Abstract
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder that currently has no approved medical therapy to address core symptoms or underling pathophysiological processes. Several compounds are under development that address both underlying pathophysiological abnormalities and core ASD symptoms. This article reviews one of these treatments, d,l-leucovorin calcium (also known as folinic acid) for treatment of folate pathway abnormalities in children with ASD. Folate is a water-soluble B vitamin that is essential for normal neurodevelopment and abnormalities in the folate and related pathways have been identified in children with ASD. One of these abnormalities involves a partial blockage in the ability of folate to be transported into the brain utilizing the primary transport mechanism, the folate receptor alpha. Autoantibodies which interfere with the function of the folate receptor alpha called folate receptor alpha autoantibodies have been identified in 58%-76% of children with ASD and independent studies have demonstrated that blood titers of these autoantibodies correlate with folate levels in the cerebrospinal fluid. Most significantly, case-series, open-label, and single and double-blind placebo-controlled studies suggest that d,l-leucovorin, a reduced folate that can bypass the blockage at the folate receptor alpha by using the reduced folate carrier, an alternate pathway, can substantially improve particular symptoms in children with ASD, especially those positive for folate receptor alpha autoantibodies. This article reviews the current evidence for treating core and associated symptoms and underlying pathophysiological mechanisms in children with ASD with d,l-leucovorin.
Introduction
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder defined by impairments in social-communication as well as the presence of restricted interests and repetitive behaviors1 with the prevalence estimated to be about 1 in 54 children in the United States (US).2 Despite early intervention with intensive behavioral therapies combined with educational approaches, only a minority of children obtain optimal outcomes3,4 and many individuals with ASD require life-long supportive care.5
Medical Therapies Targeting Core Symptoms in Autism Spectrum Disorder
Unfortunately, evidence-based medical treatments for ASD are limited with the 2 medications approved for ASD by the US Food and Drug Administration only indicated for the associated symptom of irritability rather than core symptoms of ASD. Medications commonly used for attention-deficit hyperactivity disorder can be effective but have a high adverse effects (AEs) burden and are more complicated to use in individuals with ASD and comorbid attention-deficit hyperactivity disorder.6–8 Although selective serotonin reuptake inhibitors (SSRIs) showed promise in early trials, a Cochrane review found no evidence for their efficacy for reducing repetitive thoughts and behavior for individuals with ASD.9 Pharmaceutical companies have developed medications targeting excitatory-inhibitory neurotransmission imbalances identified in animal models of ASD,10 but the first of these compounds failed to demonstrate efficacy in a double-blind placebo-controlled (DBPC) trial.11 Oxytocin, propranolol, and bumetanide show some promise in addressing the important core social interaction deficit, but the studies remain preliminary with variable outcomes.12 Thus, currently there is no approved medical therapy that targets core ASD symptoms or the pathophysiological processes that underlie ASD.13
Metabolic Targets for Treating Underlying Pathophysiology in Autism Spectrum Disorder
Several compounds which address both underlying pathophysiological abnormalities and core ASD symptoms have been developed.14 Such compounds that have undergone DBPC trials include L-Carnitine,15,16 tetrahydrobiopterin (BH4)17,18 and sulforaphane,19 but findings from these studies remain preliminary. One of the most promising treatable pathophysiological targets in ASD is abnormalities in folate metabolism, which we will concentrate on in this review.
Abnormalities in Folate Metabolism is Strongly Associated With ASD
Folate is a water-soluble B vitamin (Vitamin B9) that is essential for normal neurodevelopment.20,21 Defects in folate metabolism can cause physiological abnormalities associated with ASD such as abnormalities in the purine, methylation and redox metabolic pathways (Fig. 1).22
Figure 1.
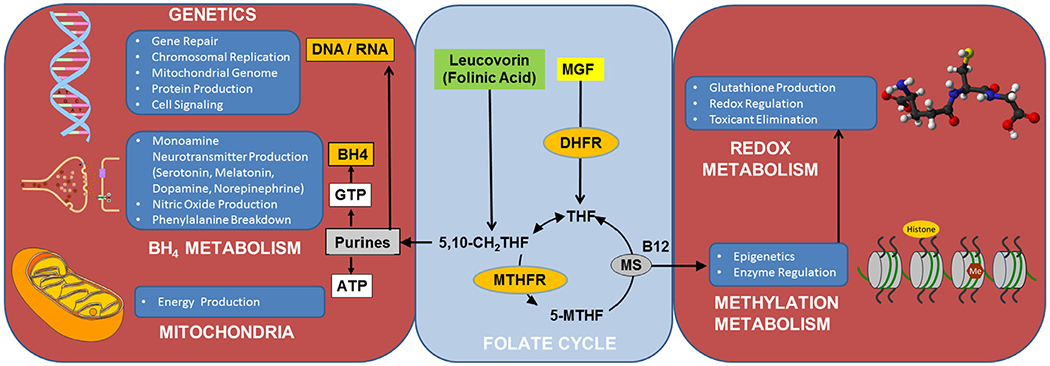
The essential role of folate in metabolism. Folate is essential for a wide variety of essential metabolic systems, including DNA and RNA synthesis and methylation, redox and tetrahydrobiopterin metabolism. ATP, adenosine triphosphate; B12, Vitamin B12 (cobalamin); BH4, tetrahydrobiopterin; DHFR, dihydrofolate reductase; DNA, deoxyribonucleic acid; GTP, guanosine triphosphate; Me, methyl group; MGF, monoglutamated folate; MS, Methionine synthase; 5-MTHF, 5-methyltetrahydrofolate; MTHFR, methylenetetrahydrofolate reductase; RNA, ribonucleic acid; 5,10-CH2THF, 5,10-methylenetetrahydrofolate; THF, tetrahydrofolate.
Purines are essential for deoxyribonucleic acid (DNA) synthesis, repair and replication. Limitations in purine production can result in de novo mutations, chromosomal instability, copy number variation and gross chromosomal abnormalities, all of which are associated with ASD.23–25 The purine guanosine 5’-triphosphate is the precursor of BH4 that is essential for monoamine neurotransmitter and nitric oxide production, both which have been shown to be abnormal in ASD.17,26,27 ASD is associated with polymorphisms in dihydrofolate reductase (DHFR in Fig. 1), an enzyme important in converting folate into its biologically active form,28 the reduced folate carrier (RFC), an important folate transporter,29 and methylenetetrahydrofolate reductase (MTHFR in Fig. 1), an enzyme critical for efficient functioning of the folate cycle,29–38 particularly in children with ASD and behavioral problems.36
ASD is associated with abnormalities in methylation metabolism (See Fig. 1), including abnormal concentrations of key methylation metabolites such as methionine, S-adenosyl-L-methionine (SAM) and S-adenosyl-L-homocysteine (SAH). Deficits in SAM may be particularly important, as SAM is the major methyl donor essential for DNA and histone methylation, the epigenetic process that regulates gene expression. Postmortem brain studies show alterations in DNA methylation in the frontal cortex39 and in other brain areas40,41 in individuals with ASD.
