Abstract
Amadori products are non-enzymatically formed by binding carbonyl groups and amino groups. Glycated amino acids generated by reacting amino acid and glucose are also in a group of Amadori products of which the transport and metabolism have been investigated mainly in mammals but not in avians. In the present study, therefore, we examined whether dietary fructosyl-valine, which is one of the glycated amino acids, orally administrated to chickens can be incorporated into blood or not. Fructosyl-valine was orally administrated to the chicken and blood samples were collected at 0, 20, 40, 60, 120 and 180 min after administration. Plasma concentration of fructosyl-valine was measured by using LC/MS. The plasma concentration of fructosyl-valine was increased by passing time from 0 to 180 min after administration, and no change was observed in the control group. Conclusively, it was clarified that fructosyl-valine orally administrated to the chicken could be absorbed from gastrointestinal tract and incorporated into blood.
Keywords: Amadori product, fructosyl-valine, gastrointestinal tract, glycation, oral administration, plasma concentration
Introduction
The amino-carbonyl reaction is a non-enzymatic reaction between carbonyl and amino groups, which results in forming Schiff base, and then forms Amadori product through Amadori rearrangement (Hodge, 1955). This reaction can be easily taken place not only in the body but also in foods (Meitinger et al., 2014). Amadori products generated from glucose and amino acids might contribute to animal's health because it has been reported that this compounds have several biological effects such as antioxidant (Ide et al., 1999), antihyperglycemia (Ha et al., 2011), and so on. Compared to mammalians, avian species have the unique characteristics such as high blood glucose concentration and high body temperature (Hazelwood and Lorenz, 1959), which would easily lead to generate Amadori products. In our previous study, advanced glycation end products (AGEs), which are generated from Amadori products, were more accumulated in spleen, liver and kidney of chickens than other tissues (Kita, 2014). These results suggest that Amadori products like AGEs could be incorporated into several tissues. It has been reported that glycated amino acids could be absorbed from gastrointestinal tract of rats (Sgarbieri et al., 1973; Hultsch et al., 2006). Although the radioactivity of Amadori products and their metabolites were measured after administrating radioactive Amadori products into rats, the actual amounts of Amadori products in tissues and plasma have not been directly measured yet. Therefore, the aim of the present study is to measure plasma concentration of fructosyl-valine in young chickens orally administrated with fructosyl-valine.
Materials and Methods
Preparation of Fructosyl-valine
Fructosyl-valine (N-α-(1-deoxyfructosyl) valine) was prepared by modifying the previous method (Wang et al., 2008). Briefly, anhydrous glucose (20 mmol), malonic acid (5 mmol) and L-valine (20 mmol) were refluxed in anhydrous methanol (30 mL) under nitrogen gas for 6 h. After filtration to remove the unreacted glucose and L-valine, the filtrate was concentrated, and the almost same volume of anhydrous acetone was added, which resulted in precipitating fructosylvaline. Precipitated fructosyl-valine was dissolved in distilled water and then precipitated with anhydrous acetone in order to eliminate unreacted valine remained with fructosylvaline. Then, the product was vacuum-dried and stored under nitrogen atmosphere until used. The identification and determination of its purity were performed using LC/MS.
Animals and Experimental Procedures
Newly hatched single-comb White Leghorn male chicks were obtained from a local hatchery (Koiwai Farm Co., Ltd, Shizukuishi, Iwate, Japan). Chicks were fed a commercial chick mash diet (crude protein: 207 g/kg, metabolizable energy: 12.1 MJ/kg; Toyohashi Feed Mills Co., Ltd, Toyohashi, Aichi, Japan) from hatching until 8 d of age in an electrically heated brooder. At this age, 40 birds were selected and used for the experiment. After fasting for 12 h, fructosyl-valine dissolved in distilled water (300 µmol/kg body weight) was orally administrated to chickens using a stomach tube. Control chickens were administrated the equivalent volume of distilled water. At 20, 40, 60, 120 and 180 min (60 and 180 min in control) after administration, blood samples were taken from heart after light anesthesia with diethylether. The samples at 0 min after administration were obtained from the chickens not administrated anything. Blood samples were centrifuged for 20 min at 5,000 × g, 4°C to separate plasma. Plasma samples were stored at −20°C until analyzed. Animal care was in compliance with applicable guidelines from the Iwate University Animal Care and Use Committee.
