Abstract
Background: Older adults are at an increased risk of frailty, but laboratory surrogates for identifying frailty in this population remain controversial and clinicians frequently encounter difficulty during frailty screening. We examined whether having a high red cell distribution width (RDW) was associated with an increased probability of frailty in older adults.
Methods: We prospectively included community-dwelling older adults between 2013 and 2016 from a single institute, with their clinical features/laboratory parameters documented. We used the Study of Osteoporotic Fractures index (malnutrition, poor physical performance, and fatigue) to delineate frailty, and harnessed multiple logistic regression to investigate whether having a high RDW (≥ 15.7%) was associated with an increased risk of having frailty among these participants.
Results: A total of 2,932 older adults (mean 73.5 ± 6.7 years; 44.6% male) were included, among whom 113 (3.9%) and 76 (2.6%) had a high RDW and presented frailty, respectively. Older adults with a high RDW were more likely to be frail (p = 0.002) and had more positive SOF items than those with normal RDW levels (p = 0.013). Those with a high RDW exhibited a significantly higher risk of having frailty (odds ratio [OR] 2.689, 95% confidence interval [CI] 1.184–6.109) compared to those without. Sensitivity analyses using RDW as a continuous variable similarly showed that RDW levels were positively associated with frailty risk (OR 1.223 per 1% RDW higher).
Conclusions: In older adults, higher RDW can be regarded as a frailty indicator, and the readiness in RDW assessment supports its screening utility.
Keywords: geriatric, frailty, frail phenotype, red cell distribution width, study of osteoporotic fractures
Background
Frailty is a degenerative phenotype occurring in conjunction with chronological aging, in the form of cumulative subclinical health deficits as well as a compromised physical capacity (1). Older adults are the most severely affected population; moreover, the construct of frailty has now been deemed applicable to those with premature aging phenotypes such as patients with diabetes mellitus (DM), chronic inflammation, and chronic kidney disease (CKD) (2). The presence of frailty is predictive of a substantially elevated risk of adverse outcomes, including mortality, hospitalization, institutionalization, incident disability, and even cardiovascular events (3–5). The prognostic importance of frailty in geriatric population cannot be over-stated, and the quest for risk factors of frailty assumes importance both from the epidemiologic and the clinical perspectives.
Different types of risk factors of frailty have been uncovered to date, ranging from clinical features to laboratory parameters. Red cell distribution width (RDW), a descriptor of red blood cell (RBC) volumetric alterations, estimates the degree of RBC anisocytosis, or the variation in RBC volumes. RBC volume usually lies between 80 and 100 fL, and pathologies involving erythropoiesis may result in progressive fluctuations of RBC volumes as well as sizes, thereby increasing RDW. RDW is normally obtained by dividing the mean corpuscular volume (MCV) standard deviation by the MCV level (6), and is regularly reported in the complete blood count panel. Traditionally much emphasis has been placed on its role in facilitating the differential diagnosis of the origin of anemia. Elevated RDW levels are frequently found in patients with anemia related to vitamin B12 or folate deficiency, while normal RDW can be observed in those with anemia of chronic disease or acute blood loss (7). Nowadays, a multitude of reports establish the utility of RDW as a marker of cardiovascular risk and overall mortality among patients with cardiovascular morbidities and older adults (8). However, there can be more adverse influences posed by aberrant RDW in older adults. As explained above, these patients are at risk for developing degenerative phenotypes, such as frailty. Whether high RDW levels are associated with an increased risk of frailty in older adults is rarely examined in the literature. In the current study, we investigated this issue through analyzing a large group of community-dwelling older adults.
Methods
Ethical Approval
The current study has been approved by the institutional review board of National Taiwan University Hospital (NO. 201802088RINC). Informed consent was deemed unnecessary by the review board due to the scrambling of participant identification at study entry and data anonymization. The study protocol adhered to the Declaration of Helsinki.
Participant Enrollment and the Study Procedure
Community-dwelling older adults (age ≥ 65 years) were identified during their annual health examination between 2013 and 2016. For those receiving repetitive health examinations during the study period, we only recorded data from their first examination. Their baseline clinical features, including sociodemographic profiles (age, gender, habits of smoking, or alcohol consumption) and comorbidities (hypertension, DM, hyperlipidemia, gout, cardiac disorders, and laboratory-confirmed CKD [having an estimated glomerular filtration rate, eGFR < 60 mL/min/1.73 m2]), were recorded during examination for subsequent analysis. Following the documentation of clinical features, participants received physical examination regarding their body height, weight, waist circumference, systolic blood pressure (SBP) and diastolic blood pressure (DBP), and pulse rate. Body mass index (BMI) was calculated using weight divided by squared height. Participants received blood tests for hemogram and serum biochemistry panels including their metabolic profile, renal function, and albumin/globulin. Renal function was estimated using serum creatinine based on the Chronic Kidney Disease – Epidemiology Collaboration (CKD-EPI) formula.