Reduced glutathione (GSH) is the major intracellular redox buffer and is essential in free radical scavenging, redox homeostasis, maintenance of protein redox conformation and regulation of redox sensitive enzyme activity. Abnormalities in GSH metabolism can result in oxidative damage to cellular DNA, protein and lipid. GSH abnormalities and markers of oxidative damage have been documented in postmortem brain regions involved in speech, emotion, and social behavior in individuals with ASD.42,43 In fact, methylation and redox abnormalities are so prevalent in ASD, biomarkers of these are proposed to be diagnostic of ASD.44,45
Cerebral Folate Deficiency and ASD
Folate transport into the brain may be compromised in ASD. The primary transporter for folate across the blood-brain barrier is the folate receptor α (FRα). Through energy dependent endocytosis, folate is transported attached to the FRα from the apical to the basolateral side of the cell against a concentration gradient (Fig. 2). Active transport is necessary because central nervous system folate concentration is several times higher than in the serum. About 15 years ago, Dr Quadros and colleagues reported in the New England Journal of Medicine a new neurometabolic disorder called cerebral folate deficiency (CFD), which is characterized by abnormally low folate levels in the cerebrospinal fluid (CSF) despite normal serum folate levels.46,47 CFD is associated with 2 types of FRα autoantibodies (FRAAs), blocking and binding, which impair FRα function.47 Serum titers of FRAAs have been correlated with CSF folate concentrations in 2 independent studies.47,48 Mitochondrial disorders, including Kearns-Sayre syndrome49,50 and mutations in the DNA polymerase subunit gamma (POLG) gene,49,51,52 are also associated with CFD due to a lack of energy for active transportation of folate.
Figure 2.
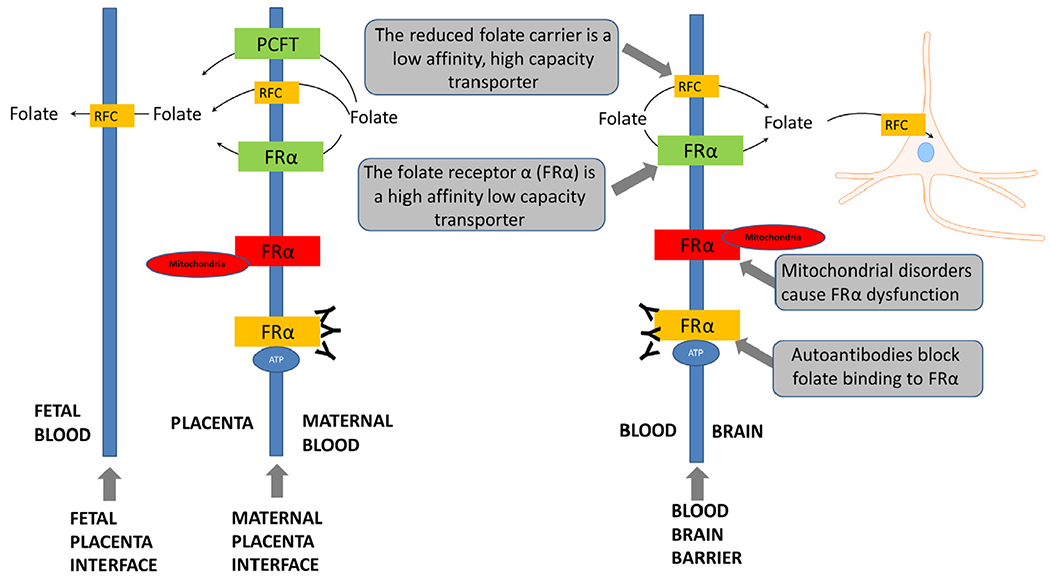
Folate transport across the blood brain barrier and the placenta. ATP, adenosine triphosphate; FRα, folate receptor α; PCFT, Proton Coupled Folate Transporter; RFC, reduced folate carrier.
In early case-series of children with CFD, many were described are having ASD symptoms.47,53 Thus, approximately 10 years ago, Drs Frye, Rossignol and Quadros studied children with ASD to determine whether a significant proportion harbored FRAAs. In our study published in Molecular Psychiatry,48 FRAAs were measured in 93 children with ASD using the assay development by Dr Quadros.47,54 As shown in Figure 3, 60% of the children with ASD had blocking and 44% had binding FRAAs. Overall, 29% were positive for both FRAAs, 46% were positive for only one FRAA and 75% were positive for at least one FRAA. In a study from Belgium, 47% of ASD children were positive for the blocking FRAA as compared to 3.3% of developmentally delayed non-ASD controls.55 In another study from Belgium, 2 groups of children with ASD demonstrated a 71.4% (n = 84) and 75.6% (n = 82) prevalence of at least one FRAA as compared to 3% of healthy children.56 In a recent study from France, 58% of children with ASD were positive for at least one FRAA.57 In a recent study from the New York City Metropolitan area, the prevalence of one or more FRAAs was 76% in children with ASD.58 The prevalence of blocking FRAAs in ASD is clearly higher than the prevalence in the general population of the US (10%-15%),48 Spain (7.2%)37,59 and Ireland (12.6%).54
Figure 3.
