Abstract
Background
Smoking has been associated with worse colorectal cancer patient survival and may potentially suppress the immune response in the tumor microenvironment. We hypothesized that the prognostic association of smoking behavior at colorectal cancer diagnosis might differ by lymphocytic reaction patterns in cancer tissue.
Methods
Using 1474 colon and rectal cancer patients within 2 large prospective cohort studies (Nurses’ Health Study and Health Professionals Follow-up Study), we characterized 4 patterns of histopathologic lymphocytic reaction, including tumor-infiltrating lymphocytes (TILs), intratumoral periglandular reaction, peritumoral lymphocytic reaction, and Crohn’s-like lymphoid reaction. Using covariate data of 4420 incident colorectal cancer patients in total, an inverse probability weighted multivariable Cox proportional hazards regression model was conducted to adjust for selection bias due to tissue availability and potential confounders, including tumor differentiation, disease stage, microsatellite instability status, CpG island methylator phenotype, long interspersed nucleotide element-1 methylation, and KRAS, BRAF, and PIK3CA mutations.
Results
The prognostic association of smoking status at diagnosis differed by TIL status. Compared with never smokers, the multivariable-adjusted colorectal cancer–specific mortality hazard ratio for current smokers was 1.50 (95% confidence interval = 1.10 to 2.06) in tumors with negative or low TIL and 0.43 (95% confidence interval = 0.16 to 1.12) in tumors with intermediate or high TIL (2-sided Pinteraction = .009). No statistically significant interactions were observed in the other patterns of lymphocytic reaction.
Conclusions
The association of smoking status at diagnosis with colorectal cancer mortality may be stronger for carcinomas with negative or low TIL, suggesting a potential interplay of smoking and lymphocytic reaction in the colorectal cancer microenvironment.
Cigarette smoking is an established risk factor for incidence of colon and rectal cancer (1). Current smoking has appeared to be a modest risk factor for colorectal cancer patient survival (2–5). Accumulating evidence indicates that smoking influences both innate and adaptive immunity. Cigarette smoke contains thousands of harmful chemicals that may potentially suppress immune cell function, thereby promoting tumor evolution (6,7). Considering evidence for the influence of smoking on the tumor immune microenvironment, we hypothesized that smoking might influence tumor progression differentially by the degree of antitumor immune response.
A variety of endogenous and exogenous factors may exert effects on the host immune response to colorectal cancer (8–11). Tumor-infiltrating lymphocytes (TIL) and T cells have been considered as indicators of the host immune response against tumor and an attractive target for immunotherapy (10,12,13). The abundance of these cells in the tumor has been associated with longer survival in colorectal cancer patients independently of stage and microsatellite instability (MSI) status (12,14,15). Recently, we reported that the incidence risk of colorectal cancer increased by smoking was stronger for tumors with lower T-cell response, suggesting a suppression effect of smoking on T-cell–mediated immunity and an important interaction of smoking and immunity in colorectal carcinogenesis (16). Immune response in the tumor microenvironment has a crucial role in suppressing tumor progression, contributing to better patient prognosis. Considering the role of immune cells, including lymphocytes, we hypothesized that smoking status at diagnosis might be associated with higher mortality in the tumors with weaker lymphocytic reactions compared with those with stronger lymphocytic reactions.
To test our hypothesis, we used 2 large US nationwide prospective cohort studies with covariate data of 4420 colorectal cancer patients and a molecular pathological epidemiology database of 1474 patients. This comprehensive dataset enabled us to examine the prognostic association of smoking status at diagnosis according to lymphocytic reaction in the colorectal cancer tissue.
Materials and Methods
Study Population
We collected data from 2 prospective cohort studies in the United States: the Nurses’ Health Study (NHS, 121 701 women followed since 1976) and the Health Professionals Follow-up Study (HPFS, 51 529 men followed since 1986) (17). In both cohorts, questionnaires have been sent to participants to update information on smoking status, other lifestyle factors, and medical history every 2 years. We used the National Death Index to confirm deaths of study participants and identify unreported lethal colorectal cancer patients.
We included 1474 patients with available data on smoking exposure at diagnosis and immune profiles including lymphocytic reaction in colorectal cancer tissue. We included both colon and rectal carcinomas based on the colorectal continuum model (18). Patients were followed-up until death or the end of follow-up (January 1, 2014, for HPFS; May 31, 2014, for NHS), whichever came first. We used the inverse probability weighting (IPW) method (19,20) and covariate data of 4420 incident colorectal cancer patients to adjust for selection bias in the 1474 patients. Previous studies using IPW in our dataset showed that results with and without IPW generated similar data (19). Study physicians reviewed medical records associated with colorectal cancer diagnoses and identified cause of death for deceased participants based on medical records and death certificates. For nonresponders who had died of colorectal cancer, we obtained permission from the next of kin and reviewed medical records to gather data on date of diagnosis, stage, tumor location, and tumor grade. Formalin-fixed paraffin-embedded tissue blocks were collected from hospitals across the United States where colorectal cancer patients underwent their primary tumor resection. A study pathologist (S.O.) blinded to other data conducted a centralized review of hematoxylin and eosin–stained tissue sections of all colorectal carcinoma patients and collected data on pathological features (21). Tumor differentiation was categorized as moderate (>50% glandular area) or poor (≤50% glandular area).
Informed consent was obtained from all participants in this analysis. The study procedures and protocols were approved by the institutional review boards at the Harvard T.H. Chan School of Public Health, Brigham and Women’s Hospital (Boston, MA), and those of participating registries as required.
Assessment of Smoking Status
Data on smoking status were collected in the 2 cohorts as reported previously (22). Current smoking status and the number of cigarettes smoked per day were reported by participants on biennial questionnaires since 1980 (NHS) and 1986 (HPFS). In the baseline questionnaires (1976 in NHS and 1986 in HPFS), participants were asked to report the age at which they began and ceased smoking (for past smokers) and the average daily consumption of cigarettes. Smoking status at colorectal cancer diagnosis was derived from the latest available questionnaire before diagnosis. Smoking status after diagnosis was derived from the available questionnaire at least more than 6 months after colorectal cancer diagnosis. We calculated cumulative pack-years of cigarettes ([cumulative average of packs per day] multiplied by [the number of years during which smoking occurred]) and duration of smoking cessation for past and current smokers (22).
Assessment of Tumor Immunity Status
Four components of lymphocytic reaction to tumors, including TIL, intratumoral periglandular reaction, peritumoral lymphocytic reaction, and Crohn’s-like lymphoid reaction, were histopathologically evaluated as previously reported (21). Briefly, the 4 lymphocytic reaction components were scored as 0, 1+, 2+, and 3+ and graded as negative or low (0), intermediate (1+), or high (2+, 3+) by a study pathologist (S.O.) based on centralized review of hematoxylin and eosin tissue sections. Review of 398 selected patients between 2 independent pathologists (S.O. and J.N. Glickman) showed good concordance on grading of histopathologic features, including lymphocytic reaction to tumor (21).
Analyses of Tumor Molecular Characteristics
Genomic DNA was extracted from formalin-fixed paraffin-embedded colorectal carcinoma tissue. MSI status was determined by polymerase chain reaction of 10 microsatellite markers (D2S123, D5S346, D17S250, BAT25, BAT26, BAT40, D18S55, D18S56, D18S67, and D18S487); MSI-high was defined as the presence of instability in at least 30% of the markers (18,23). Methylation status of 8 CpG island methylator phenotype (CIMP)-specific promoters (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1) (24) was determined by MethyLight assay using bisulfite-treated DNA (25). CIMP-high was defined as at least 6 methylated promoters of 8 promoters, and CIMP-low or negative as 0 to 5 methylated promoters (24). Methylation levels at long-interspersed nucleotide element-1 were measured by pyrosequencing using bisulfite-treated DNA (26). Polymerase chain reaction and pyrosequencing were performed for KRAS (codons 12, 13, 61, and 146), BRAF (codon 600), and PIK3CA (exons 9 and 20) (27–29).