For hemogram tests, 3 mL of whole blood were drawn gently from the peripheral veins, preserved in room temperature in ethylenediamine-tetraacetic acid (EDTA)-containing tubes, and sent to the central laboratory within 1 h for analysis (Automated Hematology Analyzer, Sysmex XN-10). The intra-assay and inter-assay variations were both lower than 2%. Samples with clot or hemolysis were discarded and re-sampling was carried out for accuracy. RDW was reported as the coefficient of variation of red cell volume (in percentages) relative to the MCV, as was validated and utilized by others (9–11).
The Assessment of Frailty
The procedure for assessing frailty in this study has been outlined previously (12). Briefly, we used the Study of Osteoporotic Fractures (SOF) index to screen for frailty; that is, the presence of frailty was defined as those with ≥ 2 out of 3 items consisting of malnutrition (weight loss ≥ 5% within 3 years), poor physical performance (inability to stand up 5 times from sitting position without arm support), and fatigue (the sense of a lack of energy) (13). We further operationalized 2 of the 3 SOF items using hypoalbuminemia (<3.5 g/dL) or underweight as a surrogate for malnutrition (Quetelet index <18.5 kg/m2), and using the occurrence of ≥ 2 fall episodes within the preceding 6 months as a surrogate for poor physical performance (12). For those who satisfied only 1 item, they were deemed as having prefrailty. Older adults with SOF-identified frailty were reported to be at risk for having an impaired quality of life, an increased risk for disability, hospitalization and mortality (3, 4). The performance of SOF index for frailty identification is similar to other renowned frailty-screening instruments including the Cardiovascular Health Study (CHS) index and the FRAIL scale in terms of outcome correlation in different populations (14, 15).
Statistical Analysis
We used SPSS version 19th for statistical analyses. For continuous variables, we described data in mean ± standard deviation, and compared data between groups using the Student's t-test (if 2 groups) and analysis of variance (ANOVA) (if > 2 groups). For categorical variables, we described data in values with percentages in parentheses, and compared data between groups using the chi-square test. These statistical tests were chosen because variables analyzed in this study were tested based on the Kolmogorov-Smirnov test.
We first divided the entire group of participants into those with a normal or a high RDW, with the threshold for determining high RDW (15.7%) based on prior studies (9, 16, 17), and compared data between those with a high and a normal RDW. We next performed univariate analyses by determining whether those with and without frailty differed regarding their clinical data, including RDW levels and the proportion of older adults with a high RDW. Finally, multiple logistic regression analyses were conducted to investigate whether having a high RDW was associated with an increased risk of frailty, incorporating significant variables (p < 0.05) in the univariate analysis and the RDW category. We did not select variables such as BMI and serum albumin, or variables used to calculate BMI in the multiple regression models, since these variables have been utilized to measure frailty as described above. If significant differences regarding serum creatinine or eGFR were noted in univariate analysis, we selected eGFR as the default option, since eGFR was calculated from serum creatinine and remained the standard approach of estimating renal function. We used the variance inflation factor (VIF) to estimate the degree of multicollinearity between variables in the regression models. Sensitivity analyses were done in two parts; first, we used RDW as a continuous variable and repeated the multiple regression analyses. Second, we used serum creatinine to replace eGFR in the multiple regression analyses, with results presented. In all analyses, two-tailed p < 0.05 were deemed statistically significant.
Results
Baseline Characteristics of Study Participants
During the study period, a total of 2,932 older adults (mean 73.5 ± 6.7 years; 44.6% male) were included (Table 1). One-fourth (24.3%) of our participants had a habit of regular drinking, while 4.7% has a habit of smoking. The most common comorbidity among these older adults was hypertension (48.8%), followed by cardiac diseases (20%) and hyperlipidemia (18%). Among 2,932 older adults, 113 (3.9%) had a high RDW (Table 1). Those with a high RDW had a significantly higher age (p = 0.002), were more likely to be diabetic (p = 0.013) but with a lower body weight (p = 0.032). Regarding hemogram, older adults with a high RDW had significantly higher RBC (p < 0.001) and platelet counts (p = 0.004) than those with a normal RDW. Serum biochemical panels showed that the high RDW group had a significantly lower serum albumin (p < 0.001), a higher albumin-to-globulin ratio (AGR) (p = 0.024), and a higher serum creatinine (p = 0.013) levels than those without, although eGFR did not differ between the former and the latter groups (Table 1). RDW levels correlated positively with age (r = 0.15, p < 0.001), pulse rate (r = 0.05, p = 0.01), leukocyte (r = 0.05, p = 0.01) and platelet counts (r = 0.06, p = 0.003) but negatively with AGR (r = −0.08, p < 0.001).
Table 1.