Deproteinization and Delipidation of Plasma Samples
The method to remove protein and lipid was described previously (Kita et al., 2013). Before deproteinization and delipidation, 62.5 µL of 1/3 mM N-methyl-valine was added into 500 µL of plasma as internal standard. Then, the plasma samples were mixed with 2 mL of acetonitrile for deproteinization. After deproteinization, lipid was removed by mixing with chloroform and methanol (2:1, v/v). For purifying fructosyl-valine, cation-exchange resin was used.
Measurement of Plasma Fructosyl-valine using LC/MS
Separation and detection of fructosyl-valine and N-methylvaline was performed using LC/MS with an electrospray positive ionization (ESI) (LCMS-8040, Shimadzu Corporation, Kyoto, Japan). The mobile phase was ultrapure water. The flow rate was 400 µL/min. The sample injection volume was 3 µL. Reverse phase HPLC column (100 × 2 mm I.D., Gemini 3 µm C18 110 Å, Phenomenex, Torrance, CA, USA) was used. Fructosyl-valine and N-methyl-valine were measured in positive ion mode using Q3 selected ion monitoring (Q3 SIM). The values for m/z of positive ion of fructosylvaline and N-methyl-valine were 280.29 and 132.17, respectively. The concentration of fructosyl-valine was corrected with the recovery rate of N-methyl-valine.
The ratio of detected fructosyl-valine in chicken plasma to the amount of fructosyl-valine orally administrated to young chickens was calculated. Calculation was performed using the ratio of 3.5–6.5 mL/100 g body weight (Whittow, 2000) as the volume of chicken plasma.
Statistical Analysis
All data are presented as mean±SE. Statistical analysis of data was performed by one-way ANOVA and Tukey's HSD test for multiple comparisons (P<0.05) using the General Linear Model Procedures of SAS (SAS version 9.3, SAS Institute, 2011).
Results
The time course change in plasma fructosyl-valine concentration in chickens orally administrated with fructosyl-valine is shown in Fig. 1. Plasma fructosyl-valine concentration in chickens administrated distilled water alone did not significantly change between before and after administration. On the other hand, when chickens were orally administrated with fructosyl-valine, plasma fructosyl-valine concentration gradually increased after administration, and significant change was observed at 180 min after administration compared to 0 min. These results suggested that fructosyl-valine could be absorbed from gastrointestinal tract and incorporated into blood. The ratio of detected fructosyl-valine in chicken plasma to the amount of fructosyl-valine orally administrated to young chickens is shown in Table 1. The ratio detected fructosyl-valine in chicken plasma was approximate 0.1% at 180 min after oral administration.
Fig. 1.
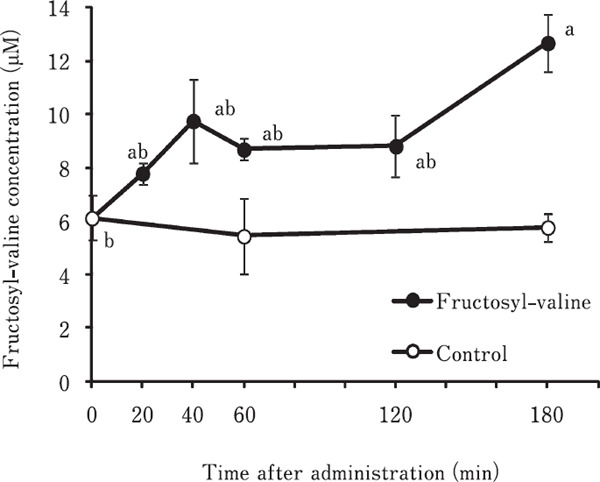
Time course change in plasma fructosyl-valine concentration in young chickens orally administrated with fructosyl-valine. Fructosyl-valine dissolved in distilled water (300 µmol/kg) was orally administrated to the crop of young chickens. Control chickens were administrated distilled water alone. At 20, 40, 60, 120 and 180 min (60 and 180 min in control) after administration, blood samples were taken from heart and plasma fructosyl-valine concentration was measured by using LC/MS. a, b Means with different superscript letters are significantly different (P<0.05). Values were means±SE. n=5.