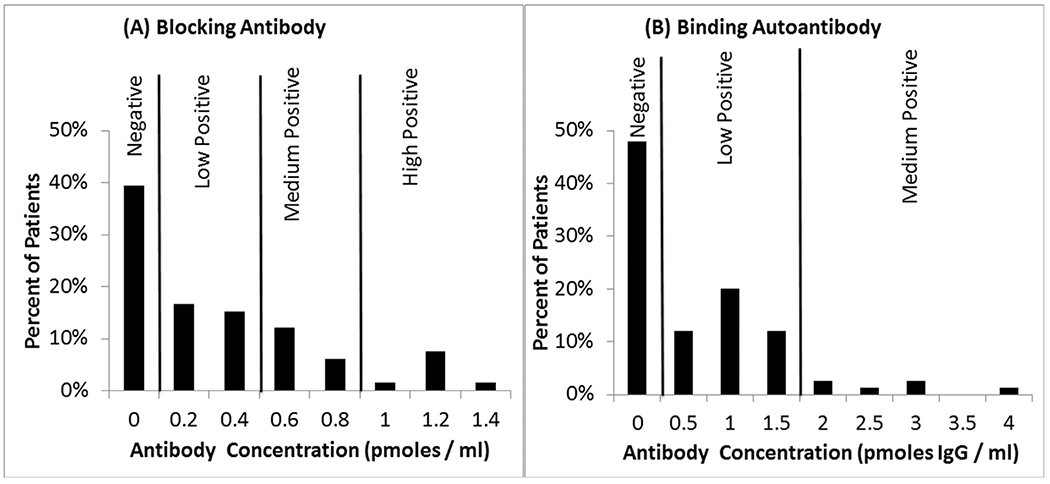
Prevalence of the folate receptor α autoantibodies in children with autism spectrum disorder in our initial study.48
d,l-Leucovorin Improves Symptoms in Children With Cerebral Folate Deficiency in Case Studies
Children with CFD demonstrate marked improvement when treated with d,l-leucovorin, a reduced folate that can cross the blood-brain barrier using the RFC when the FRα is blocked by FRAAs (See Fig. 2). Since the RFC has a lower affinity for folates than the FRα, a high-dose of a reduced folate is required for treatment. Case-reports60 and series47,61 have documented that d,l-leucovorin (0.5-2 mg/kg/day) improves neurological, behavioral and cognitive symptoms in children with CFD, including substantial improvements in language and communication in many and purported complete recovery in some.47,61
d,l-Leucovorin Improves Verbal Communication in Children With Autism Spectrum Disorder and Folate Receptor Alpha Autoantibodies
Given the high prevalence of FRAAs in children with ASD and the effectiveness and safety of d,l-leucovorin, Drs Frye and Rossignol conducted a large prospective open-label case-series in which 44 children with ASD with a positive test for at least one FRAA were treated with 2 mg/kg/day (maximum 50 mg daily) of leucovorin in 2 divided doses.48 Intervention response and AEs were assessed after a mean follow-up of 4.0 months. Parents rated intervention response using a modified Clinical Global Impression Improvement type scale called the Parent Rated Autism Symptomatic Change (PRASC) scale. We examined 9 core and associated ASD symptoms using the PRASC scale. About two-thirds of the treated children manifested some improvement in receptive and expressive language (Fig. 4). Nine FRAA positive children with ASD who were waiting for laboratory results and did not make any treatment changes served as a wait-list control group. Using Mann-Whitney U tests, significantly (P< 0.05) greater improvement was observed in treated as compared to untreated children for verbal communication, receptive and expressive language and stereotypical behavior.
Figure 4.
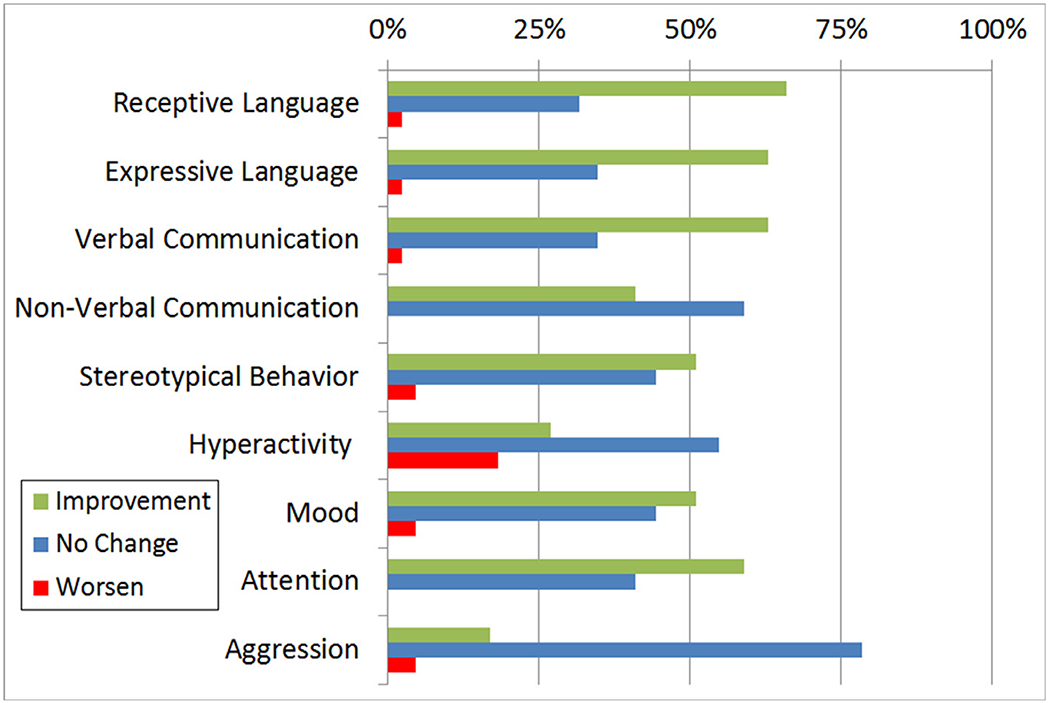
Improvement ratings on 9 cognitive-behavioral dimensions for folate receptor alpha autoantibody positive ASD children (n = 44) treated with d,l-leucovorin.
The Importance of Targeting Language and Verbal Communication in Autism Spectrum Disorder
Our open-label clinical study demonstrated that d,l-leucovorin had a prominent effect on verbal communication in children with ASD. Verbal communication is an important symptom to target for several reasons. First, improvement in verbal communication can promote better social communication. Second, deficits in language processing are associated with more severe ASD symptoms. Thus, improving language might decrease ASD symptom severity, contribute to more favorable long-term outcomes62–66 and promote improved quality of life for the family.67 Finally, improvement in delayed language may also have positive effects on brain development.68 Thus, we conducted a DBPC study to examine the effect of d,l-leucovorin on verbal communication in children with ASD that was recently published in Molecular Psychiatry (described below).69
d,l-Leucovorin Improves Verbal Communication in a Double-Blind Placebo-Controlled Study
We conducted a DBPC trial on 48 children with ASD.69 Our primary outcome measure was Verbal Communication as measured on the most ability appropriate instrument: either the Clinical Evaluation of Language Fundamentals or Preschool Language Scale. Secondary outcome measures included the Aberrant Behavior Checklist (ABC). Children were randomly assigned to receive daily d,l-leucovorin (n = 23) 2 mg/kg/day, max 50 mg/day, in 2 divided doses or placebo (n = 25) for 12 weeks. AEs were monitored every 3 weeks. All other treatments were constant for 2 months prior to entering the trial and were held constant throughout the trial. The intensity of behavioral and educational interventions was documented as minutes per week. FRAAs were measured at the beginning of the study. A linear mixed-model intent-to-treat analysis found a significant improvement in verbal communication in the d,l-leucovorin group as compared to the placebo group with a medium-to-large effect size (Cohen’s d = 0.70; Fig. 5). Adding a covariate to represent speech therapy intensity significantly improved the variance explained by the model (χ2(2) = 7.5, P < 0.01) but did not change the effect of d,l-leucovorin. Speech therapy was found to have a small effect size (Cohen’s d = 0.05). Most importantly, the absolute estimated effect of the d,l-leucovorin exceeded the minimal clinically important difference, suggesting that the effect was not only statistically significant but also clinically meaningful.
