Abstract
Aims/Introduction
The incidence of severe hypoglycemia and its risk factors including an insulin‐sensitizing adipokine, adiponectin, were prospectively investigated in Japanese patients with type 1 or insulin‐treated type 2 diabetes.
Materials and Methods
A total of 207 participants with type 1 diabetes (mean age 55 years) and 1,396 with insulin‐treated type 2 diabetes (mean age 65 years) from the local diabetes registry were followed for 5 years (follow‐up rate 99%). Severe hypoglycemia was defined as events requiring the assistance of others for recovery from hypoglycemia.
Results
The incidence of severe hypoglycemia was 9.2 per 100 person‐years in those with type 1 diabetes, and 2.3 per 100 person‐years in those with insulin‐treated type 2 diabetes, respectively. For type 1 diabetes, the risk was significant in those with a history of severe hypoglycemia within the previous year, slow eating and higher serum adiponectin (the highest vs the lowest in quartile hazard ratio 2.36, 95% confidence interval 1.22–4.69). For insulin‐treated type 2 diabetes, the risk included age ≥65 years, history of severe hypoglycemia within the previous year, alcohol consumption ≥60 g/day, larger insulin dose and higher serum adiponectin (the highest vs the lowest in quartile, hazard ratio 2.95, 95% confidence interval 1.22–4.69). For all participants, the incidence of severe hypoglycemia increased along with serum adiponectin (age‐ and sex‐adjusted hazard ratio 1.65 per 1 standard deviation increase of log serum adiponectin, 95% confidence interval 1.45–1.87).
Conclusions
The incidence of severe hypoglycemia was prospectively determined, and the association between severe hypoglycemia and higher serum adiponectin was observed in Japanese patients with type 1 and insulin‐treated type 2 diabetes.
Keywords: Adiponectin, Hypoglycemia, Insulin
The incidence of severe hypoglycemia was prospectively determined in Japanese patients with type 1 and insulin‐treated type 2 diabetes. The development of severe hypoglycemia was associated with higher serum adiponectin level.

Introduction
Although new antidiabetic drugs with fewer hypoglycemic adverse effects have been introduced into treatment for patients with type 2 diabetes mellitus, insulin therapy is essential for those with type 1 and insulin‐deficient type 2 diabetes. Therefore, the risk for developing hypoglycemia is inevitable in insulin‐treated patients, and severe hypoglycemia might be fatal through hypoglycemic encephalopathy or accident trauma 1 , 2 . In addition, episodes of severe hypoglycemia are associated with increased risks of cardiovascular disease and mortality in patients with type 2 diabetes 3 , 4 . The incidence of severe hypoglycemia and its risk factors have been widely investigated 1 , 2 , and recent surveys in Europe showed that the incidence of severe hypoglycemia declined in those with type 1 diabetes 5 and older adults with type 2 diabetes 6 .
The incidence of severe hypoglycemia has been reported in large clinical trials of intensive treatment or new drugs. However, severe hypoglycemia in the real world is more important in clinical practice and the public healthcare system, and the incidence of severe hypoglycemia is usually higher in real‐world observational studies than clinical trials 7 . Therefore, the present study using patients’ registry data was designed to investigate the incidence of severe hypoglycemia and its risk factors in participants with type 1 diabetes and insulin‐treated type 2 diabetes, which have not been studied prospectively in Japan.
Risk factors for severe hypoglycemia include long‐standing diabetes, prior hypoglycemic events, diabetic complications, comorbid conditions, excessive alcohol intakes and so on 1 , 2 . Furthermore, the susceptibility to severe hypoglycemia might be affected by insulin sensitivity. Recently, a number of adipokines secreted from adipose tissue have been identified, and their physiological function has been extensively studied. Among them, adiponectin is a potent insulin‐sensitizing adipokine, and the serum adiponectin level is reduced in those with insulin resistance 8 . However, the relationship of serum adiponectin with hypoglycemia has not been reported in insulin‐treated patients to our knowledge. In this context, we investigated whether the occurrence of severe hypoglycemia was associated with serum adiponectin level in those with type 1 diabetes and insulin‐treated type 2 diabetes.
Methods
Study participants
The Fukuoka Diabetes Registry is a multicenter prospective study of 5,131 participants with diabetes mellitus aged ≥20 years attending diabetes clinics or teaching hospitals certified by the Japan Diabetes Society in Fukuoka Prefecture, Japan (UMIN Clinical Trial Registry 000002627) 9 . The exclusion criteria were: (i) end‐stage renal failure requiring dialysis; (ii) steroid‐induced diabetes; and (iii) serious diseases other than diabetes, such as advanced malignant tumors. The registration of participants was carried out between April 2008 and October 2010. For the present investigation, 207 patients with type 1 diabetes and 1,396 patients with insulin‐treated type 2 diabetes were enrolled. The study was approved by the Kyushu University Institutional Review Board (approval no. 290, date 4 January 2008), and conforms to the provisions of the Declaration of Helsinki with written informed consent.
