Abstract
Aims/Introduction
The role of irisin in maternal glucose metabolism and how it would respond to dietary n‐3 polyunsaturated fatty acid (n‐3 PUFA) intake remains unclear. This study aimed to explore whether maternal plasma irisin is associated with glucose metabolism and whether this association is modified by dietary n‐3 PUFA.
Materials and Methods
A total of 932 pregnant women (20–28 weeks’ gestation) aged 20–45 years were recruited. Dietary n‐3 PUFA was estimated using a validated quantitative food frequency questionnaire. Plasma irisin and insulin were tested by enzyme‐linked immunosorbent assay, and insulin resistance (IR) was estimated using the homeostatic model assessment (HOMA). Gestational diabetes mellitus was diagnosed with a 75‐g oral glucose tolerance test. Adjusted multivariable linear regression and logistic regression were carried out to examine the associations between plasma irisin and glucose metabolism. The moderating effect of dietary n‐3 PUFA intake was determined by fully multiplicative models by including the interaction term.
Results
Maternal plasma irisin was negatively associated with HOMA‐IR and oral glucose tolerance test 0 h glucose level (β −0.250, −0.067; corrected P‐value for false discovery rate = 0.012, 0.018, respectively), positively associated with HOMA of insulin sensitivity (β 0.028; corrected P‐value for false discovery rate = 0.012), but not associated with postprandial glucose or the risk of gestational diabetes mellitus. Furthermore, we found a moderating effect of dietary n‐3 PUFA on the relationships of plasma irisin with HOMA‐IR and HOMA of insulin sensitivity; these associations were strengthened with increased n‐3 PUFA intake (β −0.037, 0.004; P = 0.014, 0.041, respectively).
Conclusions
Plasma irisin was negatively associated with HOMA‐IR and fasting glucose, whereas it was positively associated with HOMA of insulin sensitivity in pregnant women. We first showed that these associations were modified by dietary n‐3 PUFA intake.
Keywords: Insulin resistance, Irisin, n‐3 polyunsaturated fatty acid
Plasma irisin was negatively associated with HOMA‐IR while positively associated with HOMA‐IS in pregnant women, and we first demonstrated these associations were modified by dietary n‐3 PUFA intake.

INTRODUCTION
Maternal glucose is the major energy substrate for intrauterine growth and is transmitted in a stable stream from mother to fetus 1 , 2 . Considerable evidence shows that pregnant women with abnormal glucose metabolism, such as gestational diabetes mellitus (GDM), are at higher risk of serious adverse maternal and perinatal outcomes, and other long‐term risks, including cardiovascular diseases, obesity and type 2 diabetes mellitus 3 , 4 . Therefore, it is essential to detect the modifiable impact factors of glucose metabolism in pregnant women. A novel myokine called irisin might be one of the promising options for the prevention and management of maternal glucose metabolism 5 .
Irisin has been identified as an exercise‐mediated polypeptide, which is secreted from its precursor, fibronectin type III domain‐containing protein 5, in response to the activation of peroxisome proliferator‐activated receptor‐g coactivator 1a 6 . It has been proposed to act on white adipose cells to stimulate the uncoupling protein‐1 expression and to alter the expression of several molecules leading to brown fat‐like development 6 . This conversion of white adipocytes to brown adipocytes and the resultant increase in thermogenesis can reduce insulin resistance (IR) and improve glucose homeostasis in mice 6 , 7 . Consequently, epidemiological studies have been carried out to examine the effects of irisin on glucose metabolism in pregnancy women, yet inconsistent findings have been reported. Several studies have recently observed a negative association between circulating irisin and IR in pregnant women 8 , 9 , whereas others reported a null 10 or positive correlation 11 , 12 . With regard to GDM, a common metabolic abnormality, previous studies have found decreased irisin levels in GDM pregnancies compared with non‐diabetic controls 9 , 13 , 14 , whereas some studies suggested no difference across two groups 11 , 12 . Taken together, no consensus opinion exists regarding the association between maternal irisin and glucose metabolism in pregnant women. The equivocal results across the aforementioned studies might be due to the discrepancy of various characteristics, such as ethnicity and maternal age. Furthermore, most studies did not take the effect of dietary, physical activities and other lifestyle factors on irisin into consideration.
Dietary fatty acid, particularly the n‐3 polyunsaturated fatty acid (n‐3 PUFA), might play a crucial role in mediating the levels of plasma irisin through the activation of peroxisome proliferator‐activated receptor‐γ 6 , 15 . Similar to insulin, n‐3 PUFA can reduce IR and regulate glucose metabolism by mechanisms related to anti‐inflammatory properties and transcription factors regulation 16 , 17 . In addition, a growing body of evidence showed that dietary n‐3 PUFA supplement can enhance insulin function and improve glucose tolerance 18 , 19 . Thus, it might be plausible that maternal dietary n‐3 PUFA intake could moderate the association between circulating irisin and glucose metabolism, yet this is still an understudied area in population‐based settings.
Therefore, we were especially interested in evaluating the following: (i) the association between maternal plasma irisin and glucose metabolism; and (ii) the moderating effect of maternal dietary n‐3 PUFA intake on the association between plasma irisin and glucose metabolism in pregnant women.
METHODS
Study population
Cross‐sectional data were from the baseline examination (2017–2018) of an ongoing prospective longitudinal GDM cohort study (ClinicalTrial.gov number: NCT03023293). In brief, we recruited pregnant women (20–28 weeks’ gestation) at Yuexiu district maternal and child health hospital in Guangzhou, China. Participants were eligible for the study if they were aged 20–45 years. Individuals with pre‐existing diabetes mellitus, cardiovascular disease, thyroid disease, hematopathy, polycystic ovary syndrome, pregnancy infection, mental disorder or multiple pregnancies were excluded from the study.
A total of 982 pregnant women were enrolled. We further excluded those who had no blood samples (n = 55), or had implausibly high (>4,000 kcal) or low (<800 kcal) daily energy intakes assessed by the food frequency questionnaire (FFQ; n = 4). Thus, 923 pregnant women were included in the final analysis. The ethics committee of the School of Public Health at Sun Yat‐Sen University approved the study. All participants were carefully instructed and signed informed consent at initial enrollment.
Laboratory analysis
Fasting blood samples were collected in the morning after an overnight fast of at least 10 h, followed by centrifugation to separate plasma. Plasma samples were kept at −80°C for later laboratory analysis. The quantitative measurement of plasma irisin was carried out by enzyme‐linked immunosorbent assay (EK‐067–29; Phoenix Pharmaceuticals, Burlingame, CA, USA) with a spectrophotometric microplate reader at wavelength of 450 nm (Spark® multimode microplate reader, Tecan Trading AG, Männedorf, Switzerland). This test provided a range of detection of 0.1–1000 ng/mL, and showed an inter‐ and intra‐assay coefficient of variation of <10% and 15%, respectively.