Statistical Analyses
All statistical analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC). All P values were 2-sided. Our primary hypothesis test was an assessment of a statistical interaction between smoking status at diagnosis (ordinal; never, past, and current) and lymphocytic reaction in tumor tissue (binary classification of negative/low and intermediate/high) in a multivariable Cox proportional hazards regression model using the Wald test on the cross-product. We used the 2-sided α-level of .005 (30). The hazard ratio (HR) for smoking status at diagnosis in strata of lymphocytic reaction components using a reparameterization of the interaction term was also assessed in a single regression model (19).
Primary outcome endpoint of this study was colorectal cancer–specific mortality and the secondary endpoint was overall mortality. For colorectal cancer–specific survival analyses, deaths of other causes and patients with missing data on cause of death were censored. Survival time was defined as the period from diagnosis of colorectal cancer to death or the end of follow-up, whichever came first.
In all survival analyses, we used covariate data of 4420 incident colorectal cancer patients and the IPW method (Supplementary Methods available online) to reduce the selection bias due to the availability of tumor tissue (19,20). The probability of the availability of tumor tissue for each patient was estimated using a multivariable logistic regression model, and each patient with complete data was weighted by the inverse of the probability (19,20). The IPW-adjusted Kaplan-Meier method was used to estimate the distribution of colorectal cancer–specific and overall survivals, and the weighted log-rank test was performed (31). Similar results were obtained by Cox regression analyses without the IPW.
IPW-adjusted, multivariable Cox proportional hazards regression models were used to adjust for potential confounders and initially included the following: sex (ie, cohort), age at diagnosis, year of diagnosis, family history of colorectal cancer, body mass index at diagnosis, alcohol consumption at diagnosis; physical activity at diagnosis; processed meat intake at diagnosis; total fiber intake at diagnosis; tumor location; tumor differentiation; disease stage; MSI status; CIMP; long-interspersed nucleotide element-1 methylation level; and KRAS, BRAF, and PIK3CA mutations. A backward elimination with a threshold of P = .05 was performed to select variables for the final models. The proportionality of hazards assumption was assessed using a time-varying covariate, which is an interaction term of survival time and smoking status at diagnosis. The proportionality of hazards assumption was generally satisfied for cancer-specific survival (P > .28). All statistical tests were 2-sided.
Results
We included 1474 colorectal cancer patients with available data on smoking status at diagnosis and lymphocytic reaction among 4420 incident colorectal cancer patients in the 2 prospective cohort studies (Table 1). The frequency of smoking status at diagnosis was highly associated with that of after diagnosis. Only 6 past smokers at diagnosis (0.9%) commenced smoking after colorectal cancer diagnosis. Among current smokers at colorectal cancer diagnosis, 58.7% (71 of 121) of them had continued smoking after diagnosis. Current smokers were associated with female sex, younger age, and earlier year of diagnosis. During the median follow-up time of 11.8 years (interquartile range = 7.2-16.4 years) for all censored patients, there were 879 all-cause deaths, including 428 colorectal cancer–specific deaths.
Table 1.
Clinical, pathological, and molecular characteristics of colorectal cancer patients according to smoking status at diagnosis
| Smoking status at diagnosis |
|||||
|---|---|---|---|---|---|
| Patients | Never | Past | Current | P b | |
| Characteristicsa | (n = 1474) | (n = 601) | (n = 727) | (n = 146) | |
| Sex, No. (%) | <.001 | ||||
| Female: NHS | 844 (57.3) | 351 (58.4) | 389 (53.5) | 104 (71.2) | |
| Male: HPFS | 630 (42.7) | 250 (41.6) | 338 (46.5) | 42 (28.8) | |
| Mean age ± SD, y | 69.2 ± 9.1 | 68.8 ± 9.6 | 70.2 ± 8.6 | 65.6 ± 7.8 | <.001 |
| Year of diagnosis, No. (%) | <.001 | ||||
| 1995 or before | 514 (34.9) | 209 (34.8) | 228 (31.4) | 77 (52.7) | |
| 1996-2000 | 430 (29.2) | 173 (28.8) | 215 (29.6) | 42 (28.8) | |
| 2001-2014 | 530 (36.0) | 219 (36.4) | 284 (39.0) | 27 (18.5) | |
| Family history of colorectal cancer in first-degree relative(s), No. (%) | <.79 | ||||
| Absent | 1185 (80.6) | 487 (81.4) | 582 (80.2) | 116 (79.5) | |
| Present | 285 (19.4) | 111 (18.6) | 144 (19.8) | 30 (20.5) | |
| BMI at diagnosis, No. (%) | .18 | ||||
| <25 kg/m2 | 589 (40.0) | 249 (41.4) | 278 (38.3) | 62 (42.5) | |
| 25 to <30 kg/m2 | 607 (41.2) | 234 (38.9) | 307 (42.3) | 66 (45.2) | |
| ≥30 kg/m2 | 277 (18.8) | 118 (19.6) | 141 (19.4) | 18 (12.3) | |
| Alcohol consumption at diagnosis, No. (%) | <.001 | ||||
| 0 g/d | 498 (33.9) | 262 (43.6) | 185 (25.6) | 51 (34.9) | |
| 0 to <15 g/d | 772 (52.6) | 293 (48.8) | 408 (56.5) | 71 (48.6) | |
| ≥15 g/d | 199 (13.6) | 46 (7.7) | 129 (17.9) | 24 (16.4) | |
| Physical activity at diagnosis (METS-h/wk)c, No. (%) | .06 | ||||
| Lowest | 498 (35.6) | 203 (35.9) | 236 (33.4) | 59 (45.7) | |
| Second | 328 (23.4) | 120 (21.2) | 173 (24.5) | 35 (27.1) | |
| Third | 256 (18.3) | 109 (19.3) | 125 (17.7) | 22 (17.1) | |
| Highest | 318 (22.7) | 133 (23.5) | 172 (24.4) | 13 (10.1) | |
| Processed meat intake at diagnosis, serving/d | 0.15 ± 0.16 | 0.14 ± 0.16 | 0.15 ± 0.16 | 0.20 ± 0.17 | <.001 |