Clinical evaluation
Information regarding duration of diabetes, amount of alcohol intake, current smoking habits and leisure‐time physical activity was obtained using self‐administered questionnaires at baseline evaluation. Leisure‐time physical activity was estimated in terms of metabolic equivalent hours per week 10 . The speed of eating was evaluated by a self‐administered questionnaire (Gender Medical Research, Tokyo, Japan) 11 , as previously reported 12 . Prescribed medications were checked by reviewing participants’ medical charts. Hemoglobin A1c (HbA1c) was measured by high‐performance liquid chromatography (Tosoh Corp., Tokyo, Japan). Estimated glomerular filtration rates were determined using the equation based on age, sex and serum creatinine for Japanese people 13 , and estimated glomerular filtration rate <60 mL/min/1.73 m2 was considered as chronic kidney disease (CKD). Serum total adiponectin was measured by latex immunonephelometry (Mitsubishi Chemical Medience, Tokyo, Japan) with the intra‐assay and interassay coefficients <2%, and correlation with enzyme‐linked immunosorbent assay, r = 0.99 14 .
Assessment of severe hypoglycemia
Severe hypoglycemia was defined as events requiring the assistance of others for recovery from hypoglycemia 15 . A history of severe hypoglycemia during the year before baseline was obtained at enrollment. The development of severe hypoglycemia was investigated by self‐administered questionnaires and reviewing medical charts annually for 5 years. The reliability of self‐administered questionnaires was evaluated in the medical charts of 649 participants. There was no inconsistency of the occurrence and non‐occurrence of severe hypoglycemia between the self‐administered questionnaires and participants’ medical charts. The follow‐up period was the time from the enrollment to the first event of severe hypoglycemia, death or the planned time.
Statistical analysis
Adiponectin was log‐transformed due to a skewed distribution, back‐transformed and expressed with the 95% confidence interval (CI). Comparisons of continuous variables were carried out by Student’s t‐tests, and comparisons of categorical variables by χ2‐tests. Risks for developing severe hypoglycemia were determined using multivariable Cox proportional hazards models. Multivariate adjustments included age, sex, duration of diabetes, body mass index (BMI), history of severe hypoglycemia within the previous year, self‐reported eating speed (fast, normal, slow), HbA1c, serum adiponectin (quartile one <9.8 μg/mL, quartile two 9.8–14.5 μg/mL, quartile three 14.5–21.7 μg/mL, quartile four ≥21.7 μg/mL) and CKD in people with type 1 diabetes, and age, sex, history of severe hypoglycemia within the previous year, alcohol consumption (null, <30 g/day, 30–60 g/day, ≥60 g/day), BMI, HbA1c, serum adiponectin (quartile one <6.0 μg/mL, quartile two 6.0–8.8 μg/mL, quartile three 8.8–13.1 μg/mL, quartile four ≥13.1 μg/mL), insulin dose (quartile one <0.27 U/kg, quartile two 0.27–0.41 U/kg, quartile three 0.41–0.60 U/kg, quartile four ≥ 0.60 U/kg) and CKD in people with insulin‐treated type 2 diabetes. The results are shown as the hazard ratio (HR) with 95% CI. Statistical analyses were carried out using JMP software (version 12; SAS Institute Inc., Cary, NC, USA), and P < 0.05 was set to be statistically significant.
Results
The mean age and duration of diabetes were 55 years and 17 years for all participants with type 1 diabetes, and 65 years and 20 years for people with insulin‐treated type 2 diabetes, respectively. As shown in Figure 1, severe hypoglycemia developed in 80 of 207 participants with type 1 diabetes (median follow‐up period 5.2 years, follow‐up rate 99.5%), and 156 of 1,396 participants with insulin‐treated type 2 diabetes (median follow‐up period 5.3 years, follow‐up rate 98.5%), respectively. The incidence of severe hypoglycemia was 9.2/100 person‐years in those with type 1 diabetes, and 2.3/100 person‐years in those with insulin‐treated type 2 diabetes, respectively. Age‐ and sex‐adjusted HR in those with type 1 diabetes was 5.14 (95% CI 3.83–6.84), as compared with insulin‐treated type 2 diabetes.
Figure 1.