A 75‐g oral glucose tolerance test (OGTT) was carried out for all participants. Maternal plasma glucose levels during OGTT, including OGTT‐0 h glucose (fasting plasma glucose) and OGTT‐1 h and OGTT‐2 h glucose (postprandial glucose), were measured with the glucose oxidase method. Plasma insulin was measured using enzyme‐linked immunosorbent assay (10‐1113‐01; Mercodia, Uppsala, Sweden) by standard laboratory methods. IR was estimated using the homeostatic model assessment (HOMA) by the following formula 20 : fasting insulin (μU/mL) × OGTT‐0 h glucose (mmol/L) / 22.5; HOMA of insulin sensitivity (HOMA‐IS) was calculated as 1 / HOMA‐IR; and HOMA of β‐cell function (HOMA‐βF) was calculated as 20 × fasting plasma insulin (μU/mL) / (OGTT‐0 h glucose [mmol/L] – 3.5). GDM was diagnosed if any one of the following values was met or exceeded in the 75‐g OGTT: 0‐h glucose ≥5.10 mmol/L; 1‐h glucose ≥10.00 mmol/L; or 2‐h glucose ≥8.50 mmol/L 21 .
Dietary assessment
A validated 81‐item quantitative FFQ was used to assess women’s usual dietary intakes during the past month before OGTT. The development and validation of the FFQ have been described previously 22 . Participants were asked to report data on the frequency (per day, week or month for each food item) of intake and portion size in the face‐to‐face interview. The number of servings per frequency was shown in natural units (e.g., 1 banana), household measures (e.g., 1 spoon) or grams (e.g., 200 g of cooked vegetables). Food photographs with standard portion sizes were used for assistance during the interview. Maternal dietary nutrient intakes were computed based on the 2004 Chinese Food Composition Table (Chinese Center for Disease Control and Prevention, Beijing, China) 23 . Furthermore, we also investigated the consumption frequency and amount of nutrient supplement in the past month, and those nutrients contained were computed according to information from the manufacturer. Individual daily intake of each nutrient was adjusted for total energy intake by using the regression residual method 24 .
Assessment of covariates
We collected information including sociodemographic characteristics (e.g., age, occupation, education level and monthly household income) and lifestyle factors (e.g., maternal smoking status, physical activity). Monthly household income was divided into four groups (<4,000, 4,001–6,000, >6,001–10,000 and >10,000 RMB). The occupation was categorized into five groups (commerce and services, professionals and technicians, administrators and clerks, housewives, and others). Education level was categorized into four groups (senior high school or below, junior college, college and above college). Smoking status and alcohol use during the pregnancy were categorized into yes or no. The intensity of physical activity was expressed in metabolic equivalents according to the International Physical Activity Questionnaire 25 . Furthermore, medical conditions, including a history of gestational diabetes mellitus and family history of diabetes, were also recorded.
Anthropometric measurements were taken by trained clinical nurses. Height was measured with a standard height measuring instrument (nearest 0.1 cm) and pre‐pregnancy bodyweight with a digital panel indicator scale (nearest 0.1 kg). Pre‐pregnancy body mass index (BMI) values (kg/m2) were calculated as dividing pre‐pregnancy weight (kg) by height squared (m).
Statistical analysis
Continuous variables were reported as the mean ± standard deviation, whereas categorical variables were reported as numbers and percentages. Differences in participants’ characteristics across two groups were evaluated by t‐tests or 2‐tests as appropriate. Multivariable linear regression and logistic regression analyses were carried out to evaluate the associations between maternal plasma irisin and glucose metabolism. Model 1 was adjusted for survey years, age, gestational age and pre‐pregnancy BMI; model 2 was further adjusted for GDM in a previous pregnancy, and family history of diabetes, physical activities, smoking during pregnancy, alcohol use during pregnancy, dietary energy intake and dietary n‐3 PUFAs intake; model 3 was additionally adjusted for occupation, monthly household income and educational level. All the variables were entered simultaneously into multivariate regression models. The false discovery rate (FDR) was used for the P‐value correction on multiple comparisons using the Benjamini–Hochberg method 26 , and the corrected P‐value (P FDR) is shown in the results. Stratified analysis was carried out to show whether the association differed numerically across levels of maternal dietary n‐3 PUFA intake; pregnant women were divided into subgroups by dividing at the median of dietary n‐3 PUFA intake. The moderating effect of dietary n‐3 PUFA intake on the association between plasma irisin and glucose metabolism was determined using fully multiplicative models by including the interaction term in multivariate linear or logistic regression.
The statistical analysis was carried out by using SAS 9.4 (SAS Institute Inc, Cary, NC, USA), whereas the graph of the moderating effect was carried out by using R software version 3.5.0. All P‐values are two‐sided, and statistical significance was determined at a P‐value <0.05.
RESULTS
Characteristics of study participants
The maternal and clinical characteristics of participants are shown in Table 1. The plasma irisin concentration in pregnant women was 13.81 ± 6.12 ng/mL. Among the 932 pregnant women included in our analyses, 176 (18.89%) had GDM at 20–28 weeks’ of pregnancy. Compared with women without GDM, those with GDM had higher age, pre‐pregnancy BMI, history of GDM, glucose and insulin levels, and HOMA‐IR values, and had lower HOMA‐IS values and physical activity levels (P < 0.05; Table 1). By contrast, no statistical difference was observed among gestational age, plasma irisin levels, HOMA‐βF values and other characteristics between different GDM status categories (P > 0.05).
Table 1.