Participants' clinical features based on RDW status.
| Normal (n = 2,819) | High* (n = 113) | p-value | |
|---|---|---|---|
| Demographic profile | |||
| Age (years) | 73.4 ± 6.7 | 75.3 ± 7.5 | 0.002 |
| Sex (Male%) | 1,251 (44) | 57 (50) | 0.204 |
| Lifestyle factors | |||
| Smoking (%) | 129 (5) | 8 (7) | 0.216 |
| Drinking (%) | 689 (24) | 23 (20) | 0.321 |
| Comorbidities | |||
| Hypertension (%) | 1,381 (49) | 51 (45) | 0.422 |
| Diabetes mellitus (%) | 370 (13) | 24 (21) | 0.013 |
| Hyperlipidemia (%) | 510 (18) | 17 (15) | 0.408 |
| Chronic kidney disease (%) | 292 (10) | 14 (12) | 0.489 |
| Cardiac diseases (%) | 565 (20) | 20 (18) | 0.541 |
| Gout (%) | 142 (5) | 8 (7) | 0.334 |
| Physical parameters | |||
| Body height (cm) | 158 ± 8.2 | 157 ± 8.4 | 0.415 |
| Body weight (kg) | 59.8 ± 10.3 | 57.7 ± 10.6 | 0.032 |
| Body mass index (kg/m2) | 24 ± 3.4 | 23.3 ± 3.8 | 0.056 |
| Waist circumference (cm) | 83.4 ± 9.2 | 82.8 ± 9.9 | 0.489 |
| Systolic blood pressure (mmHg) | 128 ± 16.5 | 129 ± 19 | 0.446 |
| Diastolic blood pressure (mmHg) | 68.5 ± 11 | 68.3 ± 14.2 | 0.875 |
| Pulse rate (bpm) | 70.2 ± 10.8 | 74.2 ± 12.4 | <0.001 |
| Hemogram | |||
| Red cell count (K/μL) | 4.4 ± 0.5 | 4.9 ± 0.9 | <0.001 |
| Leukocyte (K/μL) | 5.6 ± 1.5 | 5.7 ± 1.8 | 0.769 |
| Platelet (K/μL) | 210 ± 53.1 | 225 ± 71.8 | 0.004 |
| RDW-CV (%) | 13.3 ± 0.7 | 16.7 ± 1.3 | <0.001 |
| Serum biochemistry | |||
| Albumin (g/dL) | 4.29 ± 0.26 | 4.18 ± 0.32 | <0.001 |
| Globulin (g/dL) | 2.77 ± 0.38 | 2.82 ± 0.43 | 0.181 |
| AGR | 1.54 ± 0.27 | 1.48 ± 0.29 | 0.024 |
| Glucose (mg/dL) | 101 ± 20.4 | 101 ± 24.4 | 0.699 |
| Total cholesterol (mg/dL) | 184 ± 33 | 170 ± 35.7 | <0.001 |
| Triglyceride (mg/dL) | 119 ± 63.9 | 123 ± 81.8 | 0.555 |
| Uric acid (mg/dL) | 5.8 ± 1.4 | 6 ± 1.6 | 0.262 |
| Creatinine (mg/dL) | 0.9 ± 0.4 | 1 ± 0.8 | 0.013 |
| eGFR (mL/min/1.73 m2) | 84.9 ± 21.7 | 83.3 ± 24.3 | 0.454 |
AGR, albumin to globulin ratio; CV, coefficient of variation; eGFR, estimated glomerular filtration rate; RDW, red cell distribution width.
High RDW: RDW ≥ 15.7%.
Importantly, we found that older adults with a high RDW level were more likely to have frailty (high vs. normal, 8 [7.1%] vs. 68 [2.4%], p = 0.002) and had a significantly higher number of positive SOF items than those with a normal RDW level (high vs. normal, 0.31 vs. 0.2 items, p = 0.013).
Univariate Analysis: Differences in Clinical Characteristics Between Those With and Without Frailty
Among 2,932 older adults, 76 (2.6%) were identified as having frailty (Table 2). Frail older adults had a significantly advanced age (p < 0.001), more likely to have CKD (p = 0.004) and cardiac diseases (p = 0.004), a lower body weight (p < 0.001), waist circumference (p = 0.009) and BMI (p < 0.001) than non-frail ones. Frail older adults also had significantly lower albumin (p < 0.001), AGR (p = 0.001), and eGFR (p = 0.032) but higher serum creatinine levels (p < 0.001) (Table 2) than non-frail ones. Interestingly, frail older adults had significantly lower RBC counts (p < 0.001) but a higher RDW (p = 0.001) compared to non-frail ones. We further divided participants into those without frailty, with prefrailty (1 SOF criterion) and with frailty (> 1 SOF criterion), and compared RDW levels between each group. RDW levels increased stepwise with rising frailty severity (p < 0.001; Figure 1).
Table 2.