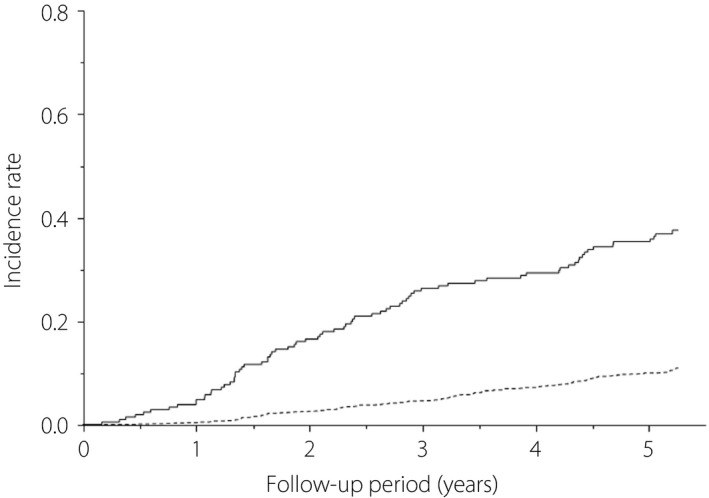
Kaplan–Meier curves for severe hypoglycemia in Japanese participants with type 1 diabetes (solid line) and insulin‐treated type 2 diabetes mellitus (broken line).
Table 1 summarizes the clinical characteristics of participants with type 1 diabetes and insulin‐treated type 2 diabetes with or without incident severe hypoglycemic events. In type 1 diabetes, there were no significant differences between those with and without severe hypoglycemia in age, sex, duration of diabetes, BMI, smoking rate, alcohol consumption, leisure‐time physical activity, HbA1c, number of insulin injections, insulin dose or the prevalence of self‐monitoring of blood glucose. However, those with a history of severe hypoglycemia within the previous year, serum adiponectin, and the prevalence of being a slow eater and CKD were increased in those with severe hypoglycemic compared with those without. For insulin‐treated type 2 diabetes, participants with severe hypoglycemia were older, had longer duration of diabetes, lower BMI, higher serum adiponectin, larger insulin dose and a higher prevalence of a history of severe hypoglycemia within the previous year, being a heavy drinker and CKD, as compared with those without. No significant differences were seen between those with and without severe hypoglycemia in sex, current smoking rate, leisure‐time physical activity, eating speed, HbA1c, users of oral hypoglycemic agents or sulfonylurea, the prevalence of self‐monitoring of blood glucose or the number of insulin injections.
Table 1.
Baseline characteristics of participants with type 1 diabetes and insulin‐treated type 2 diabetes with or without incident severe hypoglycemia
| Severe hypoglycemia | Type 1 diabetes | Insulin‐treated type 2 diabetes | ||||
|---|---|---|---|---|---|---|
| Total | − | + | Total | − | + | |
| n | 207 | 127 | 80 | 1,396 | 1,240 | 156 |
| Age (years) | 55.0 ± 14.9 | 54.2 ± 14.7 | 56.3 ± 15.2 | 65.0 ± 11.1 | 64.7 ± 11.3 | 67.3 ± 9.6** |
| <65 years | 151 (73%) | 96 (76%) | 55 (69%) | 623 (45%) | 573 (46%) | 50 (32%)** |
| 65–75 years | 37 (18%) | 24 (19%) | 13 (16%) | 543 (39%) | 475 (38%) | 68 (44%) |
| ≥75 years | 19 (9%) | 7 (6%) | 12 (15%) | 230 (16%) | 192 (15%) | 38 (24%) |
| Male | 77 (37%) | 45 (35%) | 32 (40%) | 749 (54%) | 664 (54%) | 85 (54%) |
| Duration of diabetes (years) | 17.3 ± 10.2 | 16.4 ± 10.5 | 18.6 ± 9.6 | 19.9 ± 10.9 | 19.7 ± 10.9 | 21.7 ± 10.6* |
| BMI (kg/m2) | 22.3 ± 3.1 | 22.6 ± 3.1 | 21.8 ± 3.1 | 23.7 ± 3.7 | 23.8 ± 3.7 | 23.0 ± 3.8* |
| History of severe hypoglycemia within the previous year | 44 (21%) | 16 (13%) | 28 (35%)*** | 78 (6%) | 58 (5%) | 20 (13%)*** |
| Current smoker | 46 (22%) | 28 (22%) | 18 (23%) | 290 (21%) | 259 (21%) | 31 (20%) |
| Alcohol consumption (g/day) | ||||||
| Null | 128 (62%) | 78 (61%) | 50 (63%) | 938 (67%) | 834 (67%) | 104 (67%)* |
