Abstract
Aims/Introduction
We assessed the relationship between diabetic retinopathy (DR) and/or diabetic kidney disease (DKD) according to their severity and all‐cause, cancer, vascular and non‐cancer non‐vascular mortality in real‐world patients with type 2 diabetes.
Materials and Methods
A total of 1,902 patients with type 2 diabetes were enrolled from 1995 to 1999 and followed to 2017. At baseline, DR was diagnosed in 374 patients, DKD in 529, vision‐threatening DR in 123 and advanced DKD in 287. Patients were classified by the status of DR and DKD. Multivariate Cox regression analysis was carried out.
Results
There were 266 deaths during a median follow‐up period of 18.6 years. Among these, 92 were from cancer, 78 were from vascular causes and 82 were from non‐cancer non‐vascular causes. DR and/or DKD predicted all‐cause, vascular and non‐cancer non‐vascular mortality, but not cancer mortality. Similarly, vision‐threatening DR and/or advanced DKD predicted all‐cause, vascular and non‐cancer non‐vascular mortality, but not cancer mortality. Hazard ratios for all‐cause, vascular and non‐cancer non‐vascular mortality were highest in the DR(+)DKD(+) group, and higher in the DR(−)DKD(+) and the DR(+)DKD(−) groups than in the DR(−)DKD(−) group. The results for vision‐threatening DR and advanced DKD were similar. The interaction for non‐cancer non‐vascular mortality, but not all‐cause and vascular mortality, between DR and DKD and between vision‐threatening DR and advanced DKD might be significant.
Conclusions
DR and DKD may be jointly and independently associated with all‐cause, vascular and non‐cancer non‐vascular mortality, but not cancer mortality, according to their severity in real‐world patients with type 2 diabetes.
Keywords: Diabetic kidney disease, Diabetic retinopathy, Mortality
Diabetic retinopathy and diabetic kidney disease may be jointly and independently associated with all‐cause, vascular and non‐cancer non‐vascular mortality, but not cancer mortality, according to the severity of these microvascular complications in real‐world patients with type 2 diabetes.

INTRODUCTION
Cancer and cardiovascular disease (CVD) account for a large number of causes of death worldwide. Each of these diseases is a distinct and independent disease, but they have various similarities, including a number of common risk factors, such as diabetes and obesity. Emerging evidence on chronic inflammation, oxidative stress and reactive oxygen species, and additional mechanisms (e.g., hormones, cytokines, growth factors and other metabolic reactions), indicates a shared biology of cancer and CVD 1 , 2 , 3 .
Diabetes affects CVD and the neoplastic process through several mechanisms including hyperinsulinemia, hyperglycemia, insulin‐like growth factor, and inflammation 1 . Epidemiological studies suggest that type 2 diabetes is associated with an increased risk for some cancers, such as liver, pancreatic, endometrial, colon and rectum, breast, and bladder cancers 4 , 5 . Type 2 diabetes and cancer share some risk factors, such as aging, obesity, diet and physical inactivity, but potential biological associations between these two diseases are not fully understood. Furthermore, diabetes is also related to non‐cancer non‐vascular death as a result of infectious diseases, external causes, intentional self‐harm and degenerative disorders, independent of several major risk factors 6 .
Diabetic retinopathy (DR) and diabetic kidney disease (DKD), which are specific microvascular complications of diabetes, increase the risk of CVD morbidity and mortality in patients with type 2 diabetes 7 , 8 , 9 , 10 , 11 , 12 . Among Japanese patients with diabetes, the most common cause of death is cancer 13 , 14 , 15 . Whether DR and DKD independently predict mortality, especially cancer and non‐cancer non‐vascular mortality, remains unclear. Furthermore, the effect of the biological interaction 16 , 17 , 18 , 19 between DR and DKD on mortality, according to the severity of these microvascular complications, is unclear.
Therefore, we assessed the relationship between DR and/or DKD and mortality due to all causes, cancer, vascular causes, and non‐cancer non‐vascular causes in patients with type 2 diabetes according to the severity of these microvascular complications. Additionally, the biological interaction of coexisting DR and DKD was analyzed according to their severity.
