Abstract
Background: Targeting the interaction between SDF1 and CXCR4 may provide an opportunity to intervene in the hematopoietic stem cell mobilization process.
Aim: The present study aimed to investigate the safety, efficacy, pharmacokinetic and pharmacodynamic profiles of YF-H-2015005, a CXCR4 antagonist, for the mobilization of hematopoietic stem cells (HSCs). Methods: A total of 15 patients with non-Hodgkin's lymphoma (NHL) eligible for autologous hematopoietic stem cell transplantation were enrolled. All patients achieved a partial or complete remission after the first- or second-line therapy. Granulocyte colony stimulating factor (G-CSF) was given in the morning for 8 consecutive days, and 0.24 mg/kg YF-H-2015005 was subcutaneously administered in the evening of the 4th day of G-CSF treatment for up to four days. Apheresis was performed 9-10 hours following each dose of YF-H-2015005. Results: YF-H-2015005 was rapidly absorbed and eliminated, with Tmax and t1/2 of 0.5 and 5.04 ± 1.00 hours, respectively. Moreover, the mean peripheral blood CD34+ cell counts were elevated by 2.0- to 2.9-fold from 2 to 24 hours, and reached the maximum level of 76.5 ± 53.9 cells/kg at 10 hours after YF-H-2015005 treatment. Fourteen (93%) out of 15 NHL patients achieved a minimum target of ≥2×106/kg CD34+ cells. Furthermore, there was no grades 3-4 treatment-related adverse event observed among these patients. Conclusion: YF-H-2015005 can serve as a safe, effective agent in combination with G-CSF for CD34+ hematopoietic progenitor cell mobilization in NHL patients.
Keywords: Hematopoietic Stem Cell Mobilization; Drug Evaluation; Pharmacokinetics; Safety; Lymphoma, Non-Hodgkin's
Introduction
The interaction of CXC chemokine receptor 4 (CXCR4) and its ligand CXCL12 (also referred as stromal cell-derived factor-1, SDF-1) plays an essential role in regulating the mobilization of hematopoietic stem cells (HSCs) 1,2. SDF-1 is constitutively secreted in bone marrow stromal cells, while the glycoprotein-coupled receptor CXCR4 with SDF-1 homogeneity is mainly generated by HSCs 3,4. Therefore, targeting the interaction between SDF1 and CXCR4 may provide an opportunity to intervene the mobilization of HSCs. Plerixafor, a CXCR4 antagonist, is able to enhance the steady-state release of SDF-1 into the bloodstream of both mice and non-human primates 5, and can lead to a 3- to 6-fold elevation in the absolute numbers of peripheral blood (PB) CD34+ cells in healthy volunteers 6. It is worth noting that plerixafor can exhibit high effectiveness on the mobilization of HSCs in patients with non-Hodgkin's lymphoma (NHL) or multiple myeloma 7. In 2008, plerixafor was approved by the U.S. Food and Drug Administration for the mobilization of HSCs to the PB for collection and subsequent autologous hematopoietic stem cell transplantation (HSCT) 8.
YF-H-2015005 (l,1'-[1,4-phenylenebis(methylene)]-bis-1,4,8,11-tetraazacyclotetradecane) was a CXCR4 antagonist developed by Hefei Yifan Biopharmaceuticals Inc. (Anhui, China). It displayed the structural formula C28H54N8, with a molecular mass of 502.79 g/mol. The molecular structure of YF-H-2015005 was illustrated in Figure 1. Preclinical trials have demonstrated that YF-H-2015005 is effective and safe for enhancing the mobilization of HSCs. In this study, we determined the efficacy, safety, pharmacokinetic (PK) and pharmacodynamics (PD) profiles of YF-H-2015005 in combination with granulocyte colony stimulating factor (G-CSF) for CD34+ HSC mobilization in NHL patients. Furthermore, the enhancement effect of YF-H-2015005 on HSC mobilization in NHL patients was also evaluated.
Figure 1.
Structural formula of YF-H-2015005.
Materials and Methods
Study design
The ethical approval for this open-label, single arm study was obtained from the Ethics Committee of the Peking University Cancer Hospital and Institute (Beijing, China), and all procedures were conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to study enrollment. This study was registered on www.chinadrugtrials.org.cn (registration number: CTR20170925).