| <30 | 60 (29%) | 38 (30%) | 22 (28%) | 338 (24%) | 300 (24%) | 38 (24%) |
| 30–60 | 14 (7%) | 8 (6%) | 6 (8%) | 77 (6%) | 73 (6%) | 4 (3%) |
| ≥60 | 5 (2%) | 3 (2%) | 2 (3%) | 43 (3%) | 33 (3%) | 10 (6%) |
| Leisure‐time physical activity (Met·h/week) | 16.8 ± 18.5 | 16.0 ± 16.7 | 18.6 ± 21.0 | 17.9 ± 18.9 | 18.2 ± 18.8 | 15.6 ± 19.4 |
| Self‐reported eating speed | ||||||
| Fast | 82 (40%) | 62 (49%) | 20 (25%)*** | 619 (44%) | 556 (45%) | 63 (40%) |
| Normal | 81 (39%) | 47 (37%) | 34 (43%) | 486 (35%) | 433 (35%) | 53 (34%) |
| Slow | 44 (21%) | 18 (14%) | 26 (33%) | 291 (21%) | 251 (20%) | 40 (26%) |
| HbA1c (%) | 8.09 ± 1.06 | 8.12 ± 1.08 | 8.04 ± 1.02 | 7.80 ± 1.16 | 7.80 ± 1.16 | 7.81 ± 1.14 |
| HbA1c (mmol/mol) | 64.9 ± 11.5 | 65.2 ± 11.8 | 64.4 ± 11.2 | 61.8 ± 12.7 | 61.7 ± 12.7 | 61.9 ± 12.5 |
| Serum adiponectin (μg/mL) | 14.4 (13.4–15.6) | 13.3 (12.1–14.7) | 16.4 (14.5–18.6)** | 9.6 (9.3–9.9) | 9.4 (9.1–9.7) | 11.8 (10.8–12.8)*** |
| Oral hypoglycemic agent | – | – | – | 447 (32%) | 405 (33%) | 42 (27%) |
| Sulfonylurea | – | – | – | 183 (13%) | 161 (13%) | 22 (14%) |
| No. insulin injections | 4.5 ± 0.7 | 4.5 ± 0.8 | 4.5 ± 0.7 | 3.0 ± 1.0 | 3.0 ± 1.0 | 3.0 ± 1.0 |
| Insulin dose (units/ kg bodyweight) | 0.71 ± 0.2 | 0.71 ± 0.02 | 0.70 ± 0.02 | 0.45 ± 0.25 | 0.45 ± 0.24 | 0.49 ± 0.28* |
| Basal insulin | ||||||
| Intermediate‐acting | 34 (16%) | 17 (13%) | 17 (21%) | 296 (21%) | 267 (22%) | 29 (19%) |
| Long‐acting | 157 (76%) | 100 (79%) | 57 (71%) | 320 (23%) | 289 (23%) | 31 (20%) |
| Premixed insulin | 13 (6%) | 8 (6%) | 5 (6%) | 666 (48%) | 585 (47%) | 81 (52%) |
| Insulin pump | 7 (3%) | 5 (4%) | 2 (3%) | 0 | 0 | 0 |
| Self‐monitoring of blood glucose | 196 (95%) | 120 (94%) | 76 (95%) | 1,253 (90%) | 1,117 (90%) | 136 (87%) |
| eGFR <60 mL/min/1.73 m2 | 30 (14%) | 11 (9%) | 19 (24%)** | 420 (30%) | 358 (29%) | 62 (40%) |
Values are expressed as the mean ± standard deviation. The numbers in parentheses are the percentage or interquartile range.
BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; Met, metabolic equivalent.
P < 0.05,
P < 0.01,
P < 0.001 versus those without severe hypoglycemia.
Table 2 shows the statistically significant risk factors for developing severe hypoglycemia in participants with type 1 diabetes evaluated by the multivariable Cox proportional hazards model, which included age, sex, duration of diabetes, history of severe hypoglycemia within the previous year, self‐reported eating speed, HbA1c, serum adiponectin and CKD. The risk was significant in people with a history of severe hypoglycemia within the previous year (HR 2.04, 95% CI 1.23–3.32) and high serum adiponectin (the highest vs the lowest in quartile HR 2.36, 95% CI 1.22–4.69). In contrast, fast eaters had a lower risk for severe hypoglycemic events (HR 0.56, 95% CI 0.31–0.98).
Table 2.