METHODS
Study participants
A flowchart of patients included in this study is shown in Figure 1. A total of 5,510 patients initially visited our clinic from 1995 to 1999 and were screened for eligibility. Of these 5,510 patients, 1,928 were diagnosed with type 2 diabetes and were followed up for ≥1 year. Of these 1,928 patients, 26 had missing data (14 for retinopathy, 10 for smoking and/or drinking, one for urine protein level, and one for serum lipid levels). Therefore, 1,902 patients were included in this retrospective cohort study. The 1,902 patients were subsequently followed for survival to 2017 and were provided with questionnaires.
Figure 1.
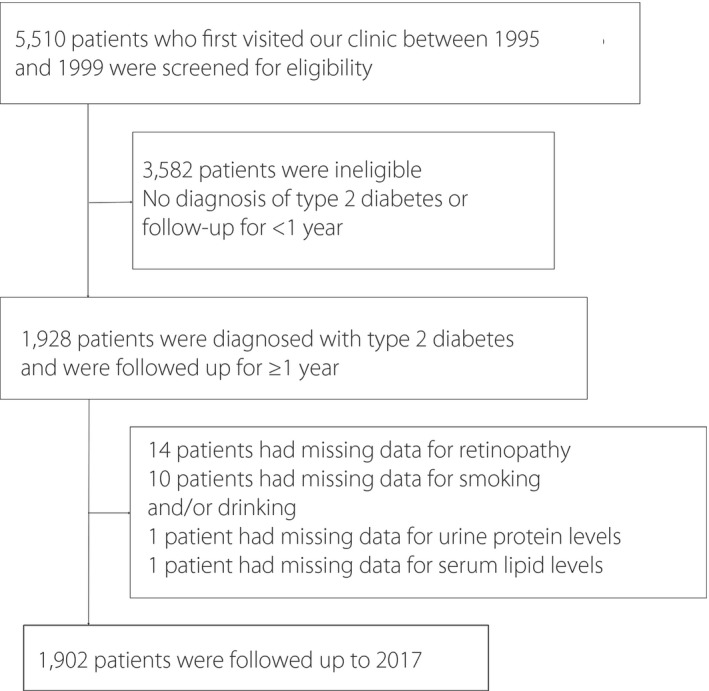
Flowchart of the patients included in the present study.
This study protocol was approved by the ethics committee of the Institute for Adult Diseases, Asahi Life Foundation in accordance with the Japanese Government’s Ethical Guidelines for Medical and Health Research Involving Human Subjects. The study protocol conforms to the provisions of the Declaration of Helsinki. The use of the opt‐out approach for consent in the clinic was approved by the committee.
Assessment of DR and DKD
At baseline, DR was evaluated by ophthalmologists with a subspecialty in diabetes. The existence and severity of DR were determined by a mydriatic indirect funduscopic examination, a slit‐lamp biomicroscopic fundus examination using a precorneal lens, and fluorescein angiography if necessary. DR treated with photocoagulation for progressive DR (severe non‐proliferative or proliferative DR) 20 before the first visit or within 1 year after the first visit was defined as vision‐threatening DR (VTDR).
Serum creatinine levels, which were measured by the Jaffe–Rate method until 11 June 1995, were converted to enzymatic method equivalents by a linear regression equation, which was derived from duplicate assays. The estimated glomerular filtration rate (eGFR) was calculated using the equation that was advocated by the Japanese Society of Nephrology 21 . Urine protein was measured by a test strip, and an automated urine chemistry analyzer (Siemens Healthcare K.K., Tokyo, Japan) was used from March 1995. Urine protein ± and 1 + correspond to urine protein concentrations of 0.15 g/L and 0.3 g/L, respectively. DKD was defined as urine protein ≥± or an eGFR <60 mL/min/1.73 m2. Advanced DKD was defined as urine protein ≥1+ or an eGFR <45 mL/min/1.73 m2.
Assessment of end‐points
End‐points were determined as death from all causes, cancer, vascular causes and non‐cancer non‐vascular causes. A complete review of medical records, which were written by the attending physicians, and questionnaire responses from family members provided these end‐points. The causes of death were identified using unified criteria. Patients who were lost to follow‐up were regarded as censored cases at their final visit to the clinic. Unknown causes of death were excluded in the analysis for cancer, vascular and non‐cancer non‐vascular mortality.