Table 1. The ratio (%)of fructosyl-valine detected in chicken plasma to the amount of fructosyl-valine orally administrated to young chickens.
| Time after administration (min) |
|||||
|---|---|---|---|---|---|
| 20 | 40 | 60 | 120 | 180 | |
| Ratio (%) | 0.02–0.037 | 0.043–0.079 | 0.03–0.056 | 0.032–0.059 | 0.077–0.143 |
Calculation was performed using the ratio of 3.5–6.5 mL / 100 g body weight (Whittow, 2000) as volume of plasma volume.
Discussion
Previously, two types of glycated tryptophan compounds in the plasma of chickens were successfully detected and quantified by using LC/MS (Makino et al., 2015). In the present study, as shown in Fig. 1, fructosyl-valine, one of glycated amino acids, was also successfully detected and measured in the chicken plasma.
In young chicken, we revealed that the concentrations of glucose and amino acids reached a peak at 20 min after oral administration with the mixture of glucose and amino acids (Ito and Kita, 2013). But, in the case of fructosyl-valine, the concentration in plasma fructosyl-valine was consecutively elevated until 180 min after oral administration. Similar results were observed in rats orally administrated with 14C-labeled leucine or 14C-labeled fructosyl-leucine (Sgarbieri et al., 1973). They measured time course change in the radioactivity of leucine and fructosyl-leucine in the serum and revealed that the radioactivity of 14C-fructosyl-leucine slowly increased by 8.5 h and then decreased gradually, which differed from that of 14C-leucine.
As shown in Table 1, only a few fructosyl-valine was detected in chicken plasma, which is very low compared to the amount of administration. In mammalians, some Amadori products generated from glucose and amino acids are poorly absorbed because absorption of these compounds might be performed from the large intestine by passive diffusion (Erbersdobler and Faist, 2001; Sgarbieri et al., 1973). In the present study, fructosyl-valine, one of the Amadori products, was also slowly absorbed into blood, which suggests this mechanism might be applied to birds.
It is reliable that plasma fructosyl-valine concentration is regulated by reversible reactions, generation and decomposition of fructosyl-valine. There are some reports as to the specific enzyme acting on glycated proteins, and this enzyme is called fructosamine 3-kinase (FN3K) (Delplanque et al., 2004; Veiga-Da-Chunha et al., 2006). FN3K phosphorylates the third carbon on the sugar moiety of fructosamines and leads to deglycation. Birds also have this enzyme (Delplanque et al., 2004), which suggests that plasma concentration of fructosyl-valine in chickens may be decreased and regulated by this enzyme. There are some reports indicating the excretion of Amadori products into urine. A large part of radiolabeled Amadori product intravenously administrated to rats was excreted into urine (Hultsch et al., 2006). This phenomenon also suggests that the plasma concentration of fructosyl-valine can be regulated by urinary excretion of this compound.
In this study, it was revealed that fructosyl-valine can be absorbed from gastrointestinal tract in chickens. As stated above, plasma fructosyl-valine concentration is changed slowly compared to glucose and amino acids, and the change in plasma fructosyl-valine would be regulated by generation, degradation and excretion of this compound. These points should be studied in the future.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (B) (JSPS KAKENHI No. 15H04580) from the Ministry of Education, Science and Culture of Japan.