Clinical features based on the presence of frailty or not.
| Without (n = 2,856) | With (n = 76) | p-value | |
|---|---|---|---|
| Demographic profile | |||
| Age (years) | 73.3 ± 6.6 | 78.8 ± 8.5 | <0.001 |
| Sex (Male%) | 1,280 (45) | 28 (37) | 0.168 |
| Lifestyle factors | |||
| Smoking (%) | 134 (5) | 3 (4) | 0.762 |
| Drinking (%) | 704 (25) | 8 (11) | 0.005 |
| Comorbidities | |||
| Hypertension (%) | 1,393 (49) | 39 (51) | 0.662 |
| Diabetes mellitus (%) | 384 (13) | 10 (13) | 0.942 |
| Hyperlipidemia (%) | 512 (18) | 15 (20) | 0.685 |
| Chronic kidney disease (%) | 291 (10) | 15 (20) | 0.007 |
| Cardiac diseases (%) | 560 (20) | 25 (33) | 0.004 |
| Gout (%) | 146 (5) | 4 (5) | 0.953 |
| Physical parameters | |||
| Body height (cm) | 158 ± 8.2 | 155 ± 9.3 | 0.001 |
| Body weight (kg) | 59.9 ± 10.1 | 52.7 ± 13.8 | <0.001 |
| Body mass index (kg/m2) | 24 ± 3.3 | 21.9 ± 4.9 | <0.001 |
| Waist circumference (cm) | 83.5 ± 9.1 | 80.6 ± 12.9 | 0.009 |
| Systolic blood pressure (mmHg) | 128 ± 16.6 | 127 ± 18.2 | 0.44 |
| Diastolic blood pressure (mmHg) | 68.5 ± 11.1 | 66.1 ± 11 | 0.059 |
| Pulse rate (bpm) | 70.3 ± 10.8 | 72.6 ± 12.7 | 0.065 |
| Hemogram | |||
| Red cell count (K/μL) | 4.5 ± 0.5 | 4.1 ± 0.5 | <0.001 |
| Leukocyte (K/μL) | 5.6 ± 1.5 | 5.5 ± 1.6 | 0.559 |
| Platelet (K/μL) | 210 ± 53.8 | 214 ± 59.3 | 0.538 |
| RDW-CV (%) | 13.4 ± 1 | 13.8 ± 1.3 | 0.001 |
| Serum biochemistry | |||
| Albumin (g/dL) | 4.3 ± 0.3 | 4 ± 0.3 | <0.001 |
| Globulin (g/dL) | 2.8 ± 0.4 | 2.9 ± 0.6 | 0.003 |
| AGR | 1.5 ± 0.3 | 1.4 ± 0.3 | 0.001 |
| Glucose (mg/dL) | 101 ± 20.5 | 99.1 ± 24 | 0.519 |
| Total cholesterol (mg/dL) | 184 ± 33 | 178 ± 39.9 | 0.127 |
| Triglyceride (mg/dL) | 120 ± 64.7 | 113 ± 63 | 0.401 |
| Uric acid (mg/dL) | 5.8 ± 1.4 | 5.6 ± 1.7 | 0.134 |
| Creatinine (mg/dL) | 0.9 ± 0.4 | 1.1 ± 1.2 | <0.001 |
| eGFR (mL/min/1.73 m2) | 85 ± 21.6 | 79.5 ± 28.9 | 0.032 |
AGR, albumin to globulin ratio; CV, coefficient of variation; eGFR, estimated glomerular filtration rate; RDW, red cell distribution width.
Figure 1.
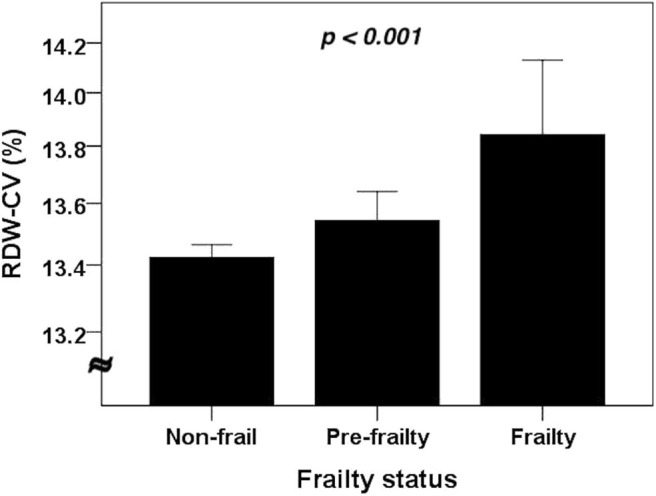
The RDW levels among older adults without and with different severities of frailty (n = 2,932). Compared using the analysis of variance (ANOVA). CV, coefficient of variation; RDW, red cell distribution width.