Measurements of covariates
We measured various covariates, including glycated hemoglobin (HbA1c) levels, blood pressure (BP), total cholesterol (TC) levels and high‐density lipoprotein cholesterol levels, as previously reported 22 . Patients who drink ≥20 g of alcohol per day were designated as drinkers.
Statistical analysis
Continuous variables are shown as the mean ± standard deviation. Categorical variables are described by number (%). The follow‐up period is expressed as the median (interquartile range) because of its skewed distribution. Differences in baseline characteristics between study participants who completed and those who did not complete follow up were analyzed. The Student’s t‐test and Wilcoxon rank‐sum test were used to analyze continuous variables, whereas the χ2‐test was used to analyze categorical variables. Differences in baseline characteristics among four groups, which were categorized according to the presence or absence of DR and DKD, or the presence or absence of VTDR and advanced DKD were also analyzed. anova and the Scheffé test were used to analyze continuous variables, and the Cochran–Armitage trend test was used to analyze categorical variables.
Kaplan–Meier curves of the four groups categorized by the DR and DKD status for all‐cause, cancer, vascular and non‐cancer non‐vascular mortality were plotted, and the distribution of survival was compared across categories using the log–rank test. The proportional hazard assumption was tested using log–log plots. Multivariate Cox proportional hazard models were used to determine the hazard ratios for all‐cause, cancer, vascular and non‐cancer non‐vascular mortality related to DR and DKD or VTDR and advanced DKD. In detail, explanatory variables included the following categories: DR(+) in model 1, DKD(+) in model 2, DR(+) and DKD(+) in model 3, and DR(–)DKD(–), DR(–)DKD(+), DR(+)DKD(–) and DR(+)DKD(+) in model 4. Similarly, VTDR(+) was included in model 5, advanced DKD(+) in model 6, VTDR(+) and advanced DKD(+) in model 7, and VTDR(–) advanced DKD(–), VTDR(–) advanced DKD(+), VTDR(+) advanced DKD(–) and VTDR(+) advanced DKD(+) in model 8. Models 1–8 were constructed for each of the four outcomes. Covariates were age, sex, duration of diabetes, body mass index (BMI), HbA1c levels, systolic BP (SBP), TC levels, high‐density lipoprotein cholesterol levels, smoking status and alcohol consumption at baseline.
Biological interaction was calculated to evaluate the combined effects of DR and DKD or those of VTDR and advanced DKD on mortality along with their independent effects 16 , 17 , 18 , 19 . Three measures of biological interaction, including the relative excess risk due to interaction (RERI), the attributable proportion due to interaction (AP) and the synergy index (S), were calculated as described by Andersson et al. 17 When there is no biological interaction, RERI = 0 and AP = 0, and S = 1 19 .
All data were analyzed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). A P‐value of <0.05 (two‐sided) was considered significant.
RESULTS
DR was diagnosed in 374 patients, DKD in 529, VTDR in 123 and advanced DKD in 287 at baseline. There were 266 deaths during a median follow‐up period of 18.6 years (interquartile range 9.7–20.7 years). Of the 266 deaths, 92 were from cancer, 78 were from vascular causes and 82 were from non‐cancer non‐vascular causes, including 27 from infection, 12 from renal failure, nine from respiratory disease, seven from digestive disease, one from diabetes and 26 from other causes (senility, drowning, a fall, cervical spine injury due to a fall, burn, heat stroke, asphyxia, multiple organ failure, amyotrophic lateral sclerosis, subdural hematomas due to a fall and a traffic accident). Additionally, 14 deaths had unknown causes. Of the 92 cancer deaths, 19 were due to lung cancer, 18 to pancreatic cancer, 10 to colon cancer, nine to liver cancer, six to stomach and prostate cancer, five to blood cancer, four to bile duct cancer, three to esophageal cancer, two to hypopharyngeal cancer, and nine to other types of cancer (one case each of the adrenal gland, bladder, brain, breast, duodenum, gallbladder, ileocecal region, kidney, uterus and unknown). The crude incidence ratios (1,000 person‐years) were 9.07 (95% confidence interval [CI] 4.81–13.33) for all‐cause mortality, 3.16 (95% CI 0.63–5.69) for cancer mortality, 2.68 (95% CI 0.35–5.01) for vascular mortality and 2.81 (95% CI 0.42–5.20) for non‐cancer non‐vascular mortality. The overall follow‐up rate was 66.9% (1,272/1,902). The baseline clinical characteristics of the patients who completed and those who did not complete follow up are shown in Table S1. A significantly higher rate of men (P = 0.008) and alcohol drinkers (P = 0.001), a significantly lower rate of patients with DKD (P = 0.002) and those with advanced DKD (P = 0.021), and a significantly lower BMI (P = 0.043) were observed in patients who completed follow up compared with those who did not complete follow up.