Maternal characteristics of the pregnant women with and without gestational diabetes mellitus
| Characteristic | Total | GDM | Normal | P‐value |
|---|---|---|---|---|
| (n = 932) | (n = 176) | (n = 756) | ||
| Survey year | ||||
| 2017, n (%) | 465 (49.79) | 78 (16.77) | 387 (83.23) | 0.107 |
| 2018, n (%) | 469 (50.21) | 98 (20.90) | 371 (79.10) | |
| Age (years) | 30.09 ± 4.87 | 32.23 ± 5.08 | 29.60 ± 4.68 | <0.001 |
| Gestational age (weeks) | 25.46 ± 2.36 | 25.19 ± 2.53 | 25.53 ± 2.31 | 0.098 |
| Pre‐pregnancy BMI (kg/m2) | 20.58 ± 2.94 | 21.63 ± 3.57 | 20.33 ± 2.72 | <0.001 |
| Physical activity, METs·(h/week) | 32.38 ± 28.32 | 27.40 ± 22.59 | 33.57 ± 29.38 | 0.002 |
| OGTT‐0 h glucose (mmol/L) | 4.42 ± 0.42 | 4.79 ± 0.56 | 4.33 ± 0.32 | <0.001 |
| OGTT‐1 h glucose (mmol/L) | 7.80 ± 1.76 | 9.81 ± 1.68 | 7.33 ± 1.41 | <0.001 |
| OGTT‐2 h glucose (mmol/L) | 6.72 ± 1.36 | 8.41 ± 1.39 | 6.33 ± 1.01 | <0.001 |
| Insulin (μU/mL) | 13.54 ± 9.06 | 15.56 ± 8.22 | 13.07 ± 9.19 | <0.001 |
| HOMA‐IR | 2.65 ± 1.43 | 3.37 ± 0.70 | 2.53 ± 1.71 | <0.001 |
| HOMA‐IS | 0.45 ± 0.18 | 0.37 ± 0.16 | 0.47 ± 0.18 | <0.001 |
| HOMA‐βF | 302.3 ± 1067.3 | 275.6 ± 167.2 | 308.6 ± 1182.7 | 0.814 |
| Plasma irisin (ng/mL) | 13.81 ± 6.12 | 14.20 ± 4.62 | 13.57 ± 5.09 | 0.110 |
| Smoking, n (%) | 40 (4.30) | 9 (5.14) | 31 (4.11) | 0.545 |
| Alcohol consumption, n (%) | 24 (2.58) | 5 (2.86) | 19 (2.52) | 0.800 |
| Total energy (MJ/day) | 7.52 ± 2.09 | 7.58 ± 2.01 | 7.51 ± 2.11 | 0.641 |
| Dietary n‐3 PUFA (g/day) † | 1.02 ± 0.67 | 1.05 ± 0.71 | 1.02 ± 0.66 | 0.940 |
| Family history of diabetes | 142 (15.27) | 30 (17.14) | 112 (14.85) | 0.448 |
| GDM in a previous pregnancy, n (%) | ||||
| Nulliparous | 355 (38.25) | 51 (29.31) | 304 (40.37) | <0.001 |
| Previous GDM | 29 (3.13) | 15 (8.62) | 14 (1.86) | |
| No previous GDM | 544 (58.62) | 108 (62.07) | 435 (57.77) | |
| Educational level, n (%) | ||||
| Senior high school or below | 171 (18.61) | 32 (19.39) | 139 (18.68) | 0.641 |
| Junior college | 195 (21.22) | 40 (22.99) | 154 (20.70) | |
| College | 499 (54.30) | 89 (51.15) | 410 (55.11) | |
| College above | 54 (5.88) | 13 (7.47) | 41 (5.51) | |
| Occupation, n (%) | ||||
| Commerce and services | 248 (27.37) | 51 (30.18) | 187 (25.37) | 0.040 |
| Professionals and technicians | 38 (4.19) | 5 (2.96) | 33 (19.00) | |
| Administrators and clerks | 184 (20.31) | 44 (26.04) | 140 (19.00) | |
| Housewives | 238 (26.27) | 33 (19.53) | 215 (29.17) | |
| Other | 198 (21.85) | 36 (21.30) | 162 (21.98) | |
| Monthly household income, n (%) | ||||
| ≤4,000 RMB | 196 (21.51) | 35 (2.35) | 161 (21.82) | 0.965 |
| 4,001–6,000 RMB | 216 (23.71) | 41 (23.84) | 174 (23.58) | |
| 6,001–10,000 RMB | 227 (24.92) | 45 (26.16) | 182 (24.66) | |
| >10,000 RMB | 272 (28.96) | 51 (29.62) | 221 (29.95) | |
Continuous variables were described by mean ± standard deviation; categorical variables were presented by number (percentage).
BMI, body mass index; GDM, gestational diabetes mellitus; HOMA‐βF, homeostatic model assessment of β‐cell function; HOMA‐IR, homeostatic model assessment of insulin resistance; HOMA‐IS, homeostatic model assessment of insulin sensitivity; OGTT, oral glucose tolerance test; PUFA, polyunsaturated fatty acid; RMB, renminbi yuan.
Energy‐adjusted by the residual method.
Association between plasma irisin and glucose metabolism
Tables 2 and 3 present the association of maternal plasma irisin with glucose levels, IR and the risk of GDM. After adjusting for potential confounding factors (model 3), maternal plasma irisin was significantly negatively associated with HOMA‐IR and OGTT‐0 h glucose levels (β −0.250, −0.067; standard error [SE] 0.082, 0.025; P FDR = 0.012, 0.018, respectively.). Furthermore, a significant positive association was found between maternal plasma irisin and HOMA‐IS (β 0.028; SE 0.007; P FDR = 0.012). In contrast, no significant relationships were observed of maternal plasma irisin with HOMA‐βF, OGTT‐postprandial glucose levels or the risk of GDM.
Table 2.
Relationships of plasma irisin with insulin related index and glucose levels
| Variable | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta ± SE | P † | P FDR ‡ | Beta ± SE | P † | P FDR ‡ | Beta ± SE | P † | P FDR ‡ | |
| Overall (n = 932) | |||||||||
| HOMA‐IR | −0.271 ± 0.078 | <0.001 | 0.003 | −0.259 ± 0.079 | 0.001 | 0.006 | −0.250 ± 0.082 | 0.002 | 0.012 |
| HOMA‐IS | 0.036 ± 0.009 | <0.001 | 0.003 | 0.031 ± 0.009 | 0.002 | 0.006 | 0.028 ± 0.007 | 0.004 | 0.012 |
| HOMA‐βF | −103.72 ± 62.1 | 0.096 | 0.144 | −104.71 ± 64.9 | 0.107 | 0.130 | −111.35 ± 68.7 | 0.106 | 0.127 |
| OGTT‐0 h glucose | −0.060 ± 0.023 | 0.012 | 0.024 | −0.056 ± 0.025 | 0.023 | 0.046 | −0.067 ± 0.025 | 0.009 | 0.018 |
| OGTT‐1 h glucose | −0.103 ± 0.097 | 0.291 | 0.291 | −0.051 ± 0.100 | 0.609 | 0.609 | −0.075 ± 0.103 | 0.466 | 0.467 |
| OGTT‐2 h glucose | 0.106 ± 0.075 | 0.156 | 0.187 | 0.135 ± 0.076 | 0.108 | 0.130 | 0.126 ± 0.077 | 0.080 | 0.120 |
| Statistical analysis | |||||||||
| High dietary n‐3 PUFA (n = 466) | |||||||||
| HOMA‐IR | −0.257 ± 0.100 | 0.011 | 0.022 | −0.214 ± 0.102 | 0.037 | 0.074 | −0.208 ± 0.104 | 0.045 | 0.090 |