The inclusion criteria of this study were: (i) NHL patients eligible for autologous HSCT, males and females, aged 18-65 years; (ii) achieving a partial or complete remission after the first- or second-line therapy; (iii) an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1; (iv) did not overlap with the toxicity of other chemotherapeutic or anti-cancer drugs; (v) an actual body weight less than 175% of ideal body weight; (vi) negative for bone marrow involvement within 45 days prior to enrollment; and (vii) negative for human immunodeficiency and hepatitis viruses.
The main exclusion criteria were as follows: (i) chronic lymphocytic leukemia; (ii) failed previous HSC collections; (iii) history of allogeneic or autologous HSCT; (iv) history of pelvic radiotherapy; and (v) secondary central nervous system involvement. In addition, NHL patients with creatinine clearance rate <50 mL/min as well as abnormal liver function test results (>2.5 times upper limit of normal) were excluded.
Combination treatment of G-CSF and YF-H-2015005
All patients were given a daily subcutaneous dose of G-CSF (10 µg/kg/day) each morning for 8 consecutive days. YF-H-2015005 with a dose of 0.24 mg/kg was subcutaneously initiated on the evening of the 4th day of G-CSF treatment and continued for up to 4 days. Apheresis was performed 9-10 hours following evening dose of YF-H-2015005. This process was continued daily for up to 4 days or until ≥2×106/kg CD34+ cells were collected.
PK and PD evaluation
For PK analysis, blood samples were collected immediately before starting YF-H-2015005 treatment, and consecutively at 0.25, 0.5, 1, 2, 4, 6, 8, 10, 16 and 24 hours following its administration and prior to apheresis. HSC apheresis was carried out using a COM.TEC instrument (Fresenius Kabi, Germany). Then, 4 mL of peripheral venous blood sample was collected into the heparin sodium tube at each time point of PK sampling. The tube was inverted 5 times and immediately placed on ice. After centrifuging at 3000 rpm for 10 minutes, the resulting plasma was kept at -70°C. The plasma from each sample was split into two approximately equal aliquots.
All samples were evaluated by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). The linear range of the assay was 2 to 1000 ng/mL. The precision values of the quality control samples (6, 40, 400 and 750 ng/mL) were all ≤8.5%, with a bias of -2.7~13.4%. The PK parameters analyzed included the area under the curve from time 0 to t (AUC0-t), area under the curve from time 0 to infinity (AUC0-∞), maximum observed concentration (Cmax), time to achieve Cmax (Tmax), apparent plasma clearance (CL), apparent volume of distribution (Vz), and apparent terminal elimination half-life (t1/2). When multiple doses of YF-H-2015005 were administered, PK parameters were consisted of the steady-state trough concentration (Cssmin), steady-state peak concentration (Cssmax), average steady-state plasma drug concentration (Cav), steady-state area under the curve (AUCss0-24), coefficient of fluctuation (Fl), and accumulation ratio (Ra). The values for all PK parameters were calculated with non-compartmental analysis using WinNonlin Professional version 6.3 software (Pharsight Corp, Mountain View, CA, USA).
For PD analysis, blood samples were collected immediately prior to dosing with YF-H-2015005 and then at 2, 4, 6, 8, 10, 16, and 24 hours after its administration. Four milliliter sample of peripheral venous blood was collected into the heparin sodium tube at each time point. The absolute number and percentage of CD34 cells were assessed by fluorescence-activated cell sorting method using a Beckman-Coulter FC500 flow cytometer and appropriate reagents.
Safety and efficacy assessments
The safety profiles were evaluated by clinical and laboratory parameters, including adverse event (AE), serious AE (SAE), treatment-related AE, treatment-related SAE, and the AEs were monitored throughout the entire study period. All the clinical signs and symptoms were recorded, and a further laboratory examination or electrocardiogram was carried out (as dictated by the occurrence of AE).
The efficacy endpoint was the proportion of NHL patients treated with YF-H-2015005 who achieved a minimum collection target of ≥2×106/kg CD34+ cells (actual body weight) within 4 days of apheresis.
Statistical analysis
All statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, New York, USA). Continuous variables were determined using the Student's t-test. Categorical variables were analyzed using the Pearson's chi-squared test or Fisher's exact test. All tests applied were two-tailed, and a p-value of less than 0.05 was deemed to be statistically significant.