The baseline clinical characteristics of patients who were classified according to the status of DR and DKD are shown in Table 1. Patients with both DR and DKD were older; less likely to be men; current smokers; drinkers; had a longer duration of diabetes; higher HbA1c, SBP, DBP and TC levels; a lower eGFR; and comprised a higher proportion of users of oral antidiabetic drugs, insulin and antihypertensive agents at baseline compared with those with neither DR nor DKD (all P < 0.05). Similar trends in patients with both DR and DKD were observed for age, the duration of diabetes, HbA1c levels, SBP, DBP, TC levels and eGFR compared with those with either DR or DKD (all P < 0.05). Additionally, patients with both VTDR and advanced DKD were older; less likely to be men; current smokers; drinkers; had a longer duration of diabetes; higher HbA1c levels, SBP, DBP and TC levels; a lower eGFR; and comprised a higher proportion of users of insulin, antihypertensive agents and lipid‐lowering agents at baseline compared with those with neither VTDR nor advanced DKD (all P < 0.05). Similar trends in patients with both VTDR and advanced DKD were observed for the duration of diabetes, HbA1c levels, SBP, TC levels and eGFR compared with those with either VTDR or advanced DKD (all P < 0.05).
Table 1.
Baseline clinical characteristics of patients who were classified according to the status of diabetic retinopathy and diabetic kidney disease
|
DR (–) DKD (–) |
DR (–) DKD (+) |
DR (+) DKD (–) |
DR (+) DKD (+) |
P‐value |
VTDR (–) Advanced DKD (–) |
VTDR (–) Advanced DKD (+) |
VTDR (+) Advanced DKD (–) |
VTDR (+) Advanced DKD (+) |
P‐value | |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 1172 | 356 | 201 | 173 | 1558 | 221 | 57 | 66 | ||
| Men (%) | 961 (82.0) | 283 (79.5) | 150 (74.6) | 133 (76.9) | 0.012 | 1259 (80.8) | 181 (81.9) | 38 (66.7) | 49 (74.2) | 0.044 |
| Age (years) | 54.5 ± 9.4 | 56.3 ± 11.2* | 57.2 ± 9.8* | 59.3 ± 10.0*† | <0.0001 | 55.2 ± 9.8 | 56.1 ± 10.9 | 59.9 ± 8.9** | 59.0 ± 9.9** | <0.0001 |
| Duration of diabetes (years) | 4.7 ± 5.7 | 4.4 ± 5.5 | 11.6 ± 9.0*† | 13.1 ± 8.4*† | <0.0001 | 5.5 ± 6.4 | 6.5 ± 7.3 | 14.1 ± 10.0**§ | 14.4 ± 7.9**§ | <0.0001 |
| HbA1c (%) (mmol/mol) | 7.9 ± 1.6 (63 ± 18) | 8.1 ± 1.6 (65 ± 18) | 8.8 ± 1.6 (73 ± 18)*† | 8.7 ± 1.6 (72 ± 18)*† | <0.0001 | 8.0 ± 1.7 (64 ± 18) | 8.3 ± 1.6 (67 ± 17) | 8.6 ± 1.5 (70 ± 17) | 9.0 ± 1.9 (75 ± 20)**§ | <0.0001 |