| Total fiber intake at diagnosis, g/d | 21.0 ± 6.9 | 22.0 ± 7.5 | 20.9 ± 6.4 | 17.2 ± 5.0 | <.001 |
| Smoking status after diagnosis, No. (%) | <.001 | ||||
| Noncurrent smoker | 1211 (94.0) | 503 (100.0) | 658 (99.1) | 50 (41.3) | |
| Current smoker | 77 (6.0) | 0 (0.0) | 6 (0.9) | 71 (58.7) | |
| Tumor location, No. (%) | .55 | ||||
| Proximal colon | 718 (48.9) | 296 (49.3) | 357 (49.3) | 65 (44.8) | |
| Distal colon | 440 (30.0) | 185 (30.8) | 213 (29.4) | 42 (29.0) | |
| Rectum | 311 (21.2) | 119 (19.8) | 154 (21.3) | 38 (26.2) | |
| AJCC disease stage, No. (%) | .04 | ||||
| I | 351 (26.1) | 151 (27.6) | 181 (27.3) | 19 (14.1) | |
| II | 429 (31.9) | 167 (30.5) | 213 (32.2) | 49 (36.3) | |
| III | 378 (28.1) | 157 (28.7) | 180 (27.2) | 41 (30.4) | |
| IV | 186 (13.8) | 72 (13.2) | 88 (13.3) | 26 (19.3) | |
| Tumor differentiation, No. (%) | .51 | ||||
| Well to moderate | 1313 (89.8) | 541 (90.6) | 647 (89.6) | 125 (87.4) | |
| Poor | 149 (10.2) | 56 (9.4) | 75 (10.4) | 18 (12.6) | |
| MSI status, No. (%) | .16 | ||||
| Non–MSI-high | 1074 (83.5) | 438 (85.9) | 528 (82.0) | 108 (81.2) | |
| MSI-high | 213 (16.6) | 72 (14.1) | 116 (18.0) | 25 (18.8) | |
| CIMP status, No. (%) | .32 | ||||
| CIMP-low or negative | 1019 (81.7) | 409 (83.5) | 506 (81.1) | 104 (78.2) | |
| CIMP-high | 228 (18.3) | 81 (16.5) | 118 (18.9) | 29 (21.8) | |
| Mean LINE-1 methylation level ± SD | 63.6 ± 10.0 | 63.5 ± 10.2 | 63.6 ± 10.0 | 63.4 ± 9.7 | .98 |
| KRAS mutation, No. (%) | .23 | ||||
| Wild type | 720 (58.6) | 291 (59.2) | 344 (56.9) | 85 (64.9) | |
| Mutant | 508 (41.4) | 201 (40.9) | 261 (43.1) | 46 (35.1) | |
| BRAF mutation, No. (%) | .73 | ||||
| Wild type | 1100 (84.6) | 442 (85.5) | 544 (83.8) | 114 (85.1) | |
| Mutant | 200 (15.4) | 75 (14.5) | 105 (16.2) | 20 (14.9) | |
| PIK3CA mutation, No. (%) | .53 | ||||
| Wild type | 1013 (83.7) | 400 (82.3) | 512 (84.5) | 101 (85.6) | |
| Mutant | 197 (16.3) | 86 (17.7) | 94 (15.5) | 17 (14.4) | |
| Tumor-infiltrating lymphocytes, No. (%) | .45 | ||||
| Negative or low | 1,100 (74.8) | 458 (76.3) | 538 (74.2) | 104 (71.7) | |
| Intermediate or high | 370 (25.2) | 142 (23.7) | 187 (25.8) | 41 (28.3) | |
| Intratumoral periglandular reaction, No. (%) | .77 | ||||
| Negative or low | 191 (13.0) | 80 (13.4) | 90 (12.4) | 21 (14.4) | |
| Intermediate or high | 1,279 (87.0) | 519 (86.6) | 635 (87.6) | 125 (85.6) | |
| Peritumoral lymphocytic reaction, No. (%) | .32 | ||||
| Negative or low | 209 (14.3) | 92 (15.4) | 93 (12.9) | 24 (16.4) | |
| Intermediate or high | 1,256 (85.7) | 506 (84.6) | 628 (87.1) | 122 (83.6) | |
| Crohn’s-like lymphoid reaction, No. (%) | .02 | ||||
| Negative or low | 915 (75.8) | 391 (80.0) | 440 (73.0) | 84 (73.0) | |
| Intermediate or high | 292 (24.2) | 98 (20.0) | 163 (27.0) | 31 (27.0) | |
Percentage (%) indicates the proportion of patients with a specific clinical, pathological, or molecular characteristic in patients or in strata of smoking status at diagnosis. AJCC = American Joint Committee on Cancer; BMI = body mass index; CIMP = CpG island methylator phenotype; HPFS = Health Professionals Follow-up Study; LINE-1 = long interspersed nucleotide element-1; METS = metabolic equivalent task score; MSI = microsatellite instability; NHS = Nurses’ Health Study.
To compare characteristics between subgroups, we used the χ2 test for categorical variables and an analysis of variance for continuous variables. To compare continuous variables, an analysis of variance was performed.
Physical activity was categorized into 4 categories (female: 0 to <5, 5-11.5, 11.5 to <22, and ≥22 METS-h/wk; male: 0 to <10, 10-22.5, 22.5 to <41.5, and ≥41.5 METS-h/wk).
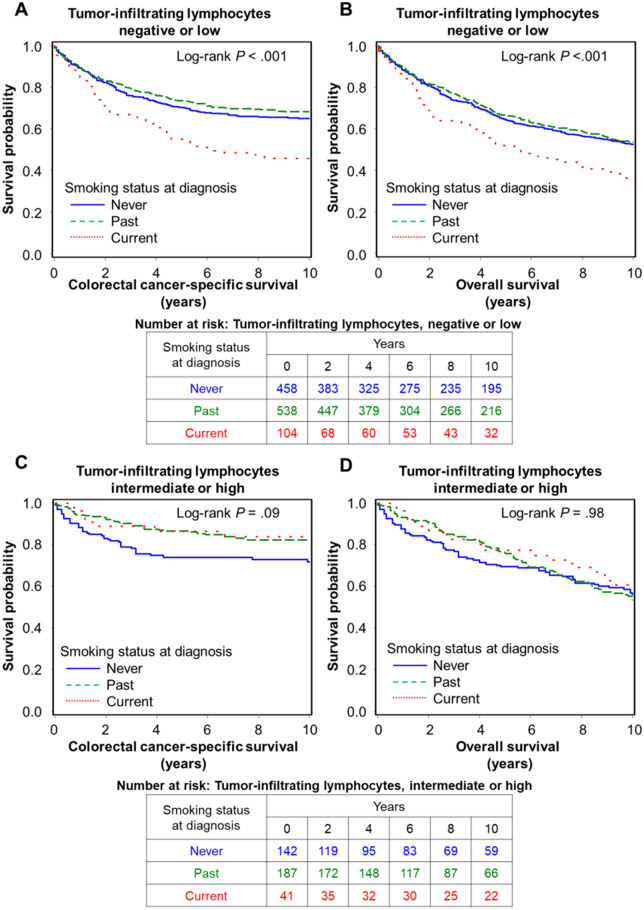
In our primary hypothesis testing, we evaluated the association of smoking status at diagnosis with colorectal cancer–specific survival according to tumor lymphocytic reaction. We found a trend of a statistical interaction between smoking status at diagnosis and TIL in relation to colorectal cancer–specific survival in the IPW-adjusted Cox model (Pinteraction = .009; Table 2). Supplementary Table 1 (available online) shows the final model of the IPW-adjusted multivariable Cox regression model. Similar findings were observed when we treated smoking status as binary variables (noncurrent [never and past] vs current; never vs ever [past and current]) (Supplementary Table 2 available online). Compared with never smokers, current smokers were associated with higher colorectal cancer–specific mortality in tumors with negative or low TIL (multivariable-adjusted HR = 1.50, 95% CI = 1.10 to 2.06) but not in tumors with intermediate or high TIL (multivariable-adjusted HR = 0.43, 95% CI = 0.16 to 1.12). Figure 1 shows IPW-adjusted Kaplan-Meier survival curves for colorectal cancer–specific and overall survival according to smoking status at diagnosis in strata of TIL grade. We observed no statistically significant interaction of smoking status at diagnosis with intratumoral periglandular reaction, peritumoral lymphocytic reaction, and Crohn’s-like lymphoid reaction, respectively. Similar results were obtained by Cox regression analyses without the IPW (Supplementary Table 3 available online). We also performed analyses stratified by sex (ie, cohort) in Supplementary Table 4 (available online) and by colon and rectum in Supplementary Table 5 (available online). The results showed that, compared with never smokers, current smokers were consistently associated with higher colorectal cancer–specific mortality in patients with negative or low TIL in each stratum of women (NHS), men (HPFS), colon cancer, and rectal cancer.
Table 2.