Figure 5.
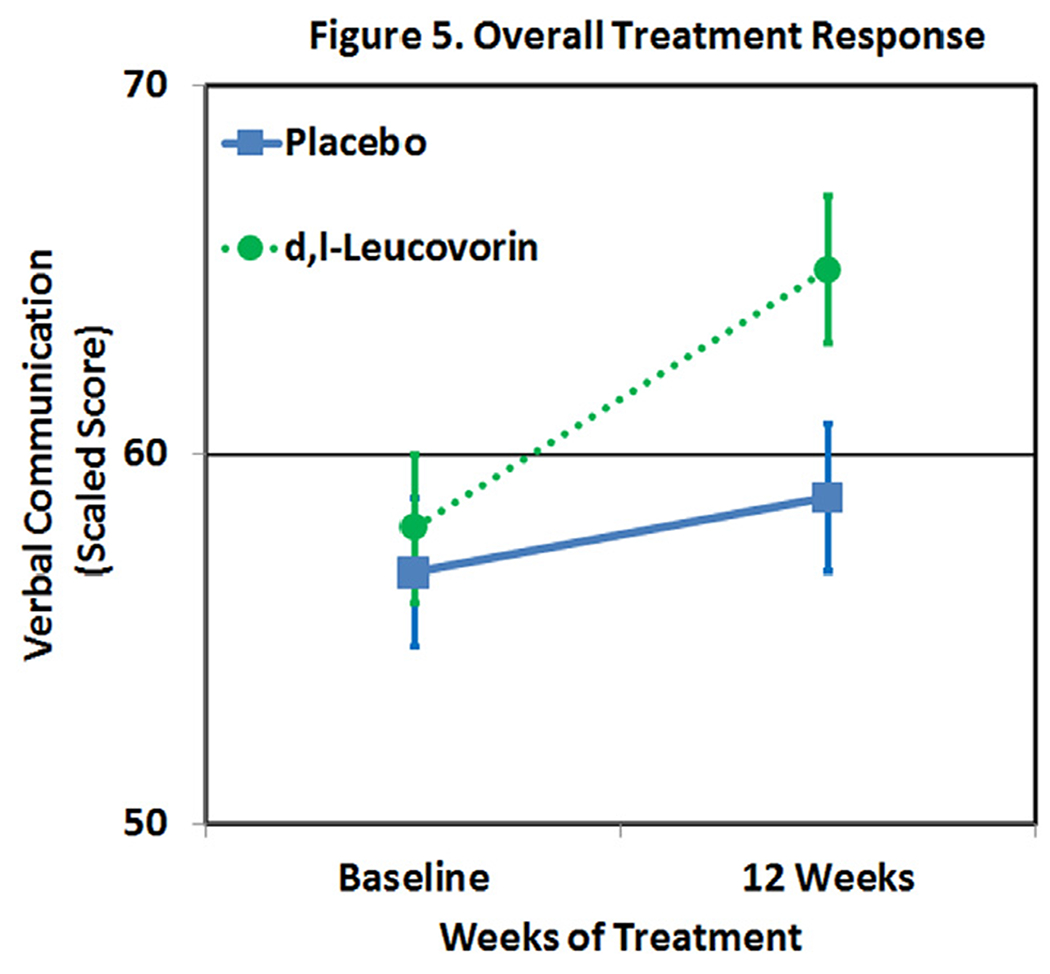
Leucovorin improves verbal communication more than placebo.
A responder analysis was performed using logistic regression with treatment response defined as an increase of 5 standardized points on the language instrument over 12 weeks. Response to treatment was significantly greater in participants treated with d,l-leucovorin as compared to placebo (65% vs 24%, P = 0.003). FRAA status (positive vs negative) was significantly associated with response to d,l-leucovorin treatment (χ2(1) = 4.92, P= 0.03) demonstrating that FRAA status was a strong candidate biomarker for predicting treatment response. The effect of d,l-leucovorin treatment in FRAA subgroups was evaluated with the mixed-model analysis. For participants that were positive for at least one FRAA, d,l-leucovorin improved verbal communication with a large effect size (Cohen’s d = 0.91). The number needed to treat (NNT) represents the number of patients that need to be treated for one patient to respond. Overall, the NNT was 2.4 but for those positive for one or more FRAAs the NNT was 1.8. These results demonstrate the importance of investigating biomarkers of folate-related metabolism.
d,l-Leucovorin Could Results in Significant Cost Savings
To demonstrate the practical significance of the d,l-leucovorin treatment, the regression coefficients were used to estimate the number of hours of speech therapy that would be equivalent to d,l-leucovorin treatment. The cost of speech therapy was estimated to be $40/hr. Three months of d,l-leucovorin was found to be equivalent to about 185 hours (about $7400) of speech therapy. Three months of d,l-leucovorin costs about $300, resulting in a savings of about $7100. The other important consideration is that many children only receive a few hours of speech therapy per week, so 185 hours could be equivalent to a year or more of speech therapy for most. In addition, this does not account for the additional educational, therapeutic and family costs for caring for a lower-functioning child with ASD for a longer period of time for those that do not make the gains seen in the treatment group.
d,l-Leucovorin Also Improves Core Symptoms of Autism Spectrum Disorder
Our open-label, case-series described above found significant improvement in the core symptom of stereotypical behavior in children with ASD who were positive for at least one FRAA with d,l-leucovorin treatment.48 In our DBPC study, the mixed-model analysis conducted on the secondary outcome measure, the parent-rated ABC aberrant behavior checklist, found improvement in Irritability, Social Withdrawal, Stereotypy, Hyperactivity, and Inappropriate Speech in the d,l-leucovorin group as compared to the placebo group.69 The improvement in social withdrawal, stereotyped behavior and inappropriate speech exceeded the minimal clinically important difference, demonstrating the clinical relevance of the effect. Both social withdrawal and stereotyped behavior are core ASD symptoms.