| BMI (kg/m2) | 23.4 ± 3.2 | 24.5 ± 4.2* | 22.5 ± 3.1*† | 23.3 ± 3.3† | <0.0001 | 23.4 ± 3.4 | 24.8 ± 4.0** | 22.2 ± 3.2§ | 22.9 ± 3.2§ | <0.0001 |
| SBP (mmHg) | 128.5 ± 18.2 | 134.2 ± 22.1* | 132.7 ± 21.5 | 143.7 ± 23.3*†‡ | <0.0001 | 129.1 ± 18.7 | 140.3 ± 24.2** | 138.9 ± 24.0** | 148.6 ± 22.6**§ | <0.0001 |
| DBP (mmHg) | 75.0 ± 11.3 | 78.5 ± 13.8* | 75.2 ± 12.0† | 79.0 ± 12.4*‡ | <0.0001 | 75.1 ± 11.4 | 81.0 ± 14.2** | 76.5 ± 12.0 | 80.7 ± 11.8** | <0.0001 |
| TC (mmol/L) | 5.52 ± 0.96 | 5.78 ± 1.07* | 5.40 ± 0.96† | 5.82 ± 1.28*‡ | <0.0001 | 5.53 ± 0.98 | 5.79 ± 1.13** | 5.38 ± 1.02 | 6.21 ± 1.37**§¶ | <0.0001 |
| HDL‐C (mmol/L) | 1.33 ± 0.35 | 1.29 ± 0.34 | 1.37 ± 0.39 | 1.34 ± 0.37 | 0.066 | 1.33 ± 0.35 | 1.25 ± 0.31** | 1.40 ± 0.42 | 1.40 ± 0.44§ | 0.002 |
| eGFR (mL/min/1.73 m2) | 82.7 ± 15.5 | 73.7 ± 22.0* | 87.2 ± 20.8*† | 71.1 ± 27.1*‡ | <0.0001 | 81.7 ± 17.9 | 75.2 ± 23.3** | 85.5 ± 18.5§ | 64.8 ± 27.7**§¶ | <0.0001 |
| Current smoker | 503 (42.9) | 133 (37.4) | 83 (41.3) | 61 (35.3) | 0.049 | 656 (42.1) | 86 (38.9) | 17 (29.8) | 21 (31.8) | 0.016 |
| Alcohol intake | 737 (62.9) | 211 (59.3) | 101 (50.3) | 73 (42.2) | <0.0001 | 949 (60.9) | 127 (57.5) | 23 (40.4) | 23 (34.9) | <0.0001 |
| Initial therapies | ||||||||||
| Oral antidiabetic drugs | 466 (39.8) | 148 (41.6) | 121 (60.2) | 87 (50.3) | <0.0001 | 661 (42.5) | 103 (46.6) | 33 (57.9) | 25 (37.9) | 0.36 |
| Insulin | 93 (7.9) | 30 (8.4) | 61 (30.4) | 77 (44.5) | <0.0001 | 161 (10.3) | 35 (15.8) | 23 (40.4) | 42 (63.6) | <0.0001 |
| Antihypertensive agents | 164 (14.0) | 108 (30.3) | 33 (16.4) | 82 (47.4) | <0.0001 | 244 (15.7) | 93 (42.1) | 11 (19.3) | 39 (59.1) | <0.0001 |
| Lipid‐lowering agents | 131 (11.2) | 54 (15.2) | 22 (11.0) | 26 (15.0) | 0.17 | 180 (11.6) | 32 (14.5) | 8 (14.0) | 13 (19.7) | 0.027 |
Values are n (%) or mean ± standard deviation. P‐values were derived from anova for continuous variables, and from the Cochran–Armitage trend test for categorical variables.
*P < 0.05 versus diabetic retinopathy (DR) (–) diabetic kidney disease (DKD) (–); † P < 0.05 versus DR (–) DKD (+); ‡ P < 0.05 versus DR (+) DKD (–); **P < 0.05 versus vision‐threatening diabetic retinopathy (VTDR) (–) advanced DKD (–); § P < 0.05 versus VTDR (–) advanced DKD (+); ¶ P < 0.05 versus VTDR (+) advanced DKD (–).
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol.