Statistically significant risk factors for developing severe hypoglycemia in Japanese participants with type 1 diabetes
| No. patients at risk | No. events | Incidence (/1,000 person‐year) | HR | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
| History of severe hypoglycemia within a previous year | – | 163 | 52 | 72.1 | Ref. | ||
| + | 44 | 28 | 187.7 | 2.00 | 1.20–3.28 | <0.01 | |
| Self‐reported eating speed | Fast | 82 | 20 | 52.1 | 0.57 | 0.31–0.99 | <0.05 |
| Normal | 81 | 34 | 100.2 | Ref. | |||
| Slow | 44 | 26 | 176.2 | 1.67 | 0.97–2.85 | 0.063 | |
| Serum adiponectin | Q1 (<9.8 μg/mL) | 51 | 16 | 66.1 | Ref. | ||
| Q2 (9.8–14.5 μg/mL) | 51 | 14 | 59.7 | 0.96 | 0.46–2.01 | NS | |
| Q3 (14.5–21.7 μg/mL) | 53 | 21 | 95.8 | 1.30 | 0.66–2.59 | NS | |
| Q4 (≥ 21.7 μg/mL) | 52 | 29 | 165.9 | 2.23 | 1.13–4.53 | <0.05 |
Multivariate adjustments included age, sex, duration of diabetes, history of severe hypoglycemia within the previous year, self‐reported eating speed (three categories), body mass index, hemoglobin A1c, serum adiponectin (quartiles) and chronic kidney disease (estimated glomerular filtration rate <60 mL/min/1.73 m2). CI, confidence interval; HR, hazard ratio; NS, not significant; Q1, quartile 1; Q2, quartile 2; Q3, quartile 3, Q4, quartile 4; Ref., reference.
Table 3 shows the statistically significant risk factors for developing severe hypoglycemia in participants with insulin‐treated type 2 diabetes evaluated by the multivariable Cox proportional hazard model, which included age, sex, history of severe hypoglycemia within a previous year, alcohol consumption, BMI, HbA1c, serum adiponectin, insulin dose and CKD. The risk was significant in those aged ≥65 years (65–75 years, HR 1.51, 95% CI 1.03–2.23; ≥75 years, HR 1.99, 95% CI 1.25–3.14, vs <65 years), those with a history of severe hypoglycemia within the previous year (HR 2.38, 95% CI 1.43–3.76), alcohol consumption ≥60 g/day (HR 2.78, 95% CI 1.32–5.26, vs abstainers), higher serum adiponectin (the highest vs the lowest in quartile, HR 2.95, 95% CI 1.72–5.23) and larger insulin dose (the highest vs the lowest in quartile, HR 1.62, 95% CI 1.02–2.60).
Table 3.
Statistically significant risk factors for developing severe hypoglycemia in Japanese participants with insulin‐treated type 2 diabetes
| No. patients at risk | No. events | Incidence (/1,000 person‐years) | HR | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
| Age | <65 years | 623 | 50 | 15.7 | Ref. | ||
| 65–75 years | 543 | 68 | 25.6 | 1.51 | 1.03–2.23 | <0.05 | |
| ≥75 years | 230 | 38 | 36.1 | 1.99 | 1.25–3.14 | <0.01 | |
| History of severe hypoglycemia within the previous year | – | 1,318 | 136 | 20.8 | Ref. | ||
| + | 78 | 20 | 57.6 | 2.38 | 1.43–3.76 | <0.01 | |
| Alcohol consumption (g/day) | Null | 938 | 104 | 22.5 | Ref. | ||
| <30 | 338 | 38 | 22.7 | 1.02 | 0.69–1.50 | NS | |
| 30–60 | 77 | 4 | 10.3 | 0.58 | 0.18–1.43 | NS | |
| ≥60 | 43 | 10 | 49.2 | 2.78 | 1.32–5.26 | <0.01 | |
| Serum adiponectin | Q1 (<6.0 μg/mL) | 342 | 20 | 11.5 | Ref. | ||
| Q2 (6.0–8.8 μg/mL) | 348 | 34 | 19.6 | 1.83 | 1.05–3.27 | <0.05 | |
| Q3 (8.8–13.1 μg/mL) | 355 | 47 | 26.7 | 2.44 | 1.43–4.31 | <0.001 | |
| Q4 (≥13.1 μg/mL) | 350 | 55 | 33.3 | 2.95 | 1.72–5.23 | <0.0001 | |
| Insulin dose | Q1 (<0.27 U/kg) | 349 | 36 | 21.1 | Ref. | ||
| Q2 (0.27–0.41 U/kg) | 349 | 32 | 18.4 | 0.83 | 0.51–1.35 | NS | |
| Q3 (0.41–0.60 U/kg) | 349 | 42 | 24.1 | 1.02 | 0.64–1.63 | NS | |
| Q4 (≥0.60 U/kg) | 349 | 46 | 26.9 | 1.62 | 1.02–2.60 | <0.05 |
Multivariate adjustments included age (three categories), sex, duration of diabetes, history of severe hypoglycemia within the previous year, alcohol consumption (four categories), body mass index, hemoglobin A1c, serum adiponectin (quartiles), insulin dose (quartiles) and chronic kidney disease (estimated glomerular filtration rate <60 mL/min/1.73 m2). CI, confidence interval; HR, hazard ratio; NS, not significant; Q1, quartile 1; Q2, quartile 2; Q3, quartile 3, Q4, quartile 4; Ref., reference.