Two other studies, one open-label and one single-blind placebo-controlled, have investigated the effect of d,l-leucovorin on core symptoms of ASD. In an open-label therapeutic trial of patients with nonsyndromic infantile ASD, patients were self-selected to undergo a comprehensive treatment protocol (n = 82) or remain untreated (n = 84). The treatment protocol involved specific treatment for various nutritional deficiencies and treatment with d,l-leucovorin at a variable dose from 0.5 to 2 mg/kg/day (maximum 50 mg daily) if the child had at least one FRAA. The Childhood Autism Rating Scale (CARS) was completed before treatment and after the treatment protocol. Overall, untreated patients, on average, did not demonstrate a change in CARS scores whereas children who underwent treatment demonstrated a decrease in CARS scores from severe to mild-to-moderate ASD.56 Although this study is not specific for treatment with d,l-leucovorin, it does demonstrate that including d,l-leucovorin in a comprehensive treatment protocol can improve outcomes in core ASD symptoms. In a recent small (n = 19) French study, children with ASD were treated with 5 mg of d,l-leucovorin twice a day or placebo for 12 weeks under single-blind conditions. The global score and the reciprocal social interaction and communication subscores on the Autism Diagnostic Observation Schedule (ADOS) improved significantly in those that were treated as compared to those individuals on placebo.57 Thus, 4 studies support the notion that treatment with d,l-leucovorin can improve core ASD symptoms.
d,l-Leucovorin Has a Low Frequency of Adverse Effects
In clinical studies, AEs to d,l-leucovorin were minimal. In our open-label study, d,l-leucovorin was discontinued in 4 children.48 Insomnia and gastroesophageal reflux led to discontinuation of d,l-leucovorin in 1 child. Three boys concurrently taking risperidone discontinued d,l-leucovorin because of worsening aggression and self-injurious behavior. However, one other patient receiving risperidone described no AEs. Although some parents rated mood (5%), stereotypical behavior (5%) and hyperactivity (17%) as mildly worse on the PRASC in some children (Fig. 4), they did not report these as AEs. The commercial form of d,l-leucovorin which contains additives, such as lactose, was used in our open-label study. From our clinical experience, we believe that inactive drug additives can cause irritable. In our DPBC study, which used a compounded form of d,l-leucovorin without additives, there were no significant differences in the prevalence of AEs between the d,l-leucovorin and placebo groups and no child treated with d,l-leucovorin discontinued the study because of AEs. However, the placebo group experienced ~30% more AEs.69 In the other 2 recent clinical studies discussed above no AEs were reported.56,57
One AE commonly reported by parents is hyperactivity and agitation with the first few weeks of d,l-leucovorin treatment. Because of this, we common recommend slowly increasing the d,l-leucovorin dose over the first 2 weeks of treatment. To investigate this AE in more detail, we examined the time course of reported excitement and agitation as an AE in our DBPC trial.69 As seen is Figure 6, the prevalence of excitement and agitation was similar in both the d,l-leucovorin and placebo groups through the first 6 weeks after which it is substantially reduced in the treatment group as compared to the placebo group at week 9 of the 12-week treatment. This clarifies the clinical experience and suggests that excitement and agitation improves after several weeks of treatment but is never significantly higher in the treatment group as compared to the placebo group at any point in time.
Figure 6.
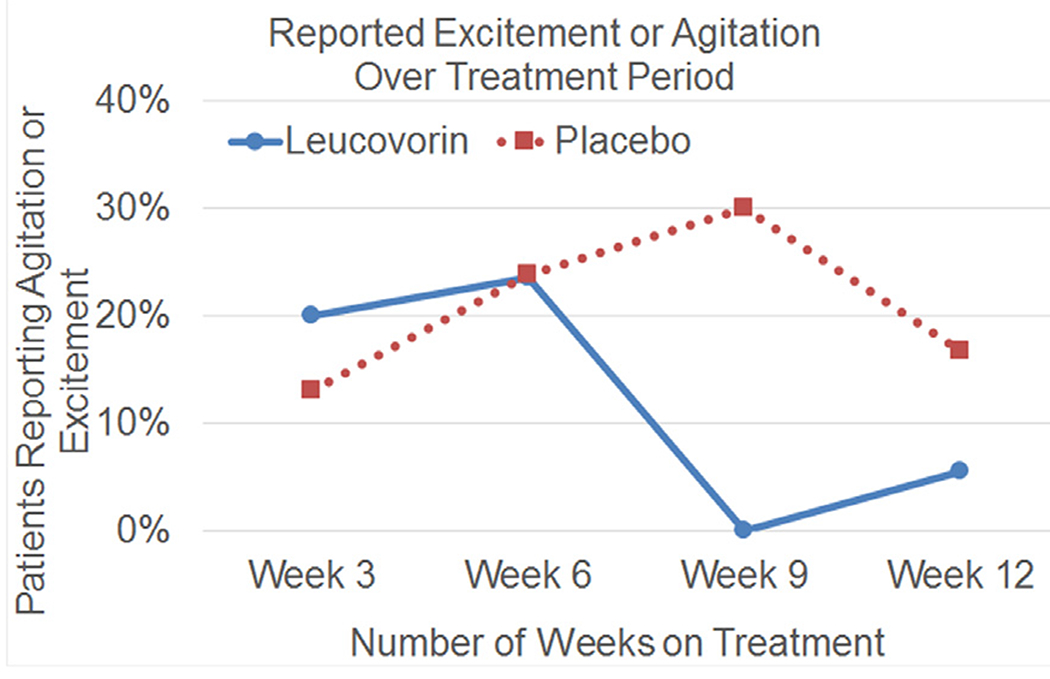
Percentage of families that reported agitation or excitement at each 3-week adverse effect check-in
Folate Improves Mitochondrial Function in Children With ASD
As previously mentioned, mitochondria have a role in folate transport into the brain. Interestingly, abnormal mitochondrial metabolism has been linked to ASD.70–73 Clinical74 and basic research studies75 suggest that folate is important in mitochondrial function. In a recent study, our group measured the function of several mitochondrial enzymes in children with ASD who were receiving various supplements that could influence mitochondrial function.76 Folate positively influenced several aspects of mitochondrial function, including increasing Complex I activity and strengthened the coupling between Complex I and Citrate Synthase. Thus, d,l-leucovorin may have a secondary effect of supporting mitochondrial function that can further enhance folate transport into the brain.