Kaplan–Meier curves of the four groups categorized by DR and DKD for all‐cause, cancer, vascular and non‐cancer non‐vascular mortality are shown in Figure 2. Compared with the DR(–)DKD(–) group, the DR(–)DKD(+) and the DR(+)DKD(–) groups had lower survival, and the DR(+)DKD(+) group had the lowest survival for all‐cause, vascular and non‐cancer non‐vascular mortality (log–rank test all P < 0.0001), but not cancer mortality (Figure 2). Additionally, Kaplan–Meier curves of the four groups categorized by VTDR and advanced DKD for all‐cause, cancer, vascular, and non‐cancer non‐vascular mortality are also shown in Figure S1). Compared with the VTDR(–) advanced DKD(–) group, the VTDR(–) advanced DKD(+) and the VTDR(+) advanced DKD(–) groups had lower survival, and the VTDR(+) advanced DKD(+) group had the lowest survival for all‐cause, vascular and non‐cancer non‐vascular mortality (log–rank test all P < 0.0001), but not cancer mortality (Figure S1).
Figure 2.
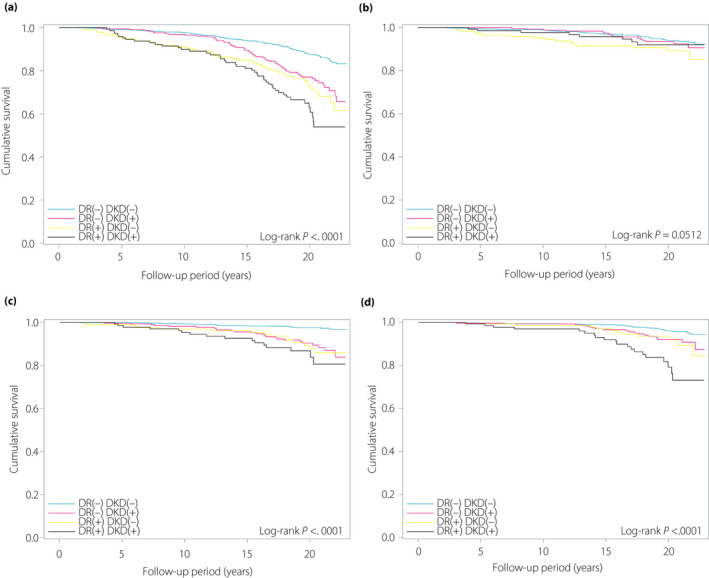
Kaplan–Meier curves according to diabetic retinopathy (DR) and diabetic kidney disease (DKD) status for all‐cause, cancer, vascular and non‐cancer non‐vascular mortality. (a) All‐cause mortality. (b) Cancer mortality. (c) Vascular mortality. (d) Non‐cancer non‐vascular mortality.
Multivariate Cox proportional hazards models for all‐cause, cancer, vascular and non‐cancer non‐vascular mortality related to DR and DKD are shown in Table 2. Age, sex, duration of diabetes, BMI, HbA1c, SBP, TC, high‐density lipoprotein cholesterol, smoking status and alcohol consumption at baseline were adjusted in all models. In model 1, the presence of DR was a significant predictor of all‐cause, vascular and non‐cancer non‐vascular mortality (P = 0.0002, P = 0.003 and P = 0.002, respectively), but not cancer mortality. In model 2, the presence of DKD was a significant predictor of all‐cause, vascular and non‐cancer non‐vascular mortality (P = 0.003, P = 0.002 and P = 0.003, respectively), but not cancer mortality. In model 3, DR and DKD simultaneously predicted all‐cause mortality (P = 0.001 and P = 0.016, respectively), vascular mortality (P = 0.014 and P = 0.007, respectively) and non‐cancer non‐vascular mortality (P = 0.008 and P = 0.011, respectively), but not cancer mortality. In model 4, patients were categorized into four groups according to the presence or absence of DR and DKD. Hazard ratios for all‐cause, vascular and non‐cancer non‐vascular mortality were highest in the DR(+)DKD(+) group (P < 0.0001, P = 0.0006 and P < 0.0001, respectively), and higher in the DR(−)DKD(+) group (P = 0.010, P = 0.0004 and P = 0.15, respectively) and the DR(+)DKD(−) group (P = 0.001, P = 0.0005 and P = 0.18, respectively) than in the DR(−)DKD(−) group.
Table 2.