Smoking status at diagnosis and colorectal cancer mortality in strata of levels of lymphocytic reaction patterns
| Characteristics | Colorectal cancer–specific mortalitya,b |
Overall mortalitya,b |
|||||
|---|---|---|---|---|---|---|---|
| No. of cases | No. of events | Univariate HR (95% CI) | Multivariable HR (95% CI)c | No. of events | Univariate HR (95% CI) | Multivariable HR (95% CI)c | |
| TIL (n = 1470) | |||||||
| Negative or low | |||||||
| Never smoker | 458 | 141 | 1.00 (referent) | 1.00 (referent) | 262 | 1.00 (referent) | 1.00 (referent) |
| Past smoker | 538 | 158 | 0.89 (0.70 to 1.13) | 0.80 (0.63 to 1.02) | 320 | 1.05 (0.87 to 1.27) | 0.95 (0.79 to 1.14) |
| Current smoker | 104 | 54 | 1.79 (1.30 to 2.45) | 1.50 (1.10 to 2.06) | 83 | 1.64 (1.24 to 2.15) | 1.93 (1.47 to 2.55) |
| Intermediate or high | |||||||
| Never smoker | 142 | 36 | 1.00 (referent) | 1.00 (referent) | 80 | 1.00 (referent) | 1.00 (referent) |
| Past smoker | 187 | 30 | 0.53 (0.32 to 0.91) | 0.54 (0.31 to 0.94) | 101 | 0.95 (0.71 to 1.28) | 0.85 (0.62 to 1.15) |
| Current smoker | 41 | 7 | 0.47 (0.20 to 1.13) | 0.43 (0.16 to 1.12) | 31 | 0.92 (0.60 to 1.41) | 1.09 (0.68 to 1.75) |
| Pinteractiond | .003 | .009 | .06 | .09 | |||
| Pinteraction (noncurrent vs current)e | .02 | .03 | .03 | .04 | |||
| Intratumoral periglandular reaction (n = 1470) | |||||||
| Negative or low | |||||||
| Never smoker | 80 | 41 | 1.00 (referent) | 1.00 (referent) | 54 | 1.00 (referent) | 1.00 (referent) |
| Past smoker | 90 | 34 | 0.64 (0.40 to 1.03) | 0.67 (0.42 to 1.07) | 51 | 1.11 (0.68 to 1.81) | 0.85 (0.56 to 1.28) |
| Current smoker | 21 | 10 | 1.02 (0.48 to 2.18) | 0.89 (0.43 to 1.82) | 14 | 1.41 (0.67 to 2.95) | 1.29 (0.60 to 2.78) |
| Intermediate or high | |||||||
| Never smoker | 519 | 136 | 1.00 (referent) | 1.00 (referent) | 288 | 1.00 (referent) | 1.00 (referent) |
| Past smoker | 635 | 153 | 0.86 (0.67 to 1.10) | 0.78 (0.60 to 1.00) | 369 | 1.03 (0.87 to 1.22) | 0.92 (0.78 to 1.09) |
| Current smoker | 125 | 52 | 1.54 (1.11 to 2.15) | 1.28 (0.90 to 1.81) | 101 | 1.41 (1.11 to 1.80) | 1.68 (1.30 to 2.17) |
| Pinteractiond | .24 | .33 | .92 | .59 | |||
| Pinteraction (noncurrent vs current)e | .46 | .41 | .93 | .71 | |||
| Peritumoral lymphocytic reaction (n = 1465) | |||||||
| Negative or low | |||||||
| Never smoker | 92 | 48 | 1.00 (referent) | 1.00 (referent) | 66 | 1.00 (referent) | 1.00 (referent) |
| Past smoker | 93 | 43 | 0.79 (0.51 to 1.22) | 0.99 (0.63 to 1.56) | 58 | 1.08 (0.65 to 1.78) | 0.93 (0.62 to 1.38) |
| Current smoker | 24 | 13 | 0.97 (0.49 to 1.94) | 0.86 (0.46 to 1.61) | 19 | 1.24 (0.67 to 2.29) | 1.38 (0.76 to 2.51) |
| Intermediate or high | |||||||
| Never smoker | 506 | 129 | 1.00 (referent) | 1.00 (referent) | 276 | 1.00 (referent) | 1.00 (referent) |
| Past smoker | 628 | 144 | 0.83 (0.65 to 1.07) | 0.73 (0.56 to 0.94) | 361 | 1.04 (0.87 to 1.23) | 0.92 (0.77 to 1.09) |
| Current smoker | 122 | 49 | 1.54 (1.10 to 2.16) | 1.29 (0.90 to 1.84) | 96 | 1.43 (1.11 to 1.84) | 1.65 (1.26 to 2.16) |
| Pinteractiond | .35 | .65 | .83 | .74 | |||
| Pinteraction (noncurrent vs current)e | .22 | .16 | .61 | .61 | |||
| Crohn’s-like lymphoid reaction (n = 1207) | |||||||
| Negative or low | |||||||
| Never smoker | 391 | 131 | 1.00 (referent) | 1.00 (referent) | 235 | 1.00 (referent) | 1.00 (referent) |
| Past smoker | 440 | 129 | 0.81 (0.63 to 1.05) | 0.81 (0.62 to 1.05) | 260 | 0.97 (0.78 to 1.19) | 0.94 (0.77 to 1.15) |
| Current smoker | 84 | 43 | 1.63 (1.16 to 2.29) | 1.37 (0.96 to 1.96) | 67 | 1.45 (1.04 to 2.01) | 1.78 (1.28 to 2.48) |
| Intermediate or high | |||||||
| Never smoker | 98 | 20 | 1.00 (referent) | 1.00 (referent) | 53 | 1.00 (referent) | 1.00 (referent) |
| Past smoker | 163 | 21 | 0.46 (0.24 to 0.88) | 0.42 (0.21 to 0.83) | 83 | 0.91 (0.66 to 1.27) | 0.72 (0.51 to 1.00) |
| Current smoker | 31 | 5 | 0.55 (0.19 to 1.61) | 0.65 (0.23 to 1.88) | 23 | 1.08 (0.72 to 1.60) | 1.30 (0.84 to 2.02) |
| Pinteractiond | .05 | .09 | .43 | .41 | |||
| Pinteraction (noncurrent vs current)e | .16 | .53 | .26 | .61 | |||
IPW was applied to reduce a bias due to the availability of tumor tissue after cancer diagnosis (see “Statistical Analysis” subsection for details). AJCC = American Joint Committee on Cancer; CI = confidence interval; HR = hazard ratio; IPW = inverse probability weighting; TIL = tumor-infiltrating lymphocytes.
Hazard ratios were estimated for each stratum on the basis of the patients with smoking status at diagnosis, using a reparameterization of the interaction term in a single regression model for the stratified analyses.
The multivariable Cox regression model initially included age, sex, year of diagnosis, family history of colorectal cancer, body mass index at diagnosis, alcohol consumption at diagnosis, physical activity at diagnosis, processed meat intake at diagnosis, total fiber intake at diagnosis, tumor location, tumor differentiation, AJCC disease stage, microsatellite instability, CpG island methylator phenotype, long interspersed nucleotide element-1 methylation level, KRAS mutation, BRAF mutation, and PIK3CA mutation. A backward elimination with a threshold of P = .05 was used to select variables in the final models.
P interaction was calculated using the Wald test for the cross-product of smoking status at diagnosis (ordinal; never, past, and current) and lymphocytic reaction status (binary; negative or low and intermediate or high) in Cox regression model.
P interaction was calculated using the Wald test for the cross-product of smoking status at diagnosis (binary; noncurrent [never and past] vs current) and lymphocytic reaction status (binary; negative/low and intermediate/high) in Cox regression model.
Figure 1.