Results
Baseline characteristics
In this study, 15 NHL patients (11 males and 4 females) were recruited, and none of them received fludrarabine or lenalidomide before HSC mobilization. The median age was 51 years. About 67% of patients had mature B-cell lymphoma. The median cycles of prior chemotherapy was 6. None received radiotherapy. Table 1 revealed the demographic and disease characteristics of the enrolled NHL patients.
Table 1.
Baseline characteristics of 15 patients treated with YF-H-2015005
| Characteristics | All patients (%) |
|---|---|
| Age, years | |
| Mean (SD) | 47.8 (10.8) |
| Median (range) | 51.0 (32.0-64.0) |
| Ethnicity, n (%) | |
| Han | 14 (93.3) |
| Others | 1 (6.7) |
| Gender, n (%) | |
| Male | 11 (73.3) |
| Female | 4 (26.7) |
| Body weight, kg | |
| Mean (SD) | 71.3 (13.0) |
| Median (range) | 74.0 (50.0-86.0) |
| Pathology type | |
| Lymphoblastic lymphoma | 1 (6.7) |
| Peripheral T/NK cell lymphoma | 4 (26.7) |
| ALK-negative ALCL/ PTCL/ AITL | 1/1/2 |
| Mature B-cell lymphoma | 10 (66.6) |
| DLBCL/MCL/BL/tFL/ Unclassified | 5/2/1/1/1 |
| Stage | |
| I | 2 (13.3) |
| II | 5 (33.4) |
| III | 2 (13.3) |
| IV | 6 (40.0) |
| Lines of chemotherapy | |
| First line | 10 (66.7) |
| Second line | 5 (33.3) |
| Cycles of chemotherapy | |
| Mean (SD) | 7 (3) |
| Median (range) | 6 (4-12) |
| Radiotherapy | |
| Yes | 0 (0.0) |
| No | 15 (100.0) |
| Disease status | |
| Complete remission | 11 (73.3) |
| Partial remission | 4 (26.7) |
AITL, angioimmunoblastic T-cell lymphoma; ALCL, ALK-, anaplastic cell lymphoma, anaplastic lymphoma kinase negative; BL, Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; PTCL NOS, peripheral T-cell lymphoma, not otherwise specified; SD, standard deviation; tFL, transformed follicular lymphoma.
PK and PD profiles of YF-H-2015005
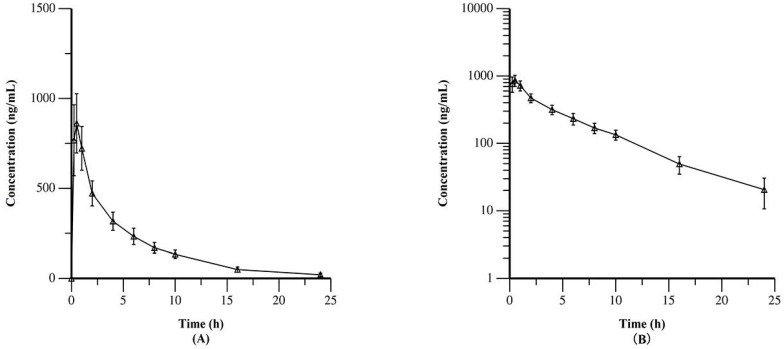
Table 2 summarized the PK data of YF-H-2015005. The results demonstrated that YF-H-2015005 was rapidly absorbed in all 15 patients without a lag time after its first administration. Moreover, its median values of Tmax and median biological half-life (t1/2) were 0.5 and 5.04 hours, respectively. Figure 2 showed the mean plasma concentrations of YF-H-2015005 over time on both linear and semilogarithmic scales. Only one patient received all 4 consecutive doses of YF-H-2015005 as stipulated in the study protocol, and hence the PK parameters of steady state were calculated based on this patient (Appendix Table S1). After administrating multiple doses of YF-H-2015005, the Cssmin, Cssmax, Tmax, t1/2, steady-state AUCss0-24, F1 and Ra of this patient were 21.6 ng/mL, 602 ng/mL, 0.5 hours, 5.83 hours, 3210 h· ng/mL, 433.9% and 1.06, respectively.
Table 2.
