital and supraoccipital bones (Jollie, 1957; Yasuda, 2002). Hogg (1978) reported that the articulation of basioccipital, exoccipital and supraoccipital bones is earlier than that of the facial bone in domestic fowl. We suggest that the early articulation of occipital bones restricts the size of the foramen magnum.
Although the ratio of GBnc and SBnc of Onagadori was not larger than that of the other breeds, its GBfm was the largest (Table 4). Adorsal notch or an extension in the foramen magnum is recognised as occipital dysplasia (Bagley et al., 1996). The dorsal notch of the foramen magnum is caused by the incomplete ossification of the ventromedial part of the supraoccipital bone (Watson et al., 1989). We suggest that the large foramen magnum in Onagadori is influenced by the imcomplete ossification of the supraoccipital bone.
Acknowledgments
The authors thank Japanese Society for the study of H. I. H. Akishinonomiya Collection for encouraging us. We are grateful to Dr. Masaoki Tsudzuki and Dr. Takao Oka (Animal Breeding and Genetics Laboratory, Hiroshima University) for their providing specimens in this study. And we thank Dr. Michiko Niimi (The Nagoya University Museum, Nagoya University) for supporting to use specimens.
References
- Akishinonomiya F, Komiya T. Livestock and Poultry in Japan. pp. 140-211. Gakken, Tokyo. 2009. (in Japanese) [Google Scholar]
- Alley TR. Head shape and the perception of cuteness. Developmental Psychology, 17: 650-654. 1981. [Google Scholar]
- Archer J, Monton S. Preferences for infant facial features in pet dogs and cats. Ethology, 117: 217-226. 2011. [Google Scholar]
- Bagley RS, Harrington ML, Tucker RL, Sande RD, Root CR, Kramer RW. Occipital dysplasia and associated cranial spinal cord abnormalities in two dogs. Veterinary Radiology and Ultrasound, 37: 359-362. 1996. [Google Scholar]
- Bock WJ. An approach to the functional analysis of bill shape. The Auk, 83: 10-51. 1966. [Google Scholar]
- Bock WJ. Functional and evolutionary morphology of woodpeckers. Ostrich, 70: 23-31. 1999. [Google Scholar]
- Burton RF. The scaling of eye size in adult birds: relationship to brain, head and body sizes. Vision Research, 48: 2345-2351. 2008. [DOI] [PubMed] [Google Scholar]
- Darroch JN, Mosimann JE. Canonical and principal components of shape. Biometrika, 72: 241-252. 1985. [Google Scholar]
- Driesch Avon den. A Guide to The Measurement of Animal Bones from Archaeological Sites. Peabody Museum of Harvard University, Cambridge: 1976. [Google Scholar]
- Dundes A. The Cockfight. University of Wisconsin Press, Madison: 1994. [Google Scholar]
- Frahm HD, Rehkämper G. Allometric comparison of the brain and brain structures in the white crested polish chicken with uncrested domestic chicken breeds. Brain, Behavior and Evolution, 52: 292-307. 1998. [DOI] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Gur RC, Sacher N. Baby schema in infant faces induces cuteness perception and motivation for caretaking in adults. Ethology, 115: 257-263. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Nishida T, Fujioka T, Tsugiyama I, Mochizuki K, Tomimoto M. Measurement of the skull of jungle and domestic fowls. Japanese Journal of Veterinary Science, 44: 1003-1006. 1982. [DOI] [PubMed] [Google Scholar]
- Hildebrandt KA, Fitzgerald HE. Facial feature determinants of perceived infant attractiveness. Infant Behavior and Development, 2: 329-339. 1979. [Google Scholar]
- Hogg DA. The articulations of the neurocranium in the postnatal skeleton of the domestic fowl (Gallus gallus domesticus). Journal of Anatomy, 127: 53-63. 1978. [PMC free article] [PubMed] [Google Scholar]
- Ichinoe K, Kuwayama T. Kakin. Soubun, Tokyo: 2007. (in Japanese) [Google Scholar]
- Jollie MT. The head skeleton of the chicken and remarks on the anatomy of this region in other birds. Journal of Morphology, 100: 389-436. 1957. [Google Scholar]
- Kuwayama T, Ogawa H, Munechika I, Kono T, Ichinoe K. Crowing characteristics of jungle fowl, Japanese native breeds and white leghorn breed of chicken. Japanese Journal of Poultry Science, 33: 89-95. 1996. [Google Scholar]
- Little AC. Manipulation of infant-like traits affects perceived cuteness of infant, adult and cat faces. Ethology, 118: 775-782. 2012. [Google Scholar]
- Lorenz K. Die angeborenen Formen möglicher Erfahrung. Zeitschrift für Tierpsychologie, 5: 235-409. 1943. [Google Scholar]
- Mlikovský J. Brain size and foramen magnum area in crows and allies (Aves: Corvidae). Acta Societatis Zoologicae Bohemicae, 67: 203-211. 2003. [Google Scholar]
- Nishida T, Hayashi Y, Fujioka T, Tsugiyama I, Mochizuki K. Osteometrical studies on the phylogenetic relationships of Japanese native fowls. Japanese Journal of Veterinary Science, 47: 25-37. 1985. [DOI] [PubMed] [Google Scholar]
- Nittono H. Abehavioral science approach to “kawaii”. Bulletin of the Graduate School of Integrated Arts and Science, Hiroshima University. I, Studies in Human Science, 4: 19-35. 2009. (in Japanese) [Google Scholar]
- Oana H. History of Japanese Domestic Fowl. Nihon-kei Kenkyusha Press, Tokyo: 1951. (in Japanese) [Google Scholar]
- Okamoto S. Zoology of Domestic Fowl. University of Tokyo Press, Tokyo: 2001. (in Japanese) [Google Scholar]
- Palacious MG, Tubaro PL. Does beak size affect acoustic frequencies in wood-creepers? The Condor, 102: 553-560. 2000. [Google Scholar]
- Samejima M, Ito S, Fujioka T. Principal component analysis of measurements in the skeleton of red jungle fowl and 12 breeds of domestic fowls: I. Cranium. Japanese Journal of Poultry Science, 25: 222-236. 1988. (in Japanese) [Google Scholar]
- Sheppy A. The colour of domestication and the designer chicken. Optics and Laser Technology, 43: 295-301. 2011. [Google Scholar]
- Stevens KN. Acoustic Phonetics. MIT Press, London: 1998. [Google Scholar]
- Watson AG, Lahunta A de, Evans HE. Dorsal notch of foramen magnum due to incomplete ossification of supraoccipital bone in dogs. Journal of Small Animal Practice, 30: 666-673. 1989. [Google Scholar]
- Yasuda M. The Anatomical Atlas of Gallus. University of Tokyo Press, Tokyo: 2002. (in Japanese) [Google Scholar]