| HOMA‐IS | 0.042 ± 0.013 | 0.001 | 0.006 | 0.039 ± 0.013 | 0.004 | 0.024 | 0.038 ± 0.013 | 0.005 | 0.015 |
| HOMA‐βF | −201.28 ± 121.82 | 0.999 | 0.999 | −203.7 ± 124.4 | 0.102 | 0.153 | −198.9 ± 128.3 | 0.122 | 0.183 |
| OGTT‐0 h glucose | −0.095 ± 0.032 | 0.003 | 0.009 | −0.086 ± 0.033 | 0.008 | 0.024 | −0.092 ± 0.033 | 0.005 | 0.015 |
| OGTT‐1 h glucose | −0.117 ± 0.139 | 0.958 | 0.999 | −0.007 ± 0.139 | 0.958 | 0.958 | −0.042 ± 0.141 | 0.768 | 0.768 |
| OGTT‐2 h glucose | 0.059 ± 0.105 | 0.571 | 0.867 | 0.078 ± 0.106 | 0.461 | 0.553 | 0.042 ± 0.106 | 0.693 | 0.768 |
| Low dietary n‐3 PUFA (n = 466) | |||||||||
| HOMA‐IR | −0.042 ± 0.062 | 0.504 | 0.605 | −0.127 ± 0.193 | 0.511 | 0.767 | −0.129 ± 0.209 | 0.533 | 0.780 |
| HOMA‐IS | 0.060 ± 0.438 | 0.169 | 0.338 | 0.018 ± 0.015 | 0.222 | 0.580 | 0.014 ± 0.015 | 0.360 | 0.720 |
| HOMA‐βF | 0.020 ± 0.015 | 0.169 | 0.338 | 19.09 ± 55.62 | 0.732 | 0.878 | 20.73 ± 61.15 | 0.735 | 0.882 |
| OGTT‐0 h glucose | −0.037 ± 0.036 | 0.297 | 0.446 | −0.039 ± 0.037 | 0.290 | 0.580 | −0.058 ± 0.039 | 0.145 | 0.435 |
| OGTT‐1 h glucose | −0.061 ± 0.144 | 0.670 | 0.670 | −0.017 ± 0.147 | 0.908 | 0.908 | −0.005 ± 0.153 | 0.975 | 0.975 |
| OGTT‐2 h glucose | 0.225 ± 0.109 | 0.040 | 0.240 | 0.249 ± 0.111 | 0.025 | 0.150 | 0.300 ± 0.115 | 0.009 | 0.054 |
Model 1 was adjusted for survey years, age, gestational age, pre‐pregnancy body mass index. Model 2 was further adjusted for gestational diabetes mellitus in a previous pregnancy, family history of diabetes, physical activities, smoking during pregnancy, alcohol consumption during pregnancy, dietary energy intake and dietary n‐3 polyunsaturated fatty acids (PUFAs) intake. Model 3 was further adjusted for occupation, monthly household income and educational level.
HOMA‐βF, homeostatic model assessment of β‐cell function; HOMA‐IR, homeostatic model assessment of insulin resistance; HOMA‐IS, homeostatic model assessment of insulin sensitivity; OGTT, oral glucose tolerance test; PUFA, polyunsaturated fatty acid; SE, standard error.
P‐value for multiple linear regression.
Corrected P‐value for false discovery rate (FDR).
Table 3.
Association between plasma irisin and the risk of gestational diabetes mellitus in pregnant women
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Overall | ||||||
| Continuous variable | 0.968 | 0.918–1.022 | 0.967 | 0.916–1.022 | 0.977 | 0.923–1.035 |
| Categories variable | ||||||
| Q1 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Q2 | 0.780 | 0.462–1.318 | 0.870 | 0.510–1.486 | 0.751 | 0.432–1.304 |
| Q3 | 0.471 | 0.168–1.316 | 0.476 | 0.164–1.385 | 0.406 | 0.132–1.253 |
| Q4 | 0.675 | 0.168–1.316 | 0.731 | 0.248–2.149 | 0.578 | 0.186–1.799 |
| Statistical analysis | ||||||
| High dietary n‐3 PUFA (n = 466) | ||||||
| Continuous variable | 1.004 | 0.949–1.063 | 0.995 | 0.936–1.058 | 0.990 | 0.923–1.062 |
| Categories variable | ||||||
| Q1 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Q2 | 0.819 | 0.409–1.643 | 0.922 | 0.451–1.886 | 0.849 | 0.409–1.760 |
| Q3 | 0.483 | 0.098–2.388 | 0.403 | 0.070–2.308 | 0.373 | 0.051–2.758 |
| Q4 | 1.095 | 0.222–5.395 | 1.105 | 0.182–5.654 | 0.882 | 0.125–6.222 |
| Low dietary n‐3 PUFA (n = 466) | ||||||
| Continuous variable | 1.037 | 0.959–1.122 | 1.047 | 0.966–1.136 | 1.033 | 0.948–1.126 |
| Categories variable | ||||||
| Q1 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Q2 | 0.975 | 0.430–2.21 | 1.150 | 0.485–2.723 | 0.870 | 0.346–2.188 |
| Q3 | 0.560 | 0.120–2.609 | 0.557 | 0.119–2.600 | 0.549 | 0.113–2.674 |
| Q4 | 0.761 | 0.157–3.697 | 0.824 | 0.170–3.999 | 0.723 | 0.723–3.676 |
Model 1 was adjusted for survey years, age, gestational age and pre‐pregnancy body mass index. Model 2 was further adjusted for gestational diabetes mellitus in a previous pregnancy, family history of diabetes, physical activities, smoking during pregnancy, alcohol use during pregnancy, dietary energy intake and dietary n‐3 PUFAs intake. Model 3 was further adjusted for occupation, monthly household income and educational level.
CI, confidence interval; OR, odds risk; PUFA, polyunsaturated fatty acid; Q, quartile.
Stratified analysis
As shown in Tables 2 and 3, we also carried out stratified analysis for the association between maternal plasma irisin and glucose metabolism. In the high dietary n‐3 PUFA intake subgroup, we found that higher maternal plasma irisin was negatively associated with lower OGTT‐0 h glucose levels (β −0.092; SE 0.033; P FDR = 0.015), whereas it was positively association with HOMA‐IS (β 0.038; SE 0.013; P FDR = 0.015). However, no significant relationships between maternal plasma irisin with other indexes were found in the stratified analysis.
Moderating effect of dietary n‐3 PUFA intake
We found a moderating effect of dietary n‐3 PUFA intakes on the relationships of maternal plasma irisin with HOMA‐IR and HOMA‐IS; these associations were strengthened with increased dietary n‐3 PUFA intakes (β −0.037, 0.004; SE 0.015, 0.002; P = 0.014, 0.041, respectively; Figure 1a,b). However, we found no significant moderating effect of maternal dietary n‐3 PUFA intake on the relationships between irisin and HOMA‐βF, glucose levels or the risk of GDM (Figures 1c ,2; Table S1).
Figure 1.