Values for PK parameters after a single dose of YF-H-2015005 (0.24 mg/kg, s.c.) (n = 15)
| PK parameters | Values (Mean ± SD) |
|---|---|
| t1/2, h | 5.04 ± 1.00 |
| Tmax, h* | 0.50 (0.25, 1.00) |
| Cmax, ng/mL | 876 ± 171 |
| AUC0-10h, h·ng/mL | 3336 ± 481.2 |
| AUC0-24h, h·ng/mL | 4166 ± 604.3 |
| AUC0-∞, h·ng/mL | 4328 ± 637.1 |
| Vz, L/kg | 0.4 ± 0.1 |
| CL, L/h/kg | 0.06 ± 0.01 |
*Indicated as the median (minimum, maximum).
AUC0-10h, area under the curve from time 0 to 10h; AUC0-24h, area under the curve from time 0 to 24h; AUC0-∞, area under the curve from time 0 to infinity; CL, apparent clearance; Cmax, maximum observed concentration; PK, pharmacokinetic; t1/2, apparent terminal half-life; Tmax, observed maximum concentration; Vz, apparent volume of distribution.
Figure 2.
Mean plasma concentrations of YF-H-2015005 over time as expressed on linear (A) and semilogarithmic (B) scales.
Table 3 summarized the PD characteristics of YF-H-2015005 in NHL patients. Following YF-H-2015005 treatment, the mean PB CD34+ cell counts increased by 2.0- to 2.9-fold over baseline from 2 to 24 hours, and reached the maximum level of 76.5 ± 53.9 cells/μL at 10 hours after dosing. As mentioned above, only 1 patient received 4 doses of YF-H-2015005. The PD characteristics of YF-H-2015005 in this patient was summarized in Appendix Table S2.
Table 3.
Changes in peripheral blood CD34+ cell count after the first dose of YF-H-2015005
| Time (hrs) | CD34+ cell count (Mean ± SD, cells/μL) |
Fold-increase in CD34+ cell count (Mean ± SD) |
|---|---|---|
| 0 | 27.2 ± 21.1 | - |
| 2 | 67.5 ± 44.7 | 2.6 ± 1.0 |
| 4 | 73.4 ± 46.3 | 2.8 ± 1.0 |
| 6 | 73.0 ± 47.9 | 2.8 ± 0.7 |
| 8 | 72.4 ± 46.4 | 2.8 ± 0.8 |
| 10 | 76.5 ± 53.9 | 2.9 ± 0.6 |
| 16 | 52.6 ± 30.4 | 2.2 ± 0.7 |
| 24 | 48.1 ± 25.4 | 2.0 ± 0.7 |
SD, standard deviation.
Efficacy of YF-H-2015005 for HSC mobilization
Fourteen (93%) patients met the endpoint of ≥2×106/kg CD 34+ cells being collected after a median of 1.0 day of apheresis (range: 1-2 days). The median amount of CD34+ cells collected was 3.3×106 cells/kg (range: 1.5-10.5×106 cells/kg). The target collection was obtained from 10 patients after only one apheresis session and from 4 patients after two apheresis sessions. Among those patients, 4 (27%) patients achieved the maximal CD34+ cell level of ≥5×106 cells/kg. The remaining 1 patient yielded 1.5×106 cells/kg following 4 days of apheresis.
Safety of YF-H-2015005
All patients had at least one AE. As shown in Table 4, treatment-related AEs were observed in 13 (87%) patients, and all of them were grade 1-2. The most frequently reported treatment-related AEs were increased lactate dehydrogenase (67%), followed by hot flashes (13%), diarrhea (7%), insomnia (7%), and hypoglycemia (7%). However, no treatment-related SAEs were observed.
Table 4.
Summary of safety
| Number (%) | |
|---|---|
| Adverse event | 15 (100.0) |
| Treatment-related adverse event | 13 (86.7) |
| Serious adverse event | 0 (0.0) |
| Treatment-related serious adverse event | 0 (0.0) |
| Common treatment-related adverse events | |
| Increased lactate dehydrogenase | 10 (66.7) |
| Hot flashes | 2 (13.3) |
| Diarrhea | 1 (6.7) |
| Insomnia | 1 (6.7) |
| Hyperhidrosis | 1 (6.7) |
Discussion
Autologous HSCT is the standard therapeutic modality for patients with NHL 9-12. The successful mobilization of PB stem cells and adequate stem cell collection are of critical importance, as the infused CD34+ cell dose can affect the engraftment of platelet and neutrophils 13. The optimal amount of CD34+ cells that causes rapid and sustained recovery is determined to be >5×106 cells/kg, while the minimum threshold amount is deemed as >2×106 cells/kg 14. However, failure to achieve sufficient HSC mobilization represents a great challenge in clinical practice, especially for supporting subsequent high-dose therapy and autologous HSCT 15. In a retrospective study 16 conducted on 1,775 patients who underwent stem cell mobilization, 47% of the patients had a suboptimal stem cell collection (CD34+ cell level <5×106 cells/kg), among which, 25% had a low collection (2-5×106 cells/kg), 10% had a poor collection (<2×106 cells/kg), and 12% failed collection. Hence, there remains an urgent need to develop more effective, safer, and better tolerated treatment regimens for the mobilization of HSCs.