Inverse probability weighting–adjusted Kaplan-Meier curves of colorectal cancer–specific and overall survival according to smoking status at diagnosis in strata of tumor-infiltrating lymphocytes. The P values were calculated using the weighted 2-sided log-rank test (2-sided). A and B) Colorectal cancer with negative or low tumor-infiltrating lymphocytes. C and D) Colorectal cancer with intermediate or high tumor-infiltrating lymphocytes.
In a secondary analysis, we evaluated the survival interaction between cumulative pack-years at diagnosis and lymphocytic reaction. We found a consistent interaction between cumulative pack-years at diagnosis and TIL in relation to colorectal cancer–specific survival (Pinteraction = .02; Table 3). Additionally, past smokers, including those who had quit within the past 10 years, did not show this interaction with survival as observed with current smoking and survival (Pinteraction = .01; Supplementary Table 6 available online). Although statistical power was limited, compared with never smokers, current smokers after diagnosis might be associated with higher colorectal cancer–specific mortality in patients with negative or low TIL, adjusting for prediagnostic cumulative pack-years (Supplementary Table 7 available online).
Table 3.
Pack-years of cigarettes at diagnosis and colorectal cancer mortality in strata of levels of lymphocytic reaction patterns
| Characteristics | Colorectal cancer–specific mortalitya,b |
Overall mortalitya,b |
|||||
|---|---|---|---|---|---|---|---|
| No. of cases | No. of events | Univariate HR (95% CI) | Multivariable HR (95% CI)c | No. of events | Univariate HR (95% CI) | Multivariable HR (95% CI)c | |
| TIL (n = 1430) | |||||||
| Negative or low | |||||||
| Pack-years = 0 | 458 | 141 | 1.00 (referent) | 1.00 (referent) | 262 | 1.00 (referent) | 1.00 (referent) |
| Pack-years = 1-19 | 260 | 71 | 0.81 (0.60 to 1.10) | 0.75 (0.54 to 1.03) | 135 | 0.87 (0.68 to 1.10) | 0.81 (0.64 to 1.03) |
| Pack-years = 20-39 | 180 | 61 | 1.07 (0.78 to 1.47) | 0.98 (0.73 to 1.31) | 116 | 1.18 (0.91 to 1.52) | 1.22 (0.96 to 1.54) |
| Pack-years ≥40 | 169 | 68 | 1.40 (1.04 to 1.88) | 1.17 (0.86 to 1.58) | 130 | 1.66 (1.31 to 2.09) | 1.47 (1.16 to 1.86) |
| Intermediate or high | |||||||
| Pack-years = 0 | 142 | 36 | 1.00 (referent) | 1.00 (referent) | 80 | 1.00 (referent) | 1.00 (referent) |
| Pack-years = 1-19 | 81 | 11 | 0.51 (0.24 to 1.08) | 0.49 (0.22 to 1.08) | 36 | 0.81 (0.53 to 1.23) | 0.79 (0.52 to 1.21) |
| Pack-years = 20-39 | 69 | 12 | 0.50 (0.25 to 1.02) | 0.60 (0.28 to 1.29) | 40 | 0.88 (0.61 to 1.27) | 0.85 (0.59 to 1.22) |
| Pack-years ≥40 | 71 | 13 | 0.57 (0.29 to 1.11) | 0.49 (0.24 to 1.01) | 54 | 1.23 (0.88 to 1.71) | 1.10 (0.76 to 1.60) |
| Pinteractiond | .006 | .02 | .08 | .07 | |||
| Intratumoral periglandular reaction (n = 1430) | |||||||
| Negative or low | |||||||
| Pack-years = 0 | 80 | 41 | 1.00 (referent) | 1.00 (referent) | 54 | 1.00 (referent) | 1.00 (referent) |
| Pack-years = 1-19 | 35 | 13 | 0.58 (0.29 to 1.14) | 0.66 (0.34 to 1.31) | 18 | 0.92 (0.48 to 1.76) | 0.74 (0.41 to 1.34) |
| Pack-years = 20-39 | 34 | 17 | 1.01 (0.57 to 1.77) | 0.91 (0.52 to 1.60) | 24 | 1.76 (1.04 to 2.97) | 1.41 (0.87 to 2.26) |
| Pack-years ≥40 | 35 | 11 | 0.60 (0.29 to 1.23) | 0.61 (0.29 to 1.28) | 19 | 0.98 (0.50 to 1.92) | 0.78 (0.40 to 1.50) |
| Intermediate or high | |||||||
| Pack-years = 0 | 519 | 136 | 1.00 (referent) | 1.00 (referent) | 288 | 1.00 (referent) | 1.00 (referent) |
| Pack-years = 1-19 | 306 | 68 | 0.80 (0.59 to 1.10) | 0.70 (0.50 to 0.98) | 152 | 0.86 (0.69 to 1.06) | 0.79 (0.63 to 0.99) |
| Pack-years = 20-39 | 215 | 56 | 0.92 (0.66 to 1.28) | 0.87 (0.63 to 1.20) | 132 | 1.04 (0.83 to 1.30) | 1.05 (0.85 to 1.30) |
| Pack-years ≥40 | 206 | 71 | 1.34 (1.00 to 1.80) | 1.13 (0.82 to 1.55) | 166 | 1.63 (1.33 to 2.00) | 1.45 (1.16 to 1.80) |
| Pinteractiond | .13 | .22 | .54 | .33 | |||
| Peritumoral lymphocytic reaction (n = 1425) | |||||||
| Negative or low | |||||||
| Pack-years = 0 | 92 | 48 | 1.00 (referent) | 1.00 (referent) | 66 | 1.00 (referent) | 1.00 (referent) |
| Pack-years = 1-19 | 40 | 20 | 0.87 (0.49 to 1.54) | 1.11 (0.61 to 2.05) | 27 | 1.32 (0.75 to 2.34) | 1.04 (0.64 to 1.68) |
| Pack-years = 20-39 | 32 | 15 | 0.88 (0.48 to 1.59) | 1.08 (0.59 to 1.99) | 21 | 1.25 (0.71 to 2.20) | 1.36 (0.83 to 2.22) |
| Pack-years ≥40 | 38 | 18 | 0.77 (0.42 to 1.41) | 0.76 (0.42 to 1.35) | 25 | 0.91 (0.48 to 1.74) | 0.84 (0.48 to 1.47) |
| Intermediate or high | |||||||
| Pack-years = 0 | 506 | 129 | 1.00 (referent) | 1.00 (referent) | 276 | 1.00 (referent) | 1.00 (referent) |
| Pack-years = 1-19 | 298 | 61 | 0.75 (0.54 to 1.04) | 0.64 (0.46 to 0.91) | 142 | 0.82 (0.66 to 1.02) | 0.75 (0.60 to 0.95) |
| Pack-years = 20-39 | 216 | 58 | 0.96 (0.69 to 1.34) | 0.87 (0.63 to 1.20) | 135 | 1.09 (0.87 to 1.37) | 1.07 (0.87 to 1.33) |
| Pack-years ≥40 | 203 | 64 | 1.28 (0.94 to 1.74) | 1.08 (0.78 to 1.50) | 160 | 1.68 (1.38 to 2.04) | 1.45 (1.16 to 1.81) |
| Pinteractiond | .20 | .45 | .11 | .13 | |||
| Crohn’s-like lymphoid reaction (n = 1174) | |||||||
| Negative/low | |||||||
| Pack-years = 0 | 391 | 131 | 1.00 (referent) | 1.00 (referent) | 235 | 1.00 (referent) | 1.00 (referent) |
| Pack-years = 1-19 | 215 | 61 | 0.77 (0.55 to 1.06) | 0.73 (0.52 to 1.04) | 108 | 0.77 (0.59 to 1.01) | 0.79 (0.61 to 1.04) |
| Pack-years = 20-39 | 135 | 46 | 1.03 (0.73 to 1.46) | 1.06 (0.77 to 1.45) | 88 | 1.14 (0.85 to 1.52) | 1.25 (0.97 to 1.60) |
| Pack-years ≥40 | 148 | 54 | 1.14 (0.83 to 1.58) | 1.01 (0.72 to 1.42) | 113 | 1.44 (1.10 to 1.87) | 1.33 (1.01 to 1.75) |
| Intermediate/high | |||||||
| Pack-years = 0 | 98 | 20 | 1.00 (referent) | 1.00 (referent) | 53 | 1.00 (referent) | 1.00 (referent) |
| Pack-years = 1-19 | 70 | 6 | 0.33 (0.12 to 0.90) | 0.29 (0.10 to 0.84) | 30 | 0.75 (0.50 to 1.13) | 0.61 (0.40 to 0.94) |
| Pack-years = 20-39 | 58 | 9 | 0.58 (0.25 to 1.35) | 0.66 (0.28 to 1.55) | 31 | 0.91 (0.60 to 1.36) | 0.90 (0.60 to 1.35) |
| Pack-years ≥40 | 59 | 11 | 0.60 (0.28 to 1.31) | 0.54 (0.25 to 1.18) | 43 | 1.32 (0.93 to 1.86) | 1.13 (0.78 to 1.64) |
| Pinteractiond | .13 | .15 | .56 | .69 | |||
IPW was applied to reduce a bias due to the availability of tumor tissue after cancer diagnosis (see “Statistical Analysis” subsection for details). AJCC = American Joint Committee on Cancer; CI = confidence interval; HR = hazard ratio; IPW = inverse probability weighting; TIL = tumor-infiltrating lymphocytes.