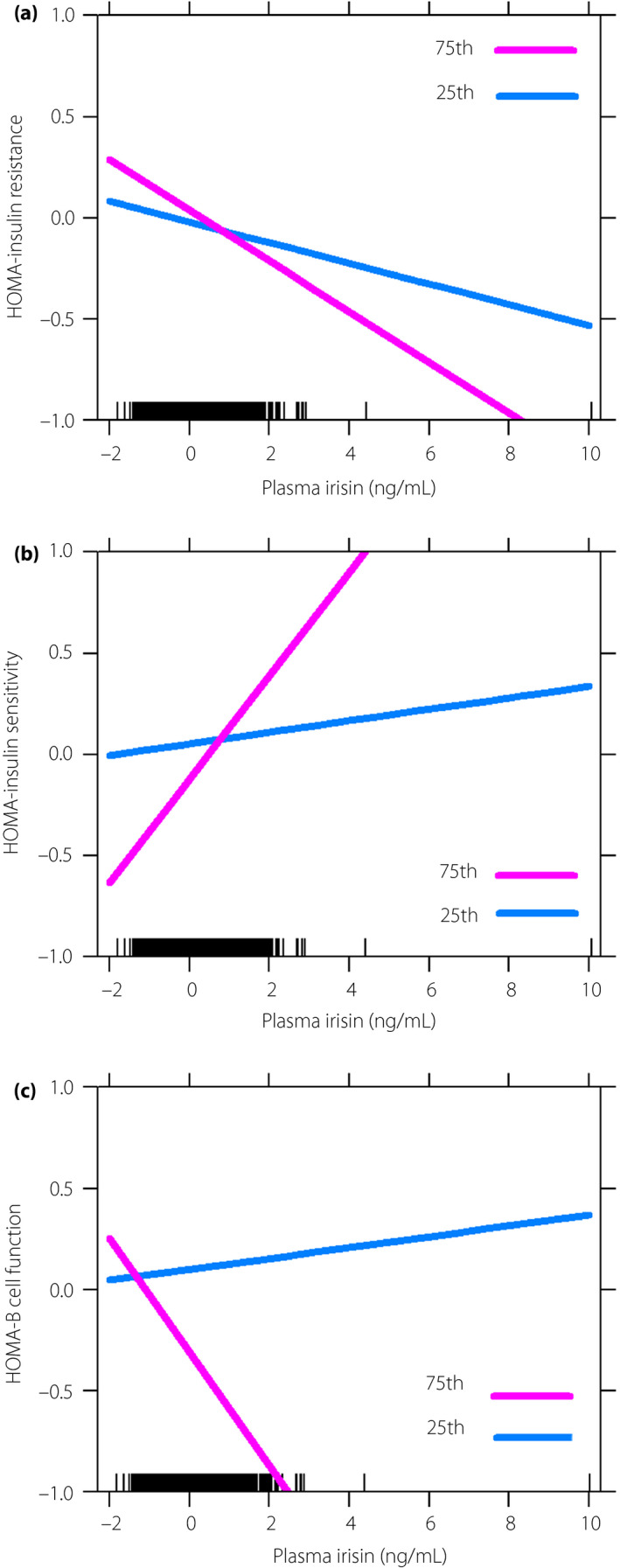
The moderating effect of n‐3 polyunsaturated fatty acid for the relationships of plasma irisin with insulin related index in pregnant women. (a) The moderating effect on homeostatic model assessment (HOMA) of insulin resistance (HOMA‐IR; β −0.037; standard error 0.015; P = 0.014). (b) The moderating effect on HOMA of insulin sensitivity (HOMA‐IS; β = 0.004; standard error 0.002; P = 0.041). (c) The moderating effect on HOMA of β‐cell function (HOMA‐βF; β −13.772; standard error 12.607; P = 0.275). Figures were plotted by using the percentile split of maternal n‐3 polyunsaturated fatty acid intake. The value of the x‐ and y‐axes were standardized. P25, 25th percentile; P75, 75th percentile.
Figure 2.
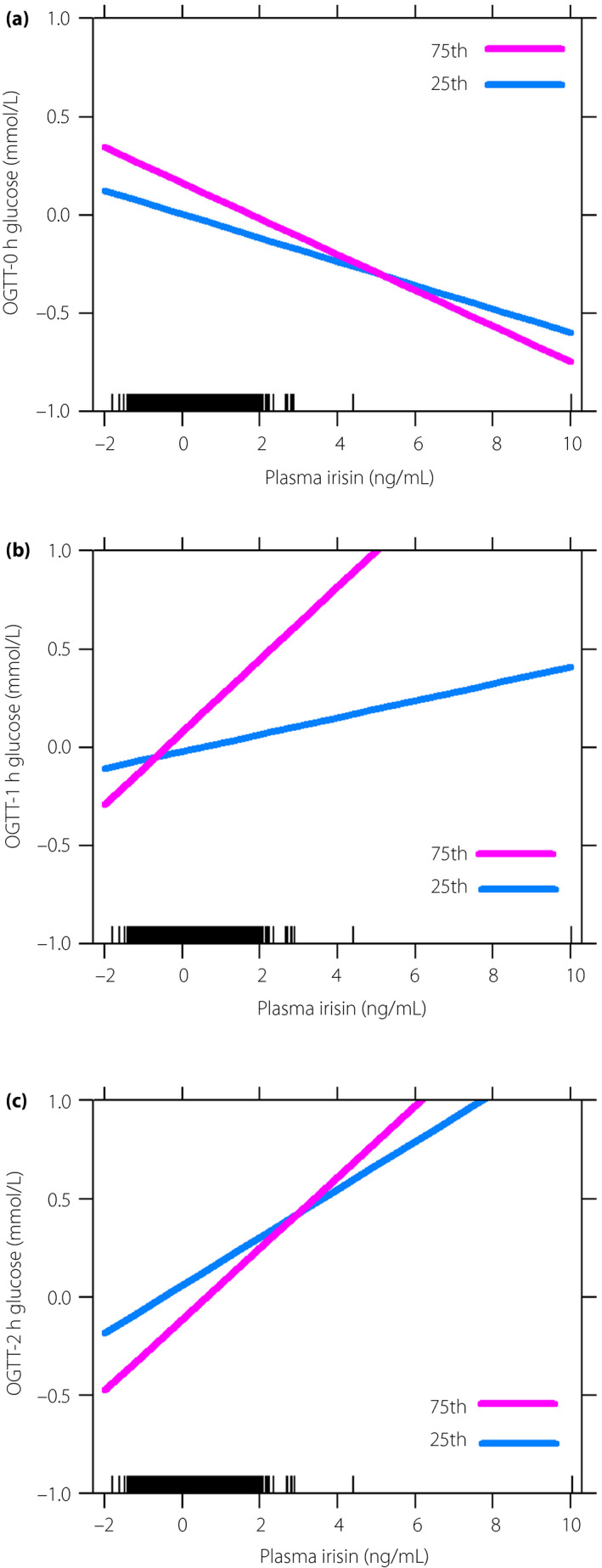
The moderating effect of n‐3 polyunsaturated fatty acid for the relationships of plasma irisin with glucose levels in pregnant women. (a) The moderating effect on oral glucose tolerance test (OGTT)‐0 h glucose level (β −0.004; standard error 0.005; P = 0. 412). (b) The moderating effect on OGTT‐1 h glucose level (β −0.003; standard error 0.019; P = 0.893). (c) The moderating effect on OGTT‐2 h glucose level (β −0.012; standard error 0.014; P = 0.395). Figures were plotted by using the percentile split of maternal n‐3 polyunsaturated fatty acid intake. The value of the x‐ and y‐axes were standardized. P25, 25th percentile; P75, 75th percentile.