The greater effectiveness of using plerixafor in combination with G-CSF compared to G-CSF treatment alone has been demonstrated by a double-blind, randomized, placebo-controlled study 17. A total of 298 NHL patients were allocated to receive G-CSF in combination with plerixafor or a placebo. The proportion of NHL patients who achieved the primary endpoint (≥5×106 CD34+ cells/kg) was higher in plerixafor group (59%) than in placebo group (20%). It has been reported that plerixafor exhibits similar efficacy in a Chinese population 18. In this randomized, double-blind study involving 101 patients with NHL, the number of patients who achieved a target collection of >5×106 CD34+ cells/kg (62% vs. 20%) or >2×106 CD34+ cells/kg (88% versus 66%) and underwent autologous transplantation (88% versus 68%) was higher in the plerixafor arm when compared to those in the placebo arm. Based on these prospective randomized studies, pre-emptive use of plerixafor for those patients at risk for HSC mobilization failure has been recommended in clinical practice guidelines 14,15,19.
In the present study, the PK and PD of YF-H-2015005 in patients with NHL were evaluated. The PK findings revealed that YF-H-2015005 was rapidly absorbed and eliminated, with a mean t1/2 of 5.04 hours. Moreover, the numbers of CD34+ cells in PB samples increased significantly after YF-H-2015005 administration. Treatment with YF-H-2015005 could result in a mean 2.9-fold elevation of PB CD34+ cells within 10 hours following YF-H-2015005 administration, by which the mean absolute PB CD34+ cells elevated from 27.2 to 76.5 cells/μL. Moreover, 14 (93%) patients achieved the endpoint for CD34+ cell collection (≥2×106 cells/kg), with a median CD34+ cell count of 3.35×106 cells/kg. These findings of PK and PD data supported the use of YF-H-2015005 dosing regimen and determined the optimal timing of apheresis.
More importantly, the target cell collection was obtained in 67% of patients after a single apheresis session and in 27% of patients after 2 apheresis sessions, which could lead to a reduction in health care costs (e.g., apheresis and hospitalization costs). Only 1 patient did not achieve the minimum target of ≥2×106 CD34+ cells/kg required for HSCT; however, similar to the other 14 patients, this patient exhibited a ≥2-fold elevation in PB CD34+ cells. In addition, all AEs were mild and transient with no SAEs reported. These findings indicated that YF-H-2015005 is an effective and safe agent for mobilizing HSCs in NHL patients.
In conclusion, our findings clearly demonstrated the potential of YF-H-2015005 as a safe, effective agent for HSC mobilization and subsequent collection by apheresis in patients with NHL. The rapid time course of YF-H-2015005-induced CD34+ cell mobilization as well as the relative paucity of adverse effects further supported the clinical applicability of this agent. The magnitude of an increased amount of PB CD34+ cells after treatment with 0.24 mg/kg YF-H-2015005 was sufficient to indicate the clinical significance of this agent for mobilizing HSCs.
Supplementary Material
Supplementary tables.
Acknowledgments
The authors wish to thank all participating patients, hemato-oncologists, pathologists, statisticians and the team of HSC mobilisation and apheresis for their invaluable contributions to this research project.