Factors Independently Associated With Having Frailty
Multiple regression analyses, accounting for significant variables in univariate analysis (age, history of drinking, CKD, cardiac diseases, waist circumference, RBC, albumin to globulin ratio [AGR], and eGFR) and the RDW category, showed that higher age was independently associated with an increased risk of frailty among older adults (odds ratio [OR] 1.091, 95% confidence interval [CI] 1.054–1.129), while higher waist circumference (OR 0.962, 95% CI 0.937–0.989) and higher RBC levels (OR 0.37, 95% CI 0.229–0.598) correlated with a lower probability (original model; Table 3). We discovered that older adults with a high RDW had a significantly higher risk of frailty (OR 2.689, 95% CI 1.184–6.109) compared to those with a normal RDW (Table 3). The VIFs of the variables included in the multiple regression analyses were between 1 and 2, excluding the possibility of multicollinearity.
Table 3.
Results from multiple logistic regression with the presence of frailty as the outcome of interest.
| Variables | OR | 95% CI | p-value |
|---|---|---|---|
| Original model* | |||
| Age | 1.091 | 1.054–1.129 | <0.001 |
| CKD | 1.735 | 1.033–2.911 | 0.037 |
| Waist circumference | 0.962 | 0.937–0.989 | 0.005 |
| RBC | 0.370 | 0.229–0.598 | <0.001 |
| High RDW | 2.689 | 1.184–6.109 | 0.018 |
| With RDW in continuous form | |||
| Age | 1.089 | 1.051–1.127 | <0.001 |
| CKD | 1.706 | 1.016–2.865 | 0.043 |
| Waist circumference | 0.962 | 0.937–0.988 | <0.001 |
| RBC | 0.375 | 0.233–0.604 | <0.001 |
| RDW (%) | 1.223 | 1.023–1.461 | 0.027 |
| With serum creatinine instead of eGFR | |||
| Age | 1.085 | 1.049–1.122 | <0.001 |
| Waist circumference | 0.958 | 0.933–0.985 | 0.002 |
| RBC | 0.426 | 0.264–0.686 | <0.001 |
| High RDW | 2.515 | 1.093–5.786 | 0.030 |
CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; OR, odds ratio; RBC, red blood cell; RDW, red cell distribution width.
Include age, history of drinking, CKD, cardiac diseases, waist circumference, RBC count, albumin to globulin ratio, RDW category, and eGFR.
Sensitivity analyses using RDW as a continuous variable showed that higher RDW levels were similarly associated with an increased frailty likelihood (OR 1.223 per 1% RDW higher, 95% CI 1.023–1.461), independent of other clinical features (Table 3). In addition, if we replaced eGFR with serum creatinine in multiple regression analysis, having a high RDW (OR 2.515, 95% CI 1.093–5.786) remained positively associated with the risk of frailty (Table 3).
Discussion
In this cross-sectional study, we enrolled 2,932 community-dwelling older adults and analyzed the relationship between RDW levels and their probability of having frailty. Participants with a high RDW had a significantly higher proportion of manifesting frailty concurrently. Using multiple regression analyses, we discovered that having a high RDW level was significantly associated with a 2- to 3-fold higher risk of frailty, independent of other clinical features. This relationship persisted independent of laboratory variables related to inflammation such as AGR, as RDW has been shown to closely correlate with the severity of inflammation (18–20). Since hemogram is a convenient laboratory test during clinical practice and RDW is routinely reported in hemogram results, we believe that RDW may have potential utility as an indicator of frailty in older adults, although this finding still needs to be affirmed in other population.
Risk factors for frailty have been extensively examined in the literature. A prior review addressing the ameliorating and aggravating factors of frailty indicated that demographic features (advanced age, certain ethnicity, and having health insurance), lifestyle issues (fruit/vegetable consumption), physical factors (activities of daily living and functional status), and laboratory parameters (uric acid) were significant modifiers of frailty in older adults (21, 22). In population with a higher incidence and prevalence of frailty such as those with CKD, another systematic review also showed that demographic features, comorbidities, disability, and specific laboratory variables (albumin and testosterone) were significant risk factors for developing frailty (1). The degrees of variations of laboratory variables such as hemoglobin, total protein, creatinine, and serum phosphate have also been shown to correlate with mobility, mortality and the severity of frailty in high-risk patients (23). The observed associations between these laboratory parameters and frailty may stem from pathophysiological intermediates such as malnutrition, sarcopenia, and anemia, all of which may co-exist and contribute to the occurrence and progression of frailty (24). In this study, we enrich the existing literature by showing that RDW may be a novel laboratory indicator for frailty in relatively healthy older adults (Table 3).
The normal ranges of multiple hemogram parameters may be influenced by factors including age, gender, body composition, and ethnicity, and RDW is no exception. A higher degree of central obesity and adiposity has been shown to correlate with an increased RDW, possibly through its concurrence with insulin resistance and a low-grade inflammation (25). This is also supported by results from Furuncuoglu et al. study, which showed that BMI had a linearly positive association with RDW levels among individuals undergoing a health checkup (26). Another population-based study disclosed that RDW had an age- and gender-sensitive reference interval, with the older adults having slightly higher RDW ranges (27). In this study, we similarly showed that those with a high RDW had a significantly advanced age and were more likely to have DM (Table 1), compatible with findings from others. Moreover, our findings lend support to the prognostic importance of RDW in older adults, despite the fact that the reference range of RDW may be higher in this population.