Folate During Gestation and ASD
Folate status during gestation appears to be a critical factor in the development of ASD. A large study of Norwegian children found that prenatal folic acid supplementation was associated with a 39% decrease in the risk of developing ASD in the offspring.77 Similarly, another large study in the US found that mothers of children with ASD had lower dietary intake of folic acid during pregnancy. Intake above 600 mcg/day was associated with a 38% decrease in the risk of the offspring developing ASD and higher intake further decreased the risk.34 Additionally, previous studies have uncovered metabolic deficits in folate-dependent pathways, including methylation and glutathione pathways, in mothers of children with ASD.78 Most compelling is an animal model of maternal FRAA exposure developed by Dr Quadros. Given that the same mechanisms for transporting folate across the blood-brain barrier also transports folate across the placenta (See Fig. 2), Dr Quadros and his group demonstrated that pregnant dams exposed to FRAAs bore offspring with ASD-like behavioral features79 and that treatment of dams with d,l-leucovorin during gestation prevented the development of ASD-like behavioral features in the offspring.80 This is consistent with the recent observation that children with ASD with mothers who have FRAAs have more severe ASD symptoms as rated by the CARS.56
Ongoing and Future Studies Using d,l-Leucovorin in the Treatment of Autism Spectrum Disorder
Although d,l-leucovorin is very promising as a treatment for ASD, further studies are needed to validate previous findings and expand the application of d,l-leucovorin in the treatment of children with ASD. First, although our first DBPC study verified that d,l-leucovorin improves verbal communication in children with ASD, we need to validate this finding in a multicenter study. A DBPC multicenter study involving Phoenix Children’s Hospital, Emory University and the Lurie Center at Mass General Hospital is ongoing to verify previous findings and to evaluation the potential for using the FRAA and other biomarkers such as single nucleotide polymorphisms known to negatively affect folate metabolism to predict response. Given that deficits in early language and social skills are fundamental in driving many ASD symptoms and that neuroplasticity is greatest in the first years of life, it is very possible that the effect of d,l-leucovorin will be greatest in young children newly diagnosed with ASD. Thus, Phoenix Children’s Hospital and State University of New York – Downstate are conducting 2 multicenter DPBC trials on very young children with ASD to determine if early l-leucovorin treatment could significantly launch recovery towards optimal outcomes and result in substantial positive repercussions over their lifetimes. Within these latter trials we are using a newly developed instrument, the Brief Observation of Social Communication Change (BOSCC) that is based on the ADOS. The BOSCC has been developed by the same scientists that developed the ADOS and is designed to be more sensitive to change in socialization than the ADOS.81 In addition, we are using advanced neuroimaging techniques to identify the change in brain circuits resulting from treatment with l-leucovorin. Lastly, in the studies on young children we are investigating the isomer l-leucovorin which may reduce any potential AEs. Although the long-term use of d,l-leucovorin and l-leucovorin is believed to be safe and has been used for decades in humans, long-term tolerability and therapeutic effect has not been evaluated. Furthermore, the optimal length of treatment is not known. Thus, further studies are needed to identify the optimal dosing and treatment schedule for this therapy and its long-term safety.
The Potential Importance of Leucovorin in the Treatment of Autism Spectrum Disorder
The studies discussed above suggest that d,l-leucovorin (aka folinic acid) is an efficacious and safe treatment for improving both core (social function, communication, stereotyped behavior) and associated (irritability, hyperactivity) ASD symptoms. The differential effect of treatment response depends on whether a child is positive for the FRAA, reinforcing the known biological mechanism of treatment with d,l-leucovorin as well as the ability to predict who will respond to the treatment. This opens up the potential for using a personalized precision medicine approach for treating children with ASD.82 The improvement in an important symptom associated with ASD, irritability, suggests that d,l-leucovorin may be an alternative to antipsychotic drugs, which have significant short- and long-term AEs in children. Additionally, d,l-leucovorin can normalize folate-dependent one-carbon metabolism by readily entering the folate cycle without being reduced by dihydrofolate reductase (DHFR in Fig. 1).83 Early studies on CFD and ASD demonstrated a strong positive effect of d,l-leucovorin in young children with ASD on both neurologic and cognitive development.22 Given the physiological abnormalities in the folate pathway associated with ASD and the potential for d,l-leucovorin to surmount these abnormalities, it is possible that d,l-leucovorin and/or l-leucovorin could correct key underlying physiological abnormalities driving ASD symptoms and in this sense be disease modifying. Given the promising and compelling data, we believe that d,l-leucovorin and/or l-leucovorin have tremendous promise in improving the health, cognition and development of children with ASD.
Acknowledgments
This work was funded by a National Institute of Child Health and Human Development grant R01-HD088528, Department of Defense grant AR180134 and Autism Speaks grant 11407.
Footnotes
Conflict of Interest: Authors declare that they have no conflict of interest.