Multivariate Cox proportional hazard models for all‐cause, cancer, vascular, and non‐cancer non‐vascular mortality in association with diabetic retinopathy and diabetic kidney disease
| All‐cause mortality | n with/without outcome | Incidence rate (1,000 person‐years) | Model 1 (266/1902) | Model 2 (266/1902) | Model 3 (266/1902) | Model 4 (266/1902) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
| DR (+) | 88/286 | 17.1 | 1.76 (1.31–2.35) | 0.0002 | 1.65 (1.23–2.22) | 0.001 | ||||
| DKD (+) | 107/422 | 14.3 | 1.49 (1.15–1.93) | 0.003 | 1.38 (1.06–1.80) | 0.016 | ||||
| Categorization into 4 groups | ||||||||||
| DR (–) DKD (–) | 116/1,056 | 6.1 | 1 | |||||||
| DR (–) DKD (+) | 62/294 | 11.9 | 1.53 (1.11–2.10) | 0.010 | ||||||
| DR (+) DKD (–) | 43/158 | 15.1 | 1.87 (1.28–2.72) | 0.001 | ||||||
| DR (+) DKD (+) † | 45/128 | 19.7 | 2.17 (1.48–3.18) | <0.0001 | ||||||
| Cancer mortality | n with/without outcome | Incidence rate (1,000 person‐years) | Model 1 (92/1,888) | Model 2 (92/1,888) | Model 3 (92/1,888) | Model 4 (92/1,888) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
| DR (+) | 24/348 | 4.7 | 1.27 (0.75–2.14) | 0.38 | 1.32 (0.77–2.25) | 0.32 | ||||
| DKD (+) | 24/501 | 3.2 | 0.87 (0.53–1.41) | 0.56 | 0.83 (0.51–1.36) | 0.46 | ||||
| Categorization into 4 groups | ||||||||||
| DR (–) DKD (–) | 52/1,111 | 2.8 | 1 | |||||||
| DR (–) DKD (+) | 16/337 | 3.1 | 1.00 (0.56–1.80) | 0.99 | ||||||
| DR (+) DKD (–) | 16/184 | 5.6 | 1.58 (0.86–2.92) | 0.14 | ||||||
| DR (+) DKD (+) | 8/164 | 3.5 | 0.90 (0.40–1.98) | 0.79 | ||||||
| Vascular mortality | n with/without outcome | Incidence rate (1,000 person‐years) | Model 1 (78/1888) | Model 2 (78/1888) | Model 3 (78/1888) | Model 4 (78/1888) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
| DR (+) | 31/341 | 6.1 | 2.24 (1.32–3.82) | 0.003 | 1.98 (1.15–3.40) | 0.014 | ||||
| DKD (+) | 41/484 | 5.5 | 2.13 (1.33–3.41) | 0.002 | 1.93 (1.20–3.11) | 0.007 | ||||
| Categorization into 4 groups | ||||||||||
| DR (–) DKD (–) | 22/1,141 | 1.2 | 1 | |||||||
| DR (–) DKD (+) | 25/328 | 4.8 | 2.92 (1.61–5.29) | 0.0004 | ||||||
| DR (+) DKD (–) | 15/185 | 5.3 | 3.51 (1.73–7.14) | 0.0005 | ||||||
| DR (+) DKD (+) ‡ | 16/156 | 7.0 | 3.56 (1.73–7.33) | 0.0006 | ||||||
| Non‐cancer non‐vascular mortality | n with/without outcome | Incidence rate (1,000 person‐years) | Model 1 (82/1888) | Model 2 (82/1888) | Model 3 (82/1888) | Model 4 (82/1888) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
| DR (+) | 31/341 | 6.1 | 2.26 (1.35–3.78) | 0.002 | 2.04 (1.21–3.44) | 0.008 | ||||
| DKD (+) | 38/487 | 5.1 | 2.01 (1.27–3.17) | 0.003 | 1.82 (1.15–2.90) | 0.011 | ||||
| Categorization into 4 groups | ||||||||||
| DR (–) DKD (–) | 33/1,130 | 1.8 | 1 | |||||||
| DR (–) DKD (+) | 18/335 | 3.5 | 1.55 (0.85–2.83) | 0.15 | ||||||
| DR (+) DKD (–) | 11/189 | 3.9 | 1.65 (0.80–3.42) | 0.18 | ||||||
| DR (+) DKD (+) § | 20/152 | 8.8 | 3.87 (2.06–7.28) | <0.0001 | ||||||
All models were adjusted for age, sex, duration of diabetes, body mass index, glycated hemoglobin, systolic blood pressure, total cholesterol, high‐density lipoprotein cholesterol, smoking status and alcohol intake at baseline.