DISCUSSION
By using a population‐based study, we observed that maternal plasma irisin was negatively associated with HOMA‐IR and OGTT‐0 h glucose levels, whereas is was positively associated with HOMA‐IS, but not associated with HOMA‐βF, OGTT‐postprandial glucose or the risk of GDM. Furthermore, the present results suggest a moderating effect of dietary n‐3 PUFA intake on relationships of maternal plasma irisin with HOMA‐IR and HOMA‐IS. To the best of our knowledge, this is the first population study to report the moderating effect of dietary n‐3 PUFA on the association between plasma irisin and glucose metabolism.
Previous studies evaluating the association between circulating irisin levels and maternal HOMA‐IR have yielded conflicting findings. Briefly, maternal irisin levels were found to be negatively correlated with HOMA‐IR in most studies 8 , 9 , although some studies suggested a null 10 or even a positive correlation 11 , 12 . However, the above studies have seldom evaluated the relationship between circulating irisin levels and maternal HOMA‐IR after adjusting for the potential confounders, including age, BMI, physical activity and nutritional status, which have been reported to affect circulating irisin levels 27 . To reduce the potential impact of confounding factors, the multiple linear regression was carried out in the present study, and an inverse association between maternal plasma irisin and HOMA‐IR was observed. The possible biological mechanism is biologically plausible. First, experimental data showed that circulating irisin levels can significantly elevate the expression of glucose transporter 4 (GLUT4) 28 , 29 , which plays a major role in regulating insulin‐mediated glucose metabolism in physiology 30 . It is thought that the expression of GLUT4 might lead to improved IS and enhanced glucose clearance, as well as offering some protection against IR 31 , 32 . Second, irisin induced browning of white adipocytes, which can lead to increased energy expenditure and thermogenesis with subsequent improvement of IS and reduced IR 33 , 34 . Finally, a few studies suggested that irisin can improve IR and glucose metabolism by increasing glycogenesis through phosphoinositide 3‐kinase/protein kinase B/glycogen synthase kinase‐3‐mediated glycogen synthase, and reducing gluconeogenesis through phosphoinositide 3‐kinase/protein kinase B/forkhead box O1‐mediated phosphoenolpyruvate carboxykinase and glucose‐6‐phosphatase downregulation 35 , 36 . Taken together, maternal plasma irisin can improve IR through multiple potential mechanism pathways, and it might be a novel and promising peptide hormone for IR and related metabolic diseases.
Maternal glucose homeostasis is maintained by a balance between glucose production and glucose uptake in peripheral tissues 37 . Decreased glucose uptake in skeletal muscle and overproduction of glucose caused by excess hepatic gluconeogenesis can contribute to high fasting plasma glucose (FPG) 38 , 39 . In the present study, we found that maternal plasma irisin was negatively associated with OGTT‐0 h glucose (FPG). There are several potential interpretations proposed for this result. Experimental data showed that the increased levels of circulating irisin within 1 h can significantly enhance glucose in the cell, which was similar to the uptake observed after exposure to insulin 40 . The increase of glucose uptake was mediated by the upregulation of expression of GLUT4 in skeletal muscle 28 , 41 . In contrast, animal research proved that irisin can inhibit hepatic gluconeogenesis by reducing the expression of glucose‐6‐phosphatase and phosphoenolpyruvate carboxykinase in the liver 37 . Altogether, maternal plasma irisin might affect FPG through enhancing glucose uptake and inhibiting gluconeogenesis.
Consistent with previous studies 10 , 11 , the present study found a non‐significant inverse relationship between maternal plasma irisin and OGTT‐1 h and OGTT‐2 h glucose levels (postprandial glucose). It is possible that postprandial glucose, compared to FPG, may be more likely influenced by external factors, such as carbohydrate intakes and gastrointestinal absorption function 42 . With regards to GDM, the prevalence has markedly been increasing in Chinese female population and has become an important public health problem in China 43 . A recent systematic review and meta‐analysis found a high incidence of GDM (14.8%) in mainland China 44 . Furthermore, some previous studies reported an even higher prevalence of GDM in economically developed areas of China, such as Sichuang (17.2%) 45 and Jiangsu (19.65%) 46 . We found similar results in Guangzhou (18.8%), which is one of the largest cities in China. Besides, a previous study found that a lower circulating irisin level exists in subjects with GDM 13 . Nevertheless, we found a non‐significant association between maternal plasma irisin and the risk of GDM. This might be partly explained by the differences in population characteristics. Alternatively, well‐documented risk factors, such as the family history of diabetes, advanced maternal age, previous history of GDM, diet and lifestyle factors also may affect the occurrence and development of GDM 47 , 48 . We speculated that although irisin can significantly improve IR and FPG, the effect on the risk of GDM remains unclear. Therefore, further study is warranted to examine a possible causal relationship between maternal irisin levels and the development of GDM.
Stratified analysis showed a diverse effect of plasma irisin on glucose metabolism in different dietary n‐3 PUFA groups. Furthermore, FDR had reduced false positive rates of the multiple linear regression in the lower dietary n‐3 PUFA intake group. Analysis of the moderating effect was carried out, and first found a moderating effect of dietary n‐3 PUFA intake on the relationships of plasma irisin with HOMA‐IR and HOMA‐IS; and the association was strengthened with increased n‐3 PUFA intakes. Although the biological mechanisms of the moderating effect of dietary n‐3 PUFA are not well understood, several possible mechanisms have been indicated. In animal experiments, dietary n‐3 PUFA intake in rats can induce the expression of the glucose‐6‐phosphate pool, which is accompanied by an increase in glucose uptake 49 . Additionally, n‐3 PUFA can maintain the activations of insulin receptor and increased GLUT4 expression in skeletal muscle cells 50 , 51 . GLUT4, an insulin dependent glucose transporter, was found almost exclusively in skeletal muscle and adipocytes. An elevated expression of GLUT4 is evidence of increased IS and decreased IR 30 . Notably, irisin can also improve IR, as evidenced by increasing GLUT4 translocation in skeletal muscle 37 . Furthermore, irisin can induce the expression of GLUT4 in human mature adipocytes 52 . Accordingly, we preliminarily propose that both dietary n‐3 PUFA and plasma irisin might affect IS and IR through GLUT4. In contrast, circulating irisin levels might be influenced by dietary n‐3 PUFA, which could play a crucial role in irisin synthesis or through the activation of peroxisome proliferator‐activated receptor‐γ 28 . It has been suggested that treatment with n‐3 PUFA for 48 h will significantly increase irisin expression in human rhabdomyosarcoma cells. Furthermore, two recent randomized clinical trials discovered that n‐3 PUFA supplementation, compared with placebo, can elevate the circulating irisin levels in patients with diabetic or coronary artery disease 53 , 54 . Combining the aforementioned two studies and the findings of the present study, we found that n‐3 PUFA not only increases the level of circulating irisin, but also enhances the positive association between irisin and insulin sensitivity, which indicates that dietary n‐3 PUFA intake might improve insulin sensitivity through irisin pathways. Further studies are required to validate our findings and elucidate the related mechanisms in pregnant women.