References
- Erbersdobler HF, Faist V. Metabolic transit of Amadori products. Nahrung/Food, 45: 177-181. 2001. [DOI] [PubMed] [Google Scholar]
- Delplanque J, Delpierre G, Opperdoes FR, Van Schaftingen E. Tissue distribution and evolution of fructosamine 3-kinase and fructosamine 3-kinase-related protein. Journal of Biological Chemistry, 279: 46606-46613. 2004. [DOI] [PubMed] [Google Scholar]
- Ha KS, Jo SH, Kang BH, Apostolidis E, Lee MS, Jang HD, Kwon YI. In vitro and in vivo antihyperglycemic effect of 2 Amadori rearrangement compounds, arginyl-fructose and arginylfructosyl-glucose. Journal of Food Science, 76: 188-193. 2011. [DOI] [PubMed] [Google Scholar]
- Hazelwood RL, Lorenz FW. Effects of fasting and insulin on carbohydrate metabolism of the domestic fowl. American Journal of Physiology, 197: 47-51. 1959. [DOI] [PubMed] [Google Scholar]
- Hodge JE. The Amadori Rearrengement. Advances in Carbohydrate Chemistry, 10: 169-205. 1955. [DOI] [PubMed] [Google Scholar]
- Hultsch C, Hellwig M, Pawelke B, Bergmann R, Rode K, Pietzsch J, Krause R, Henle T. Biodistribution and catabolism of 18F-labeled N-ε-fructoselysine as a model of Amadori products. Nuclear Medicine and Biology, 33: 865-873. 2006. [DOI] [PubMed] [Google Scholar]
- Ide N, Lau BHS, Ryu K, Matsuura H, Itakura Y. Antioxidant effects of fructosyl arginine, a Maillard reaction product in aged garlic extract. Journal of Nutritional Biochemistry, 10: 372-376. 1999. [DOI] [PubMed] [Google Scholar]
- Ito KR, Kita K. Grain proteins digested by trypsin modify plasma amino acid concentration in chickens. Journal of Poultry Science, 50: 340-345. 2013. [Google Scholar]
- Kita K, Kawashima Y, Makino R, Namauo T, Ogawa S, Muraoka H, Fujimura S. Detection of two types of glycated tryptophan compounds in the plasma of chickens fed tryptophan excess diets. Journal of Poultry Science, 50: 138-142. 2013. [Google Scholar]
- Kita K. The spleen accumulates advanced glycation end products in the chicken: Tissue comparison made with rat. Poulty Science, 93: 429-433. 2014. [DOI] [PubMed] [Google Scholar]
- Makino R, Kawashima Y, Kajita Y, Namauo T, Ogawa S, Muraoka H, Fujimura S, Kita K. Glycated tryptophan in the plasma of chickens fed tryptophan-excess diets. Journal of Poultry Science, 52: 23-27. 2015. [Google Scholar]
- Meitinger M, Hartmann S, Schieberle P. Development of stable isotope dilution assays for the quantitation of Amadori compounds in foods. Journal of Agriculture and Food Chemistry. 62: 5020-5027. 2014. [DOI] [PubMed] [Google Scholar]
- Sgarbieri VC, Amaya J, Tanaka M, Chichester CO. Nutritional consequences of the Maillard reaction. Amino acid availability from fructose leucine and fructose tryptophan in the rat. Journal of Nutrition, 103: 657-663. 1973. [DOI] [PubMed] [Google Scholar]
- Veiga-Da-Cunha M, Jacquemin P, Delpierre G, Godfraind C, Théate I, Vertommen D, Clotman F, Lemaigre F, Devuyst O, Van Schaftingen E. Increased protein glycation in fructosamine 3-kinase-deficient mice. Biochemical Journal, 399: 257-264. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lu YM, Liu BZ, He HY. Electrospray positive ionization tandem mass spectrometry of Amadori compounds. Journal of Mass Spectrometry, 43: 262-264. 2008. [DOI] [PubMed] [Google Scholar]
- Whittow GC. (eds.). Sturkie's Avian Physiology (Fifth Edition). Academic Press; 2000. [Google Scholar]