Increased RDW is a useful marker for predicting survival and cardiovascular risk among different population, including those with heart failure (28), acute myocardial infarction (29), who were critically ill (17), with pneumonia (30), and those with malignancies (31). However, none of the existing studies identify the association between aberrant RDW levels and the risk of frailty in older adults (7). The relationship between this hemogram component and frailty in older adults may be explained by several reasons; first, the RDW-frailty association may be reflective of the biologic importance of RDW similar to that of the laboratory frailty indices, which have been shown to be important outcome predictors (32, 33). Second, a high RDW level, as a surrogate of hemogram abnormalities, may influence the possibility of having frailty through the relationship between aberrant RBC, leukocyte, or platelet indices and frailty (34–36). However, the plausibility of this reason is relatively low, since we have adjusted for RBC in our regression analyses, without any risk attenuation observed. We did not measure blood cell activities among enrollees. Finally, others have suggested that a high RDW is associated with an aggravated inflammation, a greater disease burden, and higher oxidative stress, all of which potentially underlie the mechanisms of frailty development (8, 37). However, even after we accounted for laboratory-based inflammatory parameters (AGR), the relationship between a high RDW and frailty probability stands (Table 3). In light of these findings, high RDW levels may be a surrogate for a systematically adverse health status and a bodily degenerative status. Based on the ease of obtaining RDW levels during routine patient care, we believe that RDW, besides serving as an outcome predictor, can gain new applicability as a frailty indicator in older adults.
Our study has its strengths and limitations. The phenomenon that RDW independently correlates with the risk of frailty in older adults has not been examined before, and the novelty of this topic is high. The number of our participants was satisfactory, permitting adequate statistical power to analyze determinants of our outcome of interest. However, several limitations should be born in mind. First, our participants may be representative of local community citizens only; extrapolation of our findings to other population and even population of other ethnicities should be made with caution. Second, our study is cross-sectional in nature; a causal relationship between RDW and incident frailty cannot be proven. Third, we did not measure iron profile or vitamin levels among these participants, and the influences of these variables could not be completely excluded. Nonetheless, it is more likely that aberrant RDW precedes the development of frailty. We did not identify differences in the proportion of female between those with high and normal RDW levels, contrary to results from prior studies (38). We consider that this might be related to the relatively lower proportion of older adults with a high RDW in this study. In addition, only SOF index was used to screen for frailty in our participants, which can be inadequate. Other instruments such as CHS index, FRAIL scale, or Edmonton frail scale might increase the reliability of frailty-screening results. Finally, we did not measure muscle mass, arrange body composition analysis, or assess polypharmacy in these patients (39); it can be difficult to ascertain whether these factors modify the relationship we observed.
Conclusion
Among 2,932 community-dwelling older adults, we examined whether RDW affected the risk of having frailty. After adjusting for clinical features and laboratory parameters, a high RDW was found to be associated with a more than 2-fold higher probability of having frailty in these patients, while the magnitude of association paralleled the rising RDW levels. The mechanisms underlying this relationship may extend beyond inflammation and malnutrition only. Judging from our findings, we believe that RDW can be a promising marker for estimating the probability of having frailty among older adults.
Data Availability Statement
The data available for this study will not be available due to institutional regulations. Requests to access the dataset can be direction to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review board of the National Taiwan University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
C-ML and C-TC: study design. C-ML, C-TC, and S-IC: data analysis. C-ML, C-TC, S-IC, D-SH, and K-CH: article drafting. All authors approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the second core laboratory, Department of Medical research of National Taiwan University Hospital for their technical input, and the staff of the genomic research center of National Taiwan University College of Medicine for the kind assistance.
Glossary
Abbreviations
- ANOVA
analysis of variance
- AGR
albumin to globulin ratio
- CHS
Cardiovascular Health Study
- CI
confidence interval
- CKD
chronic kidney disease
- DM
diabetes mellitus
- eGFR
estimated glomerular filtration rate
- MCH
mean corpuscular hemoglobin
- MDRD
Modification of Diet in Renal Disease
- OR
odds ratio
- RBC
red blood cell
- RDW
red cell distribution width
- SOF
Study of Osteoporotic Fractures.
Footnotes
Funding. The study is financially sponsored by National Taiwan University Hospital BeiHu Branch (NO. 10905) and Ministry of Science and Technology, Taiwan (MOST 108-2314-B-002-055- and MOST 109-2314-B-002-193-MY3).