N‐3 PUFA, an essential fatty acid, must be obtained through the diet. To date, there is an important amount of scientific data related to the protective and beneficial impacts of n‐3 PUFA and their effects against inflammation, heart diseases and glucose metabolism 55 . With rising evidence supporting the benefits of increased dietary n‐3 PUFA intake, the American Dietetic Association and the Dietitians of Canada both recommend at least 500 mg/day of dietary n‐3 PUFA intake for all healthy adults, including pregnant and lactating women 56 . To obtain sufficient n‐3 PUFA, Dietary Guidelines for Chinese Residents (2016) also recommend pregnant women should consume fish at least two or three times per week 57 . The present study observed the moderation effect of dietary n‐3 PUFA intake on the relationship of maternal plasma irisin with HOMA‐IR and HOMA‐IS, which show important implications for the prevention and management of glucose metabolism in pregnant women. The recommendation of dietary n‐3 PUFA consumption in clinical consultation might show benefits for pregnant women with decreased plasma irisin levels or increased IR.
This is the first reported study to investigate the moderation effect of maternal plasma irisin and dietary n‐3 PUFA on glucose metabolism. However, several potential limitations merit further discussion. First, it is impossible to provide enough information on prospective changes in maternal glucose metabolism and plasma irisin based on a cross‐sectional study. Nevertheless, we will continue to follow our cohort to observe these changes in the future. Second, we could not rule out the possibility of unmeasured and unknown confounders. However, associations persisted after we adjusted for major confounding factors. Third, reporting bias of maternal fatty acid intake with FFQ data is inevitable. Nevertheless, we used a validated semiquantitative FFQ and photographs of foods with a standard portion size for assistance, which could minimize the bias. Finally, the reported circulating levels of irisin seem to differ greatly, even in the same species, with concentrations being reported in humans between 0.01 and 2,000 ng/mL 58 . These differences probably come from the variability in the commercial enzyme‐linked immunosorbent assay kits or populations. Furthermore, most participants of the present study were Han Chinese (96.74%), and whether these findings can also be extended to other ethnic groups remains to be determined.
The present findings suggested that maternal plasma irisin was negatively associated with HOMA‐IR and positively associated with HOMA‐IS, which was more pronounced in pregnant women who had higher intakes of n‐3 PUFA. Dietary n‐3 PUFA might play a role in modifying the possible effect of maternal plasma irisin on HOMA‐IR and HOMA‐IS. Further longitudinal studies are required to confirm this finding and explore the potential mechanisms.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Multivariable odds ratios with 95% confidence intervals for the relationship of interaction term with the risk of gestational diabetes mellitus in pregnant women.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81602862 and 81571452); the Natural Science Foundation of Guangdong Province (No. 2016A030310150 and 2019A1515011462); and LC and RG also received support from the Sanming Project of Medicine in Shenzhen (SZSM201803061).
J Diabetes Investig 2020; 11: 1326–1335
Li Cai and Weijia Wu contributed equally to this work.
References
- 1. Boden G. Fuel metabolism in pregnancy and in gestational diabetes mellitus. Obstet Gynecol Clin North Am 1996; 23: 1–10. [DOI] [PubMed] [Google Scholar]
- 2. Knopp RH. Hormone‐mediated changes in nutrient metabolism in pregnancy: a physiological basis for normal fetal development. Ann NY Acad Sci 2010; 817: 251–271. [DOI] [PubMed] [Google Scholar]
- 3. Retnakaran R, Qi Y, Sermer M, et al Glucose intolerance in pregnancy and future risk of pre‐diabetes or diabetes. Diabetes Care 2008; 31: 2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jarrett RJ. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 5: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 5. Polyzos SA, Anastasilakis AD, Efstathiadou ZA, et al Irisin in metabolic diseases. Endocrine 2018; 59: 260–274. [DOI] [PubMed] [Google Scholar]
- 6. Bostrom P, Wu J, Jedrychowski MP, et al A pgc1‐alpha‐dependent myokine that drives brown‐fat‐like development of white fat and thermogenesis. Nature 2012; 481: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang X, Enerback S, Smith U. Reduced expression of foxc2 and brown adipogenic genes in human subjects with insulin resistance. Obes Res 2003; 11: 1182–1191. [DOI] [PubMed] [Google Scholar]
- 8. Kuzmicki M, Telejko B, Lipinska D, et al Serum irisin concentration in women with gestational diabetes. Gynecol Endocrinol 2014; 30: 636–639. [DOI] [PubMed] [Google Scholar]
- 9. Yuksel MA, Oncul M, Tuten A, et al Maternal serum and fetal cord blood irisin levels in gestational diabetes mellitus. Diabetes Res Clin Pract 2014; 104: 171–175. [DOI] [PubMed] [Google Scholar]
- 10. Erol O, Erkal N, Ellidag HY, et al Irisin as an early marker for predicting gestational diabetes mellitus: a prospective study. J Matern Fetal Neonatal Med 2016; 29: 3590–3595. [DOI] [PubMed] [Google Scholar]
- 11. Ebert T, Stepan H, Schrey S, et al Serum levels of irisin in gestational diabetes mellitus during pregnancy and after delivery. Cytokine 2014; 65: 153–158. [DOI] [PubMed] [Google Scholar]
- 12. Piya MK, Harte AL, Sivakumar K, et al The identification of irisin in human cerebrospinal fluid: influence of adiposity, metabolic markers, and gestational diabetes. Am J Physiol Endocrinol Metab 2014; 306: E512–E518. [DOI] [PubMed] [Google Scholar]
- 13. Zhao L, Li J, Li ZL, et al Circulating irisin is lower in gestational diabetes mellitus. Endocr J 2015; 62: 921–926. [DOI] [PubMed] [Google Scholar]
- 14. Wang P, Ma HH, Hou XZ, et al Reduced plasma level of irisin in first trimester as a risk factor for the development of gestational diabetes mellitus. Diabetes Res Clin Pract 2018; 142: 130–138. [DOI] [PubMed] [Google Scholar]
- 15. Sanchis‐Gomar F, Perez‐Quilis C. Irisinemia: a novel concept to coin in clinical medicine? Ann Nutr Metab 2013; 63: 60–61. [DOI] [PubMed] [Google Scholar]
- 16. Fedor D, Kelley DS. Prevention of insulin resistance by n‐3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care 2009; 12: 138–146. [DOI] [PubMed] [Google Scholar]
- 17. Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 2008; 118: 2992–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohammadi E, Rafraf M, Farzadi L, et al Effects of omega‐3 fatty acids supplementation on serum adiponectin levels and some metabolic risk factors in women with polycystic ovary syndrome. Asia Pac J Clin Nutr 2012; 21: 511–518. [PubMed] [Google Scholar]
- 19. Oh DY, Talukdar S, Bae EJ, et al Gpr120 is an omega‐3 fatty acid receptor mediating potent anti‐inflammatory and insulin‐sensitizing effects. Cell 2010; 142: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 21. Yang HX. Diagnostic criteria for gestational diabetes mellitus (ws 331–2011). Chin Med J (Engl) 2012; 125: 1212–1213. [PubMed] [Google Scholar]
- 22. Zhang CX, Ho SC. Validity and reproducibility of a food frequency questionnaire among chinese women in guangdong province. Asia Pac J Clin Nutr 2009; 18: 240–250. [PubMed] [Google Scholar]
- 23. Yang YXWGPX. China Food Composition. Beijing: Peking University Medical Press, 2002. [Google Scholar]
- 24. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. AM J CLIN NUTR 1229S; 65: 1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 25. Committee IR . Guidelines for data processing and analysis of the international physical activity questionnaire (ipaq)‐short and long forms[j]. 2005.