Figure 2 shows the relationship between serum adiponectin and the age‐ and sex‐adjusted incidence of severe hypoglycemia in participants with type 1 diabetes and insulin‐treated type 2 diabetes. The incidence positively increased, along with serum adiponectin level (age‐ and sex‐adjusted HR 1.65, 95% CI 1.45–1.87 per 1 standard deviation increase of log serum adiponectin, HR adjusted by age, sex, BMI and insulin dose 1.68, 95% CI 1.47–1.92 per 1 standard deviation increase of log serum adiponectin).
Figure 2.

The relationship between serum adiponectin and the age‐ and sex‐adjusted incidence of severe hypoglycemia in participants with type 1 diabetes and insulin‐treated type 2 diabetes.
Discussion
The present study prospectively investigated the incidence of severe hypoglycemia and its risk factors in Japanese people with type 1 diabetes and insulin‐treated type 2 diabetes. There were no prospective studies to investigate the incidence of severe hypoglycemia in Japan. In addition, we showed that the development of severe hypoglycemia was significantly associated with higher serum adiponectin.
The incidence of severe hypoglycemia varies considerably according to the definition of hypoglycemia, the reporting methods, the studied population and the type of study 7 . The definition of severe hypoglycemia includes requiring assistance from others, an emergency ward visit and hospitalization. For people with type 1 diabetes, the rate of severe hypoglycemia was 4.9 events per patient‐year in 8,022 patients from 24 countries in the International Operations Hyperglycemia Assessment Tool study 16 , whereas it was 0.7–5.8 events per patient‐year in other studies 7 . Recently, however, the incidence of severe hypoglycemia has decreased in the national database in Denmark 5 , which showed that the incidence of severe hypoglycemia, defined as hospitalization for hypoglycemia, was 0.03 events per patient‐year in 2012, remarkably lower than in previous studies. The incidence of severe hypoglycemia in the present cohort was 0.09 events per patient‐year, which might be consistent with the recent Danish data. In patients with insulin‐treated type 2 diabetes, recent surveys showed that the incidence of self‐reported severe hypoglycemia requiring assistance from others was 0.1–0.2 events per patient‐year in seven European countries 17 , and 2.5 events per patient‐year in the International Operations Hyperglycemia Assessment Tool study 16 . The incidence of severe hypoglycemia was much lower in the present cohort (0.02 events per patient‐year), although the reasons for this difference were unknown. In Japan, however, the incidence of severe hypoglycemia using a retrospective Diagnosis Procedure Combination database was reported to be 0.004 per patient‐year in people with type 2 diabetes treated with oral hypoglycemic agents and/or insulin 18 , which was also very low.
There were many studies that investigated risk factors for severe hypoglycemia in patients with type 1 or type 2 diabetes 1 , 2 , 19 . The risk factors include advanced age, long duration of diabetes, tighter glycemic control, large consumption of alcohol, history of severe hypoglycemia, unawareness of hypoglycemia, CKD, autonomic neuropathy, cognitive impairment, depression and so on. Some of them were also observed in the present study, but higher serum adiponectin as a risk factor has not been previously reported. As adiponectin has a potent insulin‐sensitizing action 8 , higher adiponectin might be associated with hypoglycemia because of the enhanced insulin sensitivity. Recently, Tsuboi et al. 20 reported that higher adiponectin was associated with low glucose (≤70 mg/dL) after ingesting 75 g glucose among young healthy normal‐weight women, but they did not provide information about hypoglycemic events. In contrast, acute insulin‐induced hypoglycemia increased serum adiponectin in healthy volunteers and people with type 1 diabetes 21 . In vitro insulin induced acute release of adiponectin from a pool of insulin‐responsive, adiponectin‐containing vesicles near plasma membrane of 3T3‐L1‐differentiated adipocytes 22 . Therefore, it might be speculated that individuals with insulin overtreatment could be at risk for hypoglycemia, as well as enhanced adiponectin release from adipose tissue.