References
- 1.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. (2019) 394:1365–75. 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 2.Chao C-T, Wang J, Huang J-W, Chan D-C, Chien K-L. Hypoglycemic episodes are associated with an increased risk of incident frailty among new onset diabetic patients. J Diabetes Comp. (2020) 34:107492. 10.1016/j.jdiacomp.2019.107492 [DOI] [PubMed] [Google Scholar]
- 3.Bilotta C, Bowling A, Casè A, Pina G, Rossi S, Vergani C. Dimensions and correlates of quality of life according to frailty status: a cross-sectional study on community-dwelling older adults referred to an outpatient geriatric service in Italy. Health Qual Life Outcomes. (2010) 8:56. 10.1186/1477-7525-8-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilotta C, Nicolini P, Casè A, Pina G, Rossi S, Vergani C. Frailty syndrome diagnosed according to the Study of Osteoporotic Fractures (SOF) criteria and adverse health outcomes among community-dwelling older outpatients in Italy. a one-year prospective cohort study. Arch Gerontol Geriatr. (2012) 54:e23–8. 10.1016/j.archger.2011.06.037 [DOI] [PubMed] [Google Scholar]
- 5.Chao C-T, Wang J, Chien K-L. COGENT study group. Both pre-frailty and frailty increase healthcare utilization and adverse health outcomes in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. (2018) 17:130. 10.1186/s12933-018-0772-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lombarts AJ, Koevoet AL, Leijnse B. Basic principles and problems of haemocytometry. Ann Clin Biochem. (1986) 23:390–404. 10.1177/000456328602300404 [DOI] [PubMed] [Google Scholar]
- 7.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. (2015) 52:86–105. 10.3109/10408363.2014.992064 [DOI] [PubMed] [Google Scholar]
- 8.Patel KV, Semba RD, Ferrucci L, Newman AB, Fried LP, Wallace RB, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. (2010) 65:258–65. 10.1093/gerona/glp163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sim YE, Wee HE, Ang AL, Ranjakunalan N, Ong BC, Abdullah HR. Prevalence of preoperative anemia, abnormal mean corpuscular volume and red cell distribution width among surgical patients in Singapore, and their influence on one year mortality. PLoS ONE. (2017) 12:e0182543. 10.1371/journal.pone.0182543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellan M, Giubertoni A, Piccinino C, Dimagli A, Grimoldi F, Sguazzotti M, et al. Red cell distribution width and platelet count as biomarkers of pulmonary arterial hypertension in patients with connective tissue disorders. Dis Markers. (2019) 2019:4981982. 10.1155/2019/4981982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman JJ, Nabbe KC, van den Broek NM. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin Chem Lab Med. (2015) 53:2015–9. 10.1515/cclm-2015-0155 [DOI] [PubMed] [Google Scholar]
- 12.Chao CT, Lee YH, Li CM, Han D-S, Huang J-W, Huang K-C. Advanced age and chronic kidney disease modify the association between metabolic syndrome and frailty among community-dwelling elderly. Rejuv Res. (2019). 10.1089/rej.2019.2202. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. (2008) 168:382–9. 10.1001/archinternmed.2007.113 [DOI] [PubMed] [Google Scholar]
- 14.Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, Cauley JA, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. (2009) 57:492–8. 10.1111/j.1532-5415.2009.02137.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin SM, Aliberti MJR, Fortes-Filho SdQ, Melo JdA, Aprahamian I, Suemoto CK, et al. Comparison of 3 frailty instruments in a geriatric acute care setting in a low-middle income country. J Am Med Dir Assoc. (2018) 19:310–4. 10.1016/j.jamda.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 16.Chu Y, Yuan Z, Meng M, Zhou H, Wang C, Yang G, et al. Red blood cell distribution width as a risk factor for inhospital mortality in obstetric patients admitted to an intensive care unit: a single centre retrospective cohort study. BMJ Open. (2017) 7:e012849. 10.1136/bmjopen-2016-012849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, Lu H, Gong Y, Ying B, Cheng B. The association between red blood cell distribution width and mortality in critically Ill patients with acute kidney injury. Biomed Res Int. (2018) 2018:9658216. 10.1155/2018/9658216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zurauskaite G, Meier M, Voegeli A, Koch D, Haubitz S, Kutz A, et al. Biological pathways underlying the association of red cell distribution width and adverse clinical outcome: results of a prospective cohort study. PLoS ONE. (2018) 13:e019280. 10.1371/journal.pone.0191280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian H, Luo Z, Xiao C, Chen J, Li D, Xu H, et al. Red cell distribution width in coronary heart disease: prediction of restenosis and its relationship with inflammatory markers and lipids. Postgrad Med J. (2018) 94:489. 10.1136/postgradmedj-2018-135806 [DOI] [PubMed] [Google Scholar]