Abbreviations
- AE
adverse event
- AUC0-10h
area under the curve from time 0 to 10h
- AUC0-24h
area under the curve from time 0 to 24h
- AUC0-∞
area under the curve from time 0 to infinity
- AUCSS
steady-state area under the curve
- Cav
average steady-state plasma drug concentration
- CL
apparent clearance
- Cmax
maximum observed concentration
- Css.max
steady-state peak concentration
- Css,min
steady-state trough concentration
- CXCR4
CXC chemokine receptor 4
- ECOG
Eastern Cooperative Oncology Group
- Fl
coefficient of fluctuation
- G-CSF
granulocyte colony stimulating factor
- HSCs
hematopoietic stem cells
- NHL
non-Hodgkin's lymphoma
- PB
peripheral blood
- PD
pharmacodynamics
- PK
pharmacokinetic
- SDF-1
stromal cell-derived factor-1
- Ra
accumulation ratio
- SAE
serious AE
- t1/2
apparent terminal half-life
- Tmax
observed maximum concentration
- Vz
apparent volume of distribution
References
- 1.Karpova D, Ritchey JK, Holt MS. et al. Continuous blockade of CXCR4 results in dramatic mobilization and expansion of hematopoietic stem and progenitor cells. Blood. 2017;129:2939–2949. doi: 10.1182/blood-2016-10-746909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller TA, Pennisi S, Zwick A. et al. PIM1 inhibition effectively enhances plerixafor-induced HSC mobilization by counteracting CXCR4 upregulation and blocking CXCL12 secretion. Leukemia. 2019;33:1296–1301. doi: 10.1038/s41375-019-0428-6. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi N, Zhang TT, Nakanishi T. Involvement of CXCR4 in Normal and Abnormal Development. Cells. 2019;8(2):185. doi: 10.3390/cells8020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugiyama T, Omatsu Y, Nagasawa T. Niches for hematopoietic stem cells and immune cell progenitors. Int Immunol. 2019;31:5–11. doi: 10.1093/intimm/dxy058. [DOI] [PubMed] [Google Scholar]
- 5.Dar A, Schajnovitz A, Lapid K. et al. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25:1286–1296. doi: 10.1038/leu.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liles WC, Broxmeyer HE, Rodger E. et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 7.Stewart DA, Smith C, MacFarland R, Calandra G. Pharmacokinetics and pharmacodynamics of plerixafor in patients with non-Hodgkin lymphoma and multiple myeloma. Biol Blood Marrow Transplant. 2009;15:39–46. doi: 10.1016/j.bbmt.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Brave M, Farrell A, Ching Lin S. et al. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to peripheral blood for collection and subsequent autologous transplantation. Oncology. 2010;78:282–8. doi: 10.1159/000315736. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Ji X, Song Y. et al. Improving survival of 3760 patients with lymphoma: Experience of an academic center over two decades. Cancer Med. 2020;9(11):3765–3774. doi: 10.1002/cam4.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philip T, Guglielmi C, Hagenbeek A. et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–5. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 11.Wu M, Wang X, Xie Y. et al. Outcome and Prospective Factor Analysis of High-dose Therapy Combined with Autologous Peripheral Blood Stem Cell Transplantation in Patients with Peripheral T-cell Lymphomas. Int J Med Sci. 2018;15(9):867–874. doi: 10.7150/ijms.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Liu J, Song Y. et al. Burden of Lymphoma in China, 2006-2016: An Analysis of the Global Burden of Disease Study 2016. J Hematol Oncol. 2019;12(1):115. doi: 10.1186/s13045-019-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2014;28:31–40. doi: 10.1016/j.blre.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duong HK, Savani BN, Copelan E. et al. Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014;20:1262–73. doi: 10.1016/j.bbmt.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Douglas KW, Gilleece M, Hayden P. et al. UK consensus statement on the use of plerixafor to facilitate autologous peripheral blood stem cell collection to support high-dose chemoradiotherapy for patients with malignancy. J Clin Apher. 2018;33:46–59. doi: 10.1002/jca.21563. [DOI] [PubMed] [Google Scholar]
- 16.Gertz MA, Wolf RC, Micallef IN, Gastineau DA. Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone Marrow Transplant. 2010;45:1396–403. doi: 10.1038/bmt.2009.370. [DOI] [PubMed] [Google Scholar]
- 17.DiPersio JF, Micallef IN, Stiff PJ. et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27:4767–73. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Huang H, Chen H. et al. Plerixafor and granulocyte-colony-stimulating factor for mobilization of hematopoietic stem cells for autologous transplantation in Chinese patients with non-Hodgkin's lymphoma: a randomized Phase 3 study. Transfusion. 2018;58:81–87. doi: 10.1111/trf.14426. [DOI] [PubMed] [Google Scholar]
- 19.Mohty M, Hübel K, Kröger N. et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(7):865–872. doi: 10.1038/bmt.2014.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.