Regarding eating behavior, fast eating has been implicated to be associated with obesity 11 . Therefore, eating slowly has been recommended for preventing obesity. In the present study, however, slow eating tended to be associated with severe hypoglycemia in patients with type 1 diabetes. Rather, fast eating was associated with a lower occurrence of severe hypoglycemia. Although further studies will be required to confirm the findings, postprandial glucose profile might better match with the timing of glucose‐lowering action of rapid‐acting insulin in fast eaters than in slow eaters.
The strength of the current study is a prospective cohort protocol with a high follow‐up rate (99%), but the present study had several limitations. First, participants were recruited from specialist diabetes clinics in Fukuoka prefecture. Therefore, the incidence of severe hypoglycemia in our study might be different from the nationwide survey data. However, there were no other large‐scale prospective cohort studies of registries or the general population to our knowledge. Second, the number of participants was limited in our cohort, especially type 1 diabetes, because of its very low incidence in Japan (1.7/100,000 per year). Therefore, the relationship between serum adiponectin and the age‐ and sex‐adjusted incidence of severe hypoglycemia (Figure 2) was not investigated separately for type 1 and type 2 diabetes. Third, insulin sensitivity was not evaluated in the present study. The homeostatic model assessment index can be used as the parameter of insulin sensitivity in epidemiological studies, but was inapplicable to type 1 and insulin‐treated type 2 diabetes because of the profound insulin deficiency. Fourth, the current study included only Japanese people. As there is a racial difference in the susceptibility to severe hypoglycemia 23 , the findings of the present study might not be applied to other races. Finally, some clinical practices at registration might be different from the present day; for example, premixed insulin and intermediate‐acting insulin were often used, and the continuous blood glucose monitoring system was not introduced. These might affect the incidence of severe hypoglycemia 24 , 25 .
In conclusion, we investigated the incidence of severe hypoglycemia in Japanese people with type 1 diabetes and insulin‐treated type 2 diabetes, which was lower than other studies. In addition, we showed the association between severe hypoglycemia and higher serum adiponectin level, suggesting the importance of insulin sensitivity for insulin‐induced severe hypoglycemia.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors thank Tamaki Jodai‐Kitamura, Ai Murao‐Kimura, Yutaka Kiyohara, Yasufumi Doi, Toshiharu Ninomiya, Shigenobu Kanba, Dongchon Kang, Shuzo Kumagai, Shinako Kaizu, Yoichiro Hirakawa, Chisa Matsumoto and Chie Kitaoka (Kyushu University); Nobutaka Tsutsu and Nobuhiro Sasaki (Fukuoka Red Cross Hospital); Kiyohide Nunoi, Yuichi Sato, Yuji Uchizono, Ayumi Yamauchi, Kaori Itoh and Chie Kono (St. Mary’s Hospital); Sakae Nohara, Hirofumi Imoto and Kazushi Amano (Steel Memorial Yawata Hospital); Daisuke Gotoh, Toshitaka Himeno and Masae Toyonaga (Kyushu Central Hospital); Noriyasu Shinohara and Ayako Tsutsumi (Fukuoka Higashi Medical Center); Yasuhiro Idewaki, Masahiro Nakano, Mina Matsuo, Shoko Morimoto and Tomoko Hyodo (Hakujyuji Hospital); Masae Minami (Clinic Minami Masae); Miya Wada (Wada Miya Naika Clinic); Yoshifumi Yokomizo (Yokomizo Naika Clinic); Masanori Kikuchi and Yohei Kikuchi (Kikuchi Naika Clinic); Riku Nomiyama (Suzuki Naika Clinic); Shin Nakamura (Nakamura Naika Clinic); Kenji Tashiro (Oshima Eye Hospital); Mototaka Yoshinari (Yoshinari Naika Clinic); Kojiro Ichikawa (Fukutsu Naika Clinic); and Teruo Omae (Hisayama Research Institute For Lifestyle Diseases). The authors also thank the clinical research coordinators, Chiho Ohba (Hisayama Research Institute For Lifestyle Diseases); Kayoko Sekioka and Yoko Nishioka (Kyushu University); and those in the administration office, Tomoko Matake (Hisayama Research Institute For Lifestyle Diseases) and Junko Ishimatsu (Kyushu University). This work was supported in part by The Japan Society for the Promotion of Science KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant numbers 23249037 and 23659353 to MI, and 16K00861 to HF), and the Junior Scientist Development Grant supported by the Japan Diabetes Society (to YK).