Hazard ratios were estimated for each stratum on the basis of the patients with pack-years of cigarettes at diagnosis, using a reparameterization of the interaction term in a single regression model for the stratified analyses.
The multivariable Cox regression model initially included age, sex, year of diagnosis, family history of colorectal cancer, body mass index at diagnosis, alcohol consumption at diagnosis, physical activity at diagnosis, processed meat intake at diagnosis, total fiber intake at diagnosis, tumor location, tumor differentiation, AJCC disease stage, microsatellite instability, CpG island methylator phenotype, long interspersed nucleotide element-1 methylation level, KRAS mutation, BRAF mutation, and PIK3CA mutation. A backward elimination with a threshold of P = .05 was used to select variables in the final models.
P interaction was calculated using the Wald test for the cross-product of pack-years of cigarettes at diagnosis (ordinal; 0, 1-19, 20-39, and ≥40) and lymphocytic reaction status (binary; negative or low and intermediate or high) in Cox regression model.
We constructed prediction models for 5-year colorectal cancer–specific and overall survival based on smoking status at diagnosis, TIL status, and other clinical prognostic variables (Supplementary Methods; Supplementary Table 8 available online).
Discussion
We conducted this study to test the hypothesis that the association of smoking status at diagnosis with mortality might differ by lymphocytic reaction patterns in tumor tissue. Utilizing 1474 colorectal cancer patients with detailed information on host lifestyle risk factors as well as tumor pathological and molecular data among 4420 incident colorectal cancer patients in the 2 US prospective cohort studies, we found that current smokers had worse colorectal cancer–specific survival in the negative or low TIL group but not in the intermediate or high TIL group. Similar survival interactions were obtained between TIL grade and pack-years at diagnosis. Although validation in independent datasets is warranted, our findings provided population-based evidence that current smoking influences colorectal cancer mortality via modified effects by host immunity.
Cigarette smoke is a complex mixture of more than 4500 chemicals, many of which have been shown to modulate the function of immune cells (7,32), suggesting that these chemicals can result in the suppression of antitumor immunity, including both innate and adaptive immune response. These chemicals can enter the local tissue microenvironment and constitute etiologic field effects (33–36). Nicotine, which is a major component of cigarette smoke, and its metabolite cotinine can facilitate tumor progression by impairment of the immune system. CHRN (cholinergic receptors nicotinic subunits, also known as nicotinic acetylcholine receptors)-mediated signaling, for example, activates several tumor-promoting networks such as RAS (RAS type GTPase family)-RAF (RAF kinase)-MAPK (mitogen-activated protein kinases) and JAK (jak family tyrosine kinases)-STAT3 (signal transducer and activator of transcription 3) pathways (32,37,38). An experimental study showed that nicotine can increase immune suppression mediated by regulatory T cells via cholinergic receptor nicotinic alpha 7 subunit (39). In addition, immunosuppression effects in nicotine-treated rats were sustained for several weeks after cessation of nicotine administration, indicating that the immunosuppression effects of nicotine can have sustained effects after initial nicotine exposure (40). Acrolein, which is a toxic unsaturated aldehyde constituent of cigarette smoke, could suppress antitumor immunity through altering neutrophil function, resulting in decreasing responsiveness of CD8+ T cells to T-cell receptor triggering (41,42). Other chemicals, such as benzo (α) pyrene and hydroquinone, can also inhibit T-cell immune response (43). A population-based study showed that the number and activity of natural killer cells were decreased in regular smokers compared with nonsmokers (44). The function of the dendritic cells was suppressed in a dose- and time-dependent manner by cigarette smoke (45,46). Thus, a better understanding of the effect of smoking on antitumor immunity could have considerable clinical implications. Our findings suggest that colorectal cancer subtypes with a high-level lymphocytic reaction may be less sensitive to the adverse prognostic effects of cigarette smoking, whereas tumors with low-level lymphocytic reaction can be immunologically incompetent and may be more vulnerable to the deleterious effects of smoking.
Moreover, the immunosuppressive effect of smoking may synergize with inferior survival for colorectal cancers with weaker lymphocytic reactions. Recent studies suggest that high-level antitumor immune response may potentially contribute to better response to not only immunotherapy but also chemotherapy (12,47). Chemotherapy and radiotherapy have been shown to induce more tumor cell death in tumors containing higher numbers of immune cells than in tumors containing fewer immune cells (47). Studies also showed that current smoking was associated with shorter disease-free survival among colon cancer patients who were receiving fluorouracil-based adjuvant chemotherapy (48,49), suggesting that smoking might cause treatment resistance (50,51). Given our data suggesting the interactive influences of smoking and TIL on tumor progression, it is of interest to examine in future studies whether smoking and TIL may jointly modify responsiveness to various forms of therapy. Accordingly, integrated analyses of tumor and host characteristics, including immune profile and lifestyle factors such as smoking behavior, have become important to identify individuals with potentially better response to therapeutic and lifestyle interventions (10). Our data strengthen the link between smoking and colorectal cancer mortality, demonstrating survival interactions according to tumor immune profile and enhancing our understanding of the mechanisms through which smoking may exert its neoplastic effects via the immune system. Our data support that smoking at diagnosis may influence host antitumor responses, especially the lymphocytic reaction, influencing colorectal cancer mortality. In addition, our findings can have clinical implications. The potential health benefits of smoking cessation are underscored for cancer prevention and interception, although our findings suggest that patients with more than 10 years of smoking cessation may not have strong survival benefits relative to continuing smoking within the negative or low TIL subgroup compared with patients with less than 10 years of smoking cessation. Particularly, individuals with tumors presenting with negative or low TIL may represent a patient population in need of additional measures to improve survival, including advocacy for immediate smoking cessation or the identification of more aggressive treatment strategies compared with never or past smokers with limited pack-years of exposure.