References
- 1.APA: Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association, 2013 [Google Scholar]
- 2.Maenner MJ, Shaw KA, Baio J, et al. : Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ 69:1–12, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fein D, Barton M, Eigsti IM, et al. : Optimal outcome in individuals with a history of autism. J Child Psychol Psychiatry. 54:195–205, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fountain C, Winter AS, Bearman PS: Six developmental trajectories characterize children with autism. Pediatrics 129:e1112–e1120, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magiati I, Tay XW, Howlin P: Cognitive, language, social and behavioural outcomes in adults with autism spectrum disorders: a systematic review of longitudinal follow-up studies in adulthood. Clin Psychol Rev 34:73–86, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Mahajan R, Bernal MP, Panzer R, et al. : Clinical practice pathways for evaluation and medication choice for attention-deficit/hyperactivity disorder symptoms in autism spectrum disorders. Pediatrics 130(Suppl 2): S125–S138, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Research Units on Pediatric Psychopharmacology Autism, N: Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry 62:1266–1274, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Scahill L, McCracken JT, King BH, et al. : Extended-Release Guanfacine for Hyperactivity in Children With Autism Spectrum Disorder. Am J Psychiatry. 172:1197–1206, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Williams K, Brignell A, Randall M, et al. : Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev 2013:CD004677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb S: Drugmakers dance with autism. Nat Biotechnol 28:772–774, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Frye RE: Clinical potential, safety, and tolerability of arbaclofen in the treatment of autism spectrum disorder. Drug Healthc Patient Saf 6:69–76, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frye RE: Social Skills Deficits in Autism Spectrum Disorder: Potential Biological Origins and Progress in Developing Therapeutic Agents. CNS Drugs 32:713–734, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh A, Michalon A, Lindemann L, et al. : Drug discovery for autism spectrum disorder: challenges and opportunities. Nat Rev Drug Discov. 12:777–790, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Frye RE, Rossignol DA: Identification and Treatment of Pathophysiological Comorbidities of Autism Spectrum Disorder to Achieve Optimal Outcomes. Clin Med Insights Pediatr 10:43–56, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geier DA, Kern JK, Davis G, et al. : A prospective double-blind, randomized clinical trial of levocarnitine to treat autism spectrum disorders. Med Sci Monit 17:PI15–PI23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahmy SF, El-hamamsy MH, Zaki OK, et al. : l-Carnitine supplementation improves the behavioral symptoms in autistic children. Research in Autism Spectrum Disorders. 7:159–166, 2013 [Google Scholar]
- 17.Frye RE, Huffman LC, Elliott GR: Tetrahydrobiopterin as a novel therapeutic intervention for autism. Neurotherapeutics 7:241–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klaiman C, Huffman L, Masaki L, et al. : Tetrahydrobiopterin as a treatment for autism spectrum disorders: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 23:320–328, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Singh K, Connors SL, Macklin EA, et al. : Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci U S A. 111:15550–15555, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenblatt JM, Huffman LC, Reiss AL: Folic acid in neurodevelopment and child psychiatry. Prog Neuropsychopharmacol Biol Psychiatry 18:647–660, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Black MM: Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull 29:S126–S131, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frye RE, Slattery JC, Quadros EV: Folate metabolism abnormalities in autism: potential biomarkers. Biomark Med 11:687–699, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Devlin B, Scherer SW: Genetic architecture in autism spectrum disorder. Curr Opin Genet Dev 22:229–237, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Neale BM, Kou Y, Liu L, et al. : Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 485:242–245, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders SJ, Murtha MT, Gupta AR, et al. : De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 485:237–241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frye RE: Central tetrahydrobiopterin concentration in neurodevelopmental disorders. Front Neurosci 4:52, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frye RE, DeLatorre R, Taylor HB, et al. : Metabolic effects of sapropterin treatment in autism spectrum disorder: a preliminary study. Transl Psychiatry. 3:e237, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams M, Lucock M, Stuart J, et al. : Preliminary evidence for involvement of the folate gene polymorphism 19bp deletion-DHFR in occurrence of autism. Neurosci Lett. 422:24–29, 2007 [DOI] [PubMed] [Google Scholar]
- 29.James SJ, Melnyk S, Jernigan S, et al. : Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 141B:947–956, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frustaci A, Neri M, Cesario A, et al. : Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med. 52:2128–2141, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Boris M, Goldblatt A, Galanko J, et al. : Association of MTHFR gene variants with autism. Journal of American Physicians and Surgeons. 9:106–108, 2004 [Google Scholar]
- 32.Mohammad NS, Jain JM, Chintakindi KP, et al. : Aberrations in folate metabolic pathway and altered susceptibility to autism. Psychiatr Genet. 19:171–176, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Guo T, Chen H, Liu B, et al. : Methylenetetrahydrofolate reductase polymorphisms C677T and risk of autism in the Chinese Han population. Genet Test Mol Biomarkers. 16:968–973, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt RJ, Tancredi DJ, Ozonoff S, et al. : Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 96:80–89, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Solehdin F, Cohen IL, et al. : Population- and family-based studies associate the MTHFR gene with idiopathic autism in simplex families. J Autism Dev Disord. 41:938–944, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Goin-Kochel RP, Porter AE, Peters SU, et al. : The MTHFR 677C–>T polymorphism and behaviors in children with autism: exploratory genotype-phenotype correlations. Autism Res. 2:98–108, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Pasca SP, Dronca E, Kaucsar T, et al. : One carbon metabolism disturbances and the C677T MTHFR gene polymorphism in children with autism spectrum disorders. J Cell Mol Med. 13:4229–4238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pu D, Shen Y, Wu J: Association between MTHFR gene polymorphisms and the risk of autism spectrum disorders: a meta-analysis. Autism Res 6:384–392, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Nagarajan RP, Hogart AR, Gwye Y, et al. : Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 1:e1–11, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lintas C, Sacco R, Persico AM: Differential methylation at the RELN gene promoter in temporal cortex from autistic and typically developing post-puberal subjects. J Neurodev Disord 8:18, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nardone S, Sams DS, Zito A, et al. : Dysregulation of Cortical Neuron DNA Methylation Profile in Autism Spectrum Disorder. Cereb Cortex. 27:5739–5754, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose S, Melnyk S, Pavliv O, et al. : Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry. 2:e134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sajdel-Sulkowska EM, Xu M, McGinnis W, et al. : Brain region-specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD). Cerebellum. 10:43–48, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Howsmon DP, Kruger U, Melnyk S, et al. : Classification and adaptive behavior prediction of children with autism spectrum disorder based upon multivariate data analysis of markers of oxidative stress and DNA methylation. PLoS Comput Biol. 13:e1005385, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howsmon DP, Vargason T, Rubin RA, et al. : Multivariate techniques enable a biochemical classification of children with autism spectrum disorder versus typically-developing peers: A comparison and validation study. Bioeng Transl Med. 3:156–165, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramaekers VT, Hausler M, Opladen T, et al. : Psychomotor retardation, spastic paraplegia, cerebellar ataxia and dyskinesia associated with low 5-methyltetrahydrofolate in cerebrospinal fluid: a novel neurometabolic condition responding to folinic acid substitution. Neuropediatrics. 33:301–308, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Ramaekers VT, Rothenberg SP, Sequeira JM, et al. : Autoantibodies to folate receptors in the cerebral folate deficiency syndrome. N Engl J Med. 352:1985–1991, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Frye RE, Sequeira JM, Quadros EV, et al. : Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol Psychiatry. 18:369–381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batllori M, Molero-Luis M, Ormazabal A, et al. : Cerebrospinal fluid monoamines, pterins, and folate in patients with mitochondrial diseases: systematic review and hospital experience. J Inherit Metab Dis. 41:1147–1158, 2018 [DOI] [PubMed] [Google Scholar]