J Diabetes Investig 2020; 11: 1258–1264
Clinical Trial Registry University Hospital Medical Information Registry‐UMIN000002627
References
- 1. Silbert R, Salcido‐Montenegro A, Rodriguez‐Gutierrez R, et al Hypoglycemia among patients with type 2 diabetes: epidemiology, risk factors, and prevention strategies. Curr Diab Rep 2018; 18: 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heller S, Novodvorsky P. Hypoglycaemia in diabetes. Medicine 2019; 47: 52–58. [Google Scholar]
- 3. Lee AK, Warren B, Lee CJ, et al The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care 2018; 41: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis SN, Duckworth W, Emanuele N, et al Effects of severe hypoglycemia on cardiovascular outcomes and death in the Veterans Affairs Diabetes Trial. Diabetes Care 2019; 42: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ishtiak‐Ahmed K, Carstensen B, Pedersen‐Bjergaard U, et al Incidence trends and predictors of hospitalization for hypoglycemia in 17,230 adult patients with type 1 diabetes: a Danish Register Linkage Cohort Study. Diabetes Care 2017; 40: 226–232. [DOI] [PubMed] [Google Scholar]
- 6. Zhong VW, Juhaeri J, Cole SR, et al Incidence and trends in hypoglycemia hospitalization in adults with type 1 and type 2 diabetes in England, 1998–2013: a retrospective cohort study. Diabetes Care 2017; 40: 1651–1660. [DOI] [PubMed] [Google Scholar]
- 7. Pedersen‐Bjergaard U, Thorsteinsson B. Reporting severe hypoglycemia in type 1 diabetes: facts and pitfalls. Curr Diab Rep 2017; 17: 131–142. [DOI] [PubMed] [Google Scholar]
- 8. Kadowaki T, Yamauchi T, Kubota N, et al Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006; 116: 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohkuma T, Fujii H, Iwase M, et al Impact of sleep duration on obesity and the glycemic level in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes Care 2013; 36: 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ainsworth BE, Haskell WL, Whitt MC, et al Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000; 32: S498–504. [DOI] [PubMed] [Google Scholar]
- 11. Otsuka R, Tamakoshi K, Yatsuya H, et al Eating fast leads to obesity: findings based on self‐administered questionnaires among middle‐aged Japanese men and women. J Epidemiol 2006; 16: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohkuma T, Fujii H, Iwase M, et al Impact of eating rate on obesity and cardiovascular risk factors according to glucose tolerance status: the Fukuoka Diabetes Registry and the Hisayama Study. Diabetologia 2013; 56: 70–77. [DOI] [PubMed] [Google Scholar]
- 13. Matsuo S, Imai E, Horio M, et al Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 14. Nishimura A, Sawai T. Determination of adiponectin in serum using a latex particle‐enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta 2006; 371: 163–168. [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association Working Group on Hypoglycemia . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005; 28: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 16. Khunti K, Alsifri S, Aronson R, et al Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin‐treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab 2016; 18: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ostenson CG, Geelhoed‐Duijvestijn P, Lahtela J, et al Self‐reported non‐severe hypoglycaemic events in Europe. Diabet Med 2014; 31: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuika I, Takekazu K, Eisei O, et al Incidence rate and patient characteristics of severe hypoglycemia in treated type 2 diabetes mellitus patients in Japan: retrospective diagnosis procedure combination database analysis. J Diabetes Investig 2018; 9: 925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Diabetes Association . 6. Glycemic targets: standards of medical care in diabetes‐2019. Diabetes Care 2019; 42(Suppl. 1): S61–S70. [DOI] [PubMed] [Google Scholar]
- 20. Tsuboi A, Minato S, Yano M, et al Higher circulating adiponectin and lower orosomucoid were associated with postload glucose ≤70 mg/dL, a possible inverse marker for dysglycemia, in young Japanese women. BMJ Open Diabetes Res Care. 2019; 7: e000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gogitidze Joy N, Hedrington MS, Briscoe VJ, et al Effects of acute hypoglycemia on inflammatory and pro‐atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 2010; 33: 1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim CY, Hong W, Han W. Adiponectin is released via a unique regulated exocytosis pathway from a pre‐formed vesicle pool on insulin stimulation. Biochem J. 2015; 471: 381–389. [DOI] [PubMed] [Google Scholar]
- 23. Lee AK, Lee CJ, Huang ES, et al Risk factors for severe hypoglycemia in black and white adults with diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 2017; 40: 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holman RR, Thorne KI, Farmer AJ, et al Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007; 357: 1716–1730. [DOI] [PubMed] [Google Scholar]
- 25. Šoupal J, Petruželková L, Grunberger G, et al Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow‐up from the COMISAIR Study. Diabetes Care 2020; 43: 37–43. [DOI] [PubMed] [Google Scholar]