Our study has several limitations. First, there was the possibility of unmeasured confounding. Second, data on cancer treatment were limited. However, distribution of treatment including the use of chemotherapy and molecular targeting therapy and its regimen unlikely substantially differed by lymphocytic reaction pattern in cancer tissue, because these data were not available at the time of cancer treatment decisions. Third, data on cancer recurrence were not available in this study. Given that median cancer recurrence (metastasis) was approximately 10 to 20 months (52), colorectal cancer–specific survival can be a reasonable clinical outcome for colorectal cancer in a population-based study with long-term follow-up. Fourth, given that current smoking at diagnosis was associated with smoking after diagnosis, we could not evaluate whether quitting after diagnosis would confer the same benefit as not smoking at the time of diagnosis. Lastly, the availability of tissue after colorectal cancer diagnosis might have introduced bias. However, IPW methods were used in all survival analyses to specifically reduce this potential bias.
The major strength of this study was the use of a molecular pathological epidemiology (8–10) database of colorectal carcinoma patients in 2 US nationwide prospective cohort studies that integrate clinicopathological and molecular features, long-term survival data, and lifestyle factors, including smoking status and tumor immune profile. The concept of molecular pathological epidemiology has been used widely (53–58). In particular, examining the interactions between environmental exposures and immune cells in the tumor microenvironment is an important research direction (59) and may inform strategies to improve clinical outcomes. This comprehensive database allowed us to examine an interactive prognostic association of smoking status at diagnosis and tumor lymphocytic reactions and control for a variety of potential confounders and selection bias due to tumor tissue availability. Our study population was derived from a large number of colorectal cancer patients from hospitals throughout the United States, which can contribute to increased generalizability of our findings. Nevertheless, our findings should be validated in independent studies.
In conclusion, the association of smoking behavior at diagnosis with colorectal cancer survival appears to differ by the host immune system, and tumors with negative or low lymphocytic reaction resulted in poor survival among current smokers compared with never smokers. Our findings emphasize the link between smoking and tumor immunity, both of which may interactively influence colorectal cancer progression.
Funding
This work was supported by US National Institutes of Health (NIH) grants (P01 CA87969; UM1 CA186107; P01 CA55075; UM1 CA167552; U01 CA167552; P50 CA127003 to C.S.F.; R01 CA118553 to C.S.F.; R01 CA169141 to C.S.F.; R01 CA137178 to A.T.C.; K24 DK098311 to A.T.C.; R35 CA197735 to S.O.; R01 CA151993 to S.O.; R01 CA248857 to S.O.; K07 CA190673 to R.N.; K07 CA188126 to X.Z., and R01 CA225655 to J.K.L.); by Cancer Research UK’s Grand Challenge Award (OPTIMISTICC; UK C10674/A27140 to M.G. and S.O.); by Nodal Award (2016-20) from the Dana-Farber Harvard Cancer Center (to S.O.); by Stand Up to Cancer Colorectal Cancer Dream Team Translational Research Grant (SU2C-AACR-DT22-17 to C.S.F. and M.G.), administered by the American Association for Cancer Research, a scientific partner of SU2C; and by grants from the Project P Fund, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance and SU2C. K.F. and K.H. were supported by fellowship grants from the Uehara Memorial Foundation. K.F. was supported by fellowship grants from the Grant of The Clinical Research Promotion Foundation (2018). Y.C. and L.L. were supported by a scholarship grant from Chinese Scholarship Council. K.H. was supported by fellowship grants from the Mitsukoshi Health and Welfare Foundation. T.U. and K.A. were supported by a grant from Overseas Research Fellowship (201960541 to T.U.; 201860083 to K.A) from Japan Society for the Promotion of Science. A.T.C. is a Stuart and Suzanne Steele MGH Research Scholar. M.G. is supported by an ASCO Conquer Cancer Foundation Career Development Award.
Notes
Role of the funders: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Disclosures: A.T.C. previously served as a consultant for Bayer Healthcare and Pfizer Inc. This study was not funded by Bayer Healthcare or Pfizer Inc. J.A.M has received institutional research funding from Boston Biomedical, has served as an advisor/consultant to Ignyta and COTA Healthcare, and served on a grant review panel for the National Comprehensive Cancer Network funded by Taiho Pharmaceutical. C.S.F. previously served as a consultant for Agios, Bain Capital, Bayer, Celgene, Dicerna, Five Prime Therapeutics, Gilead Sciences, Eli Lilly, Entrinsic Health, Genentech, KEW, Merck, Merrimack Pharmaceuticals, Pfizer Inc, Sanofi, Taiho, and Unum Therapeutics; C.S.F. also serves as a Director for CytomX Therapeutics and owns unexercised stock options for CytomX and Entrinsic Health. R.N. is currently employed by Pfizer Inc; she contributed to this study before she became an employee of Pfizer Inc. M.G. receives research funding from Bristol-Myers Squibb and Merck. This study was not funded by any of these commercial entities. No other conflicts of interest exist. The other authors declare that they have no conflicts of interest.
Author contributions: All authors contributed to review and revision. M.G., J.A.N., and S.O.: developed the main concept and designed the study. R.N., A.T.C., C.S.F., M.G., and S.O.: wrote grant applications. K.F., Y.C., K.H., T.U., J.K., T.H., L.L., J.B., D.A.D, M.S., A.T.C., E.L.G., J.A.M., C.S.F., R.N., J.K.L., J.A.N., and S.O.: were responsible for collection of tumor tissue, and acquisition of epidemiologic, clinical and tumor tissue data, including histopathological, immunohistochemical, and immunofluorescent characteristics. K.F., Y.C., T.U., J.K., K.H., C.S.F., R.N., X.Z., K.W., and S.O.: performed data analysis and interpretation. K.F., Y.C., K.H., T.U., J.K., and S.O.: drafted the manuscript. M.Z., K.A., J.P.V., M.C.L., S.G., S.S., N.A., T.S.T., M.S., R.N., M.G., J.A.N., X.Z., K.W., and S.O.: contributed to editing and critical revision for important intellectual contents.