- 26. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc 1995; 57: 289–300. [Google Scholar]
- 27. Roca‐Rivada A, Castelao C, Senin LL, et al Fndc5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013; 8: e60563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vaughan RA, Gannon NP, Barberena MA, et al Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes Metab 2014; 16: 711–718. [DOI] [PubMed] [Google Scholar]
- 29. Huh JY, Dincer F, Mesfum E, et al Irisin stimulates muscle growth‐related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes (Lond) 2014; 38: 1538–1544. [DOI] [PubMed] [Google Scholar]
- 30. Larance M, Ramm G, James DE. The glut4 code. Mol Endocrinol 2008; 22: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Atkinson BJ, Griesel BA, King CD, et al Moderate glut4 overexpression improves insulin sensitivity and fasting triglyceridemia in high‐fat diet‐fed transgenic mice. Diabetes 2013; 62: 2249–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yen‐Hao C, Saleh H, Jung‐Min L, et al Mirna‐93 inhibits glut4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 2013; 2278–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen JQ, Huang YY, Gusdon AM, et al Irisin: a new molecular marker and target in metabolic disorder. Lipids Health Dis 2015; 14: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fisher FM, Kleiner S, Douris N, et al Fgf21 regulates pgc‐1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 2012; 26: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu TY, Shi CX, Gao R, et al Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the pi3k/akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci (Lond) 2015; 129: 839–850. [DOI] [PubMed] [Google Scholar]
- 36. Tang H, Yu R, Liu S, et al Irisin inhibits hepatic cholesterol synthesis via ampk‐srebp2 signaling. EBioMedicine 2016; 6: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xin C, Liu J, Zhang J, et al Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the ampk signaling pathway. Int J Obes (Lond) 2016; 40: 443–451. [DOI] [PubMed] [Google Scholar]
- 38. DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non‐insulin‐dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism 1989; 38: 387–395. [DOI] [PubMed] [Google Scholar]
- 39. Perriello G, Pampanelli S, Del SP, et al Evidence of increased systemic glucose production and gluconeogenesis in an early stage of niddm. Diabetes 1997; 46: 1010–1016. [DOI] [PubMed] [Google Scholar]
- 40. Huh JY, Mougios V, Kabasakalis A, et al Exercise‐induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through ampk activation. J Clin Endocrinol Metab 2014; 99: E2154–E2161. [DOI] [PubMed] [Google Scholar]
- 41. Zisman A, Peroni OD, Abel ED, et al Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 2000; 6: 924–928. [DOI] [PubMed] [Google Scholar]
- 42. American diabetes association . Postprandial blood glucose. Diabetes Care 2001; 24: 775–778. [DOI] [PubMed] [Google Scholar]
- 43. Zhang F, Dong L, Zhang CP, et al Increasing prevalence of gestational diabetes mellitus in chinese women from 1999 to 2008. Diabet Med 2011; 28: 652–657. [DOI] [PubMed] [Google Scholar]
- 44. Gao C, Sun X, Lu L, et al Prevalence of gestational diabetes mellitus in mainland china: a systematic review and meta‐analysis. J Diabetes Investig 2019; 10: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang JZGZR. A longitudinal study on the difference of perinatal period weight gain between gestational diabetes mellitus and normal pregnant women. Chin J Obstet Gynecol Pediatr 2016; 12: 680–685. (Chinese). [Google Scholar]
- 46. Xu XZXJY. The incidence and risk factors of gestational diabetes mellitus in 2748 hospitalized pregnant women. Acta Univ Med Nanjing 2015; 35: 695–698. (Chinese). [Google Scholar]
- 47. Ben‐Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med 2004; 21: 103–113. [DOI] [PubMed] [Google Scholar]
- 48. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr 1975S; 94: 1975S–1979S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Storlien LH, Kraegen EW, Chisholm DJ, et al Fish oil prevents insulin resistance induced by high‐fat feeding in rats. Science 1987; 237: 885–888. [DOI] [PubMed] [Google Scholar]
- 50. Taouis M, Dagou C, Ster C, et al N‐3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol Endocrinol Metab 2002; 282: E664–E671. [DOI] [PubMed] [Google Scholar]
- 51. Vaughan RA. Conjugated linoleic acid or omega 3 fatty acids increase mitochondrial biosynthesis and metabolism in skeletal muscle cells. Lipids Health Dis 2012; 11: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huh JY, Panagiotou G, Mougios V, et al Fndc5 and irisin in humans: i. Predictors of circulating concentrations in serum and plasma and ii. Mrna expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012; 61: 1725–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ansari S, Djalali M, Mohammadzadeh HN, et al The effect of n‐3 polyunsaturated fatty acids supplementation on serum irisin in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled trial. Int J Endocrinol Metab 2017; 15: e40614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Agh F, Mohammadzadeh HN, Djalali M, et al Omega‐3 fatty acid could increase one of myokines in male patients with coronary artery disease: a randomized, double‐blind, placebo‐controlled trial. Arch Iran Med 2017; 20: 28. [PubMed] [Google Scholar]
- 55. Gogus U, Smith C. N‐3 omega fatty acids: a review of current knowledge. Int J Food Sci Technol 2010; 45: 417–436. [Google Scholar]
- 56. Kris‐Etherton PM, Innis S, American Dietetic Association , et al Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc 2007; 107: 1599–1611. [PubMed] [Google Scholar]
- 57. Wang SS, Lay S, Yu HN, et al Dietary guidelines for Chinese residents (2016): comments and comparisons. J Zhejiang Univ Sci B 2016; 17: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Perakakis N, Triantafyllou GA, Fernandez‐Real JM, et al Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol 2017; 13: 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Multivariable odds ratios with 95% confidence intervals for the relationship of interaction term with the risk of gestational diabetes mellitus in pregnant women.