- 50.Perez-Duenas B, Ormazabal A, Toma C, et al. : Cerebral folate deficiency syndromes in childhood: clinical, analytical, and etiologic aspects. Arch Neurol. 68:615–621, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Hasselmann O, Blau N, Ramaekers VT, et al. : Cerebral folate deficiency and CNS inflammatory markers in Alpers disease. Mol Genet Metab. 99:58–61, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Samanta D, Ramakrishnaiah R, Frye RE: Complex heterozygous polymerase gamma mutation and cerebral folate deficiency in a child with refractory partial status. Neurol India 67:259–260, 2019 [DOI] [PubMed] [Google Scholar]
- 53.Ramaekers VT, Blau N: Cerebral folate deficiency. Dev Med Child Neurol 46:843–851, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Molloy AM, Quadros EV, Sequeira JM, et al. : Lack of association between folate-receptor autoantibodies and neural-tube defects. N Engl J Med. 361:152–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramaekers VT, Quadros EV, Sequeira JM: Role of folate receptor autoantibodies in infantile autism. Mol Psychiatry 18:270–271, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Ramaekers VT, Sequeira JM, DiDuca M, et al. : Improving Outcome in Infantile Autism with Folate Receptor Autoimmunity and Nutritional Derangements: A Self-Controlled Trial. Autism Res Treat. 2019:7486431, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Renard E, Leheup B, Guéant-Rodriguez RM, et al. : Folinic acid improves the score of Autism in the EFFET placebo-controlled randomized trial. Biochimie 173:57–61, 2020. in press [DOI] [PubMed] [Google Scholar]
- 58.Quadros EV, Sequeira JM, Brown WT, et al. : Folate receptor autoantibodies are prevalent in children diagnosed with autism spectrum disorder, their normal siblings and parents. Autism Res. 11:707–712, 2018 [DOI] [PubMed] [Google Scholar]
- 59.dos Santos PA, Longo D, Brandalize AP, et al. : MTHFR C677T is not a risk factor for autism spectrum disorders in South Brazil. Psychiatr Genet. 20:187–189, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Moretti P, Sahoo T, Hyland K, et al. : Cerebral folate deficiency with developmental delay, autism, and response to folinic acid. Neurology. 64:1088–1090, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Ramaekers VT, Blau N, Sequeira JM, et al. : Folate receptor autoimmunity and cerebral folate deficiency in low-functioning autism with neurological deficits. Neuropediatrics. 38:276–281, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Mukaddes NM, Tutkunkardas MD, Sari O, et al. : Characteristics of children who lost the diagnosis of autism: a sample from istanbul, Turkey. Autism Res Treat 2014:472120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tager-Flusberg H, Rogers S, Cooper J, et al. : Defining spoken language benchmarks and selecting measures of expressive language development for young children with autism spectrum disorders. J Speech Lang Hear Res 52:643–652, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luyster R, Qiu S, Lopez K, et al. : Predicting outcomes of children referred for autism using the MacArthur-Bates Communicative Development Inventory. J Speech Lang Hear Res 50:667–681, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Szatmari P, Bryson SE, Boyle MH, et al. : Predictors of outcome among high functioning children with autism and Asperger syndrome. J Child Psychol Psychiatry. 44:520–528, 2003 [DOI] [PubMed] [Google Scholar]
- 66.Anderson DK, Lord C, Risi S, et al. : Patterns of growth in verbal abilities among children with autism spectrum disorder. J Consult Clin Psychol. 75:594–604, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Tilford JM, Payakachat N, Kovacs E, et al. : Preference-based health-related quality-of-life outcomes in children with autism spectrum disorders: a comparison of generic instruments. Pharmacoeconomics. 30:661–679, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai MC, Lombardo MV, Ecker C, et al. : Neuroanatomy of Individual Differences in Language in Adult Males with Autism. Cereb Cortex. 25:3613–3628, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frye RE, Slattery J, Delhey L, et al. : Folinic acid improves verbal communication in children with autism and language impairment: a randomized double-blind placebo-controlled trial. Mol Psychiatry. 23:247–256, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frye RE, Rossignol DA: Mitochondrial dysfunction can connect the diverse medical symptoms associated with autism spectrum disorders. Pediatr Res 69:41R–47R, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rose S, Niyazov DM, Rossignol DA, et al. : Clinical and Molecular Characteristics of Mitochondrial Dysfunction in Autism Spectrum Disorder. Mol Diagn Ther. 22:571–593, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rossignol DA, Frye RE: Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry 17:290–314, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh K, Singh IN, Diggins E, et al. : Developmental regression and mitochondrial function in children with autism. Ann Clin Transl Neurol. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ormazabal A, Casado M, Molero-Luis M, et al. : Can folic acid have a role in mitochondrial disorders? Drug Discov Today 20:1349–1354, 2015 [DOI] [PubMed] [Google Scholar]
- 75.Morscher RJ, Ducker GS, Li SH, et al. : Mitochondrial translation requires folate-dependent tRNA methylation. Nature. 554:128–132, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Delhey LM, Nur Kilinc E, Yin L, et al. The Effect of Mitochondrial Supplements on Mitochondrial Activity in Children with Autism Spectrum Disorder. J Clin Med. 62017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suren P, Roth C, Bresnahan M, et al. : Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. Jama. 309:570–577, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.James SJ, Melnyk S, Jernigan S, et al. : Abnormal transmethylation/transsulfuration metabolism and DNA hypomethylation among parents of children with autism. J Autism Dev Disord. 38:1966–1975, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sequeira JM, Desai A, Berrocal-Zaragoza MI, et al. : Exposure to Folate Receptor Alpha Antibodies during Gestation and Weaning Leads to Severe Behavioral Deficits in Rats: A Pilot Study. PLoS One. 11: e0152249, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desai A, Sequeira JM, Quadros EV: Prevention of behavioral deficits in rats exposed to folate receptor antibodies: implication in autism. Mol Psychiatry 22:1291–1297, 2017 [DOI] [PubMed] [Google Scholar]
- 81.Grzadzinski R, Carr T, Colombi C, et al. : Measuring Changes in Social Communication Behaviors: Preliminary Development of the Brief Observation of Social Communication Change (BOSCC). J Autism Dev Disord. 46:2464–2479, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Frye RE, Vassall S, Kaur G, et al. : Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 7:792, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boarman DM, Baram J, Allegra CJ: Mechanism of leucovorin reversal of methotrexate cytotoxicity in human MCF-7 breast cancer cells. Biochem Pharmacol 40:2651–2660, 1990 [DOI] [PubMed] [Google Scholar]