- 20.Goyal H, Lippi G, Gjymishka A, John B, Chhabra R, May E. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol. (2017) 23:4879–91. 10.3748/wjg.v23.i27.4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao C-T, Hsu Y-H, Chang P-Y, He Y-T, Ueng R-S, Lai C-F, et al. Simple self-report FRAIL scale might be more closely associated with dialysis complications than other frailty screening instruments in rural chronic dialysis patients. Nephrology. (2015) 20:321–8. 10.1111/nep.12401 [DOI] [PubMed] [Google Scholar]
- 22.Feng Z, Lugtenberg M, Franse C, Fang X, Hu S, Jin C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: a systematic review of longitudinal studies. PLoS ONE. (2017) 12:e0178383 10.1371/journal.pone.0178383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakazato Y, Kurane R, Hirose S, Watanabe A, Shimoyama H. Variability of laboratory parameters is associated with frailty markers and predicts non-cardiac mortality in hemodialysis patients. Clin Exp Nephrol. (2015) 19:1165–78. 10.1007/s10157-015-1108-0 [DOI] [PubMed] [Google Scholar]
- 24.Calvani R, Marini F, Cesari M, Tosato M, Anker SD, von Haehling S, et al. Biomarkers for physical frailty and sarcopenia: state of the science and future developments. J Cachexia Sarcopenia Muscle. (2015) 6:278–86. 10.1002/jcsm.12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vuong J, Qiu Y, La M, Clarke G, Swinkels DW, Cembrowski G. Reference intervals of complete blood count constituents are highly correlated to waist circumference: should obese patients have their own “normal values?” Am J Hematol. (2014) 89:671–7. 10.1002/ajh.23713 [DOI] [PubMed] [Google Scholar]
- 26.Furuncuoglu Y, Tulgar S, Dogan AN, Cakar S, Tulgar YK, Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci. (2016) 20:1300–6. [PubMed] [Google Scholar]
- 27.Lee Eun J, Kim M, Lee E, Jeon K, Lee J, Lee J-S, et al. A comparison of complete blood count reference intervals in healthy elderly vs. younger Korean adults: a large population study. Clin Chem Lab Med. (2019) 57:716–29. 10.1515/cclm-2018-0649 [DOI] [PubMed] [Google Scholar]
- 28.Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJV, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM program and the duke databank. J Am Coll Cardiol. (2007) 50:40–7. 10.1016/j.jacc.2007.02.067 [DOI] [PubMed] [Google Scholar]
- 29.Dabbah S, Hammerman H, Markiewicz W, Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. (2010) 105:312–7. 10.1016/j.amjcard.2009.09.027 [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Chung HJ, Kim K, Jo YH, Rhee JE, Kim YJ, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med. (2013) 31:72–9. 10.1016/j.ajem.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 31.Xu W-Y, Yang X-B, Wang W-Q, Bai Y, Long J-Y, Lin J-Z, et al. Prognostic impact of the red cell distribution width in esophageal cancer patients: a systematic review and meta-analysis. World J Gastroenterol. (2018) 24:2120–9. 10.3748/wjg.v24.i19.2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blodgett JM, Theou O, Howlett SE, Rockwood K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. Geroscience. (2017) 39:447–55. 10.1007/s11357-017-9993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao C-T, Huang J-W, Chiang C-K, Hung K-Y. Applicability of laboratory deficit-based frailty index in predominantly older patients with end-stage renal disease under chronic dialysis: a pilot test of its correlation with survival and self-reported instruments. Nephrology. (2020) 25:73–81. 10.1111/nep.13583 [DOI] [PubMed] [Google Scholar]
- 34.Sánchez-Flores M, Marcos-Pérez D, Lorenzo-López L, Maseda A, Millan-Calenti JC, Bonassi S, et al. Frailty syndrome and genomic instability in older adults: suitability of the cytome micronucleus assay as a diagnostic tool. J Gerontol Biol Sci Med Sci. (2018) 73:864–72. 10.1093/gerona/glx258 [DOI] [PubMed] [Google Scholar]
- 35.Ruan Y, Guo Y, Kowal P, Lu Y, Liu C, Sun S, et al. Association between anemia and frailty in 13,175 community-dwelling adults aged 50 years and older in China. BMC Geriatr. (2019) 19:327. 10.1186/s12877-019-1342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernández B, Fuentes E, Palomo I, Alarcon M. Increased platelet function during frailty. Exp Hematol. (2019) 77:12–25. 10.1016/j.exphem.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 37.da Silva Garrote-Filho M, Bernardino-Neto M, Penha-Silva N. Influence of erythrocyte membrane stability in atherosclerosis. Curr Atheroscler Rep. (2017) 19:17. 10.1007/s11883-017-0653-2 [DOI] [PubMed] [Google Scholar]
- 38.Alis R, Fuster O, Rivera L, Romagnoli M, Vaya A. Influence of age and gender on red blood cell distribution width. Clin Chem Lab Med. (2015) 53:e25–8. 10.1515/cclm-2014-0756 [DOI] [PubMed] [Google Scholar]
- 39.Chao C-T, Tsai H-B, Wu C-Y, Lin Y-F, Hsu N-C, Chen JS, et al. Cumulative cardiovascular polypharmacy is associated with the risk of acute kidney injury in elderly patients. Medicine. (2015) 94:e1251. 10.1097/MD.0000000000001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data available for this study will not be available due to institutional regulations. Requests to access the dataset can be direction to the corresponding author.