Supplementary Material
References
- 1. Liang PS, Chen TY, Giovannucci E.. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124(10):2406–2415. [DOI] [PubMed] [Google Scholar]
- 2. Jayasekara H, English DR, Haydon A, et al. Associations of alcohol intake, smoking, physical activity and obesity with survival following colorectal cancer diagnosis by stage, anatomic site and tumor molecular subtype. Int J Cancer. 2018;142(2):238–250. [DOI] [PubMed] [Google Scholar]
- 3. Yang B, Jacobs EJ, Gapstur SM, et al. Active smoking and mortality among colorectal cancer survivors: The Cancer Prevention Study II nutrition cohort. J Clin Oncol. 2015;33(8):885–893. [DOI] [PubMed] [Google Scholar]
- 4. Walter V, Jansen L, Hoffmeister M, et al. Smoking and survival of colorectal cancer patients: systematic review and meta-analysis. Ann Oncol. 2014;25(8):1517–1525. [DOI] [PubMed] [Google Scholar]
- 5. Zhu Y, Yang SR, Wang PP, et al. Influence of pre-diagnostic cigarette smoking on colorectal cancer survival: overall and by tumour molecular phenotype. Br J Cancer. 2014;110(5):1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qiu F, Liang CL, Liu H, et al. Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget. 2017;8(1):268–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Talhout R, Schulz T, Florek E, et al. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health. 2011;8(2):613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ogino S, Nowak JA, Hamada T, et al. Insights into pathogenic interactions among environment, host, and tumor at the crossroads of molecular pathology and epidemiology. Annu Rev Pathol Mech Dis. 2019;14(1):83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamada T, Nowak JA, Milner DA Jr, et al. Integration of microbiology, molecular pathology, and epidemiology: a new paradigm to explore the pathogenesis of microbiome-driven neoplasms. J Pathol. 2019;247(5):615–628. doi: 10.1002/path.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogino S, Nowak JA, Hamada T, et al. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut. 2018;67(6):1168–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koh H, Hamada T, Song M, et al. Physical activity and colorectal cancer prognosis according to tumor-infiltrating T cells. JNCI Cancer Spectr. 2018;2(4):pky058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pages F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–2139. [DOI] [PubMed] [Google Scholar]
- 13. Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232(2):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haruki K, Kosumi K, Li P, et al. An integrated analysis of lymphocytic reaction, tumour molecular characteristics and patient survival in colorectal cancer. Br J Cancer. 2020;122(9):1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222(4):350–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamada T, Nowak JA, Masugi Y, et al. Smoking and risk of colorectal cancer sub-classified by tumor-infiltrating T cells. J Natl Cancer Inst. 2019;111(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61(6):847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamada T, Cao Y, Qian ZR, et al. Aspirin use and colorectal cancer survival according to tumor CD274 (programmed cell death 1 ligand 1) expression status. J Clin Oncol. 2017;35(16):1836–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seaman SR, White IR.. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. [DOI] [PubMed] [Google Scholar]
- 21. Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15(20):6412–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishihara R, Morikawa T, Kuchiba A, et al. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am J Epidemiol. 2013;178(1):84–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58(1):90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogino S, Kawasaki T, Kirkner GJ, et al. Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn. 2007;9(3):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogino S, Kawasaki T, Brahmandam M, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8(2):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Irahara N, Nosho K, Baba Y, et al. Precision of pyrosequencing assay to measure LINE-1 methylation in colon cancer, normal colonic mucosa, and peripheral blood cells. J Mol Diagn. 2010;12(2):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogino S, Kawasaki T, Brahmandam M, et al. Sensitive sequencing method for KRAS mutation detection by pyrosequencing. J Mol Diagn. 2005;7(3):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Imamura Y, Lochhead P, Yamauchi M, et al. Analyses of clinicopathological, molecular, and prognostic associations of KRAS codon 61 and codon 146 mutations in colorectal cancer: cohort study and literature review. Mol Cancer. 2014;13(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benjamin DJ, Berger JO, Johannesson M, et al. Redefine statistical significance. Nat Hum Behav. 2018;2(1):6–10. [DOI] [PubMed] [Google Scholar]
- 31. Xie J, Liu C.. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089–3110. [DOI] [PubMed] [Google Scholar]
- 32. Sanner T, Grimsrud TK.. Nicotine: carcinogenicity and effects on response to cancer treatment-a review. Front Oncol. 2015;5:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lochhead P, Chan AT, Nishihara R, et al. Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression. Mod Pathol. 2015;28(1):14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Curtius K, Wright NA, Graham TA.. An evolutionary perspective on field cancerization. Nat Rev Cancer. 2018;18(1):19–32. [DOI] [PubMed] [Google Scholar]
- 35.AEGIS Study Team. Shared gene expression alterations in nasal and bronchial epithelium for lung cancer detection. J Natl Cancer Inst. 2017;109(7):djw327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roy HK, Turzhitsky V, Wali R, et al. Spectral biomarkers for chemoprevention of colonic neoplasia: a placebo-controlled double-blinded trial with aspirin. Gut. 2017;66(2):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schaal C, Chellappan SP.. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res. 2014;12(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grando SA. Connections of nicotine to cancer. Nat Rev Cancer. 2014;14(6):419–429. [DOI] [PubMed] [Google Scholar]
- 39. Wang DW, Zhou RB, Yao YM, et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J Pharmacol Exp Ther. 2010;335(3):553–561. [DOI] [PubMed] [Google Scholar]
- 40. Geng Y, Savage SM, Razani-Boroujerdi S, et al. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J Immunol. 1996;156(7):2384–2390. [PubMed] [Google Scholar]
- 41. Governa V, Trella E, Mele V, et al. The interplay between neutrophils and CD8(+) T cells improves survival in human colorectal cancer. Clin Cancer Res. 2017;23(14):3847–3858. [DOI] [PubMed] [Google Scholar]
- 42. Urso P, Wirsiy YG, Zhang W, et al. Alterations in CD4+, CD8+, Vgamma3, Vgammadelta, and/or Valpha betaT-lymphocyte expression in lymphoid tissues of progeny after in utero exposure to benzo(alpha)pyrene. J Immunotoxicol. 2008;5(3):293–306. [DOI] [PubMed] [Google Scholar]
- 43. McCue JM, Lazis S, John Cohen J, et al. Hydroquinone and catechol interfere with T cell cycle entry and progression through the G1 phase. Mol Immunol. 2003;39(16):995–1001. [DOI] [PubMed] [Google Scholar]
- 44. Mian MF, Lauzon NM, Stampfli MR, et al. Impairment of human NK cell cytotoxic activity and cytokine release by cigarette smoke. J Leukoc Biol. 2008;83(3):774–784. [DOI] [PubMed] [Google Scholar]
- 45. Robbins CS, Dawe DE, Goncharova SI, et al. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Am J Respir Cell Mol Biol. 2004;30(2):202–211. [DOI] [PubMed] [Google Scholar]
- 46. Givi ME, Folkerts G, Wagenaar GT, et al. Cigarette smoke differentially modulates dendritic cell maturation and function in time. Respir Res. 2015;16(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Galon J, Bruni D.. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218. [DOI] [PubMed] [Google Scholar]
- 48. Phipps AI, Shi Q, Newcomb PA, et al. Associations between cigarette smoking status and colon cancer prognosis among participants in North Central Cancer Treatment Group Phase III Trial N0147. J Clin Oncol. 2013;31(16):2016–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCleary NJ, Niedzwiecki D, Hollis D, et al. Impact of smoking on patients with stage III colon cancer: results from cancer and leukemia group B 89803. Cancer. 2010;116(4):957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ye YN, Wu WK, Shin VY, et al. A mechanistic study of colon cancer growth promoted by cigarette smoke extract. Eur J Pharmacol. 2005;519(1-2):52–57. [DOI] [PubMed] [Google Scholar]
- 51. Yuge K, Kikuchi E, Hagiwara M, et al. Nicotine induces tumor growth and chemoresistance through activation of the PI3K/Akt/mTOR pathway in bladder cancer. Mol Cancer Ther. 2015;14(9):2112–2120. [DOI] [PubMed] [Google Scholar]
- 52. Meyerhardt JA, Mayer RJ.. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352(5):476–487. [DOI] [PubMed] [Google Scholar]
- 53. Wang ST, Cui WQ, Pan D, et al. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J Gastroenterol. 2020;26(6):562–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hughes LAE, Simons C, van den Brandt PA, et al. Lifestyle, diet, and colorectal cancer risk according to (Epi)genetic instability: current evidence and future directions of molecular pathological epidemiology. Curr Colorectal Cancer Rep. 2017;13(6):455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rescigno T, Micolucci L, Tecce MF, et al. Bioactive nutrients and nutrigenomics in age-related diseases. Molecules. 2017;22(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carr PR, Alwers E, Bienert S, et al. Lifestyle factors and risk of sporadic colorectal cancer by microsatellite instability status: a systematic review and meta-analyses. Ann Oncol. 2018;29(4):825–834. [DOI] [PubMed] [Google Scholar]
- 57. Gunter MJ, Alhomoud S, Arnold M, et al. Meeting report from the joint IARC-NCI international cancer seminar series: a focus on colorectal cancer. Ann Oncol. 2019;30(4):510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Myte R, Gylling B, Haggstrom J, et al. Metabolic factors and the risk of colorectal cancer by KRAS and BRAF mutation status. Int J Cancer. 2019;145(2):327–337. [DOI] [PubMed] [Google Scholar]
- 59. Ogino S, Giannakis M.. Immunoscore for (colorectal) cancer precision medicine. Lancet. 2018;391(10135):2084–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.