CI, confidence interval; DKD, diabetic kidney disease; DR, diabetic retinopathy; HR, hazard ratio.
Biological interaction: relative excess risk as a result of interaction −0.225 (−1.191–0.740); attributable proportion due to interaction −0.104 (−0.567–0.360); synergy index 0.838 (0.397–1.773).
Biological interaction: relative excess risk as a result of interaction −1.869 (−4.890–1.152); attributable proportion due to interaction −0.525 (−1.515–0.465); synergy index 0.578 (0.243–1.373).
Biological interaction: relative excess risk as a result of interaction 1.673 (−0.562–3.908); attributable proportion due to interaction 0.432 (0.012–0.852); synergy index 2.394 (0.666–8.605).
Multivariate Cox proportional hazards models for all‐cause, cancer, vascular and non‐cancer non‐vascular mortality related to VTDR and advanced DKD are shown in Table 3. The covariates of all models in Table 3 were the same as those shown in Table 2. In model 5, the presence of VTDR was a significant predictor of all‐cause, vascular and non‐cancer non‐vascular mortality (P < 0.0001, P = 0.001 and P < 0.0001, respectively), but not cancer mortality. In model 6, the presence of advanced DKD was a significant predictor of all‐cause, vascular and non‐cancer non‐vascular mortality (P = 0.0001, P = 0.003 and P < 0.0001, respectively), but not cancer mortality. In model 7, VTDR and advanced DKD simultaneously predicted all‐cause mortality (P = 0.0001 and P = 0.002, respectively), vascular mortality (P = 0.007 and P = 0.013, respectively) and non‐cancer non‐vascular mortality (P = 0.0009 and P = 0.0003, respectively), but not cancer mortality. In model 8, patients were categorized into four groups according to the presence or absence of VTDR and advanced DKD. Hazard ratios for all‐cause, vascular and non‐cancer non‐vascular mortality were highest in the VTDR(+) advanced DKD(+) group (all P < 0.0001), and higher in the VTDR(−) advanced DKD(+) group (P = 0.018, P = 0.041 and P = 0.005, respectively) and the DVTR(+) advanced DKD(−) group (P = 0.019, P = 0.11, and P = 0.047, respectively) than in the VTDR(−) advanced DKD(−) group.
Table 3.
Multivariate Cox proportional hazard models for all‐cause, cancer, vascular, and non‐cancer non‐vascular mortality in association with vision‐threatening DR and advanced diabetic kidney disease
| All‐cause mortality | n with/without outcome | Incidence rate (1,000 person‐years) | Model 5 (266/1902) | Model 6 (266/1902) | Model 7 (266/1902) | Model 8 (266/1902) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
| VTDR (+) | 39/84 | 24.5 | 2.38 (1.64–3.46) | <0.0001 | 2.11 (1.44–3.09) | 0.0001 | ||||
| Advanced DKD (+) | 66/221 | 16.6 | 1.79 (1.33–2.40) | 0.0001 | 1.61 (1.19–2.17) | 0.002 | ||||
| Categorization into 4 groups | ||||||||||
| VTDR (–) Advanced DKD (–) | 183/1,375 | 7.5 | 1 | |||||||
| VTDR (–) Advanced DKD (+) | 44/177 | 13.8 | 1.52 (1.07–2.15) | 0.018 | ||||||
| VTDR (+) Advanced DKD (–) | 17/40 | 21.5 | 1.88 (1.11–3.18) | 0.019 | ||||||
| VTDR (+) Advanced DKD (+) † | 22/44 | 27.4 | 3.66 (2.28–5.88) | <0.0001 | ||||||
| Cancer mortality | n with/without outcome | Incidence rate (1,000 person‐years) | Model 5 (92/1888) | Model 6 (92/1888) | Model 7 (92/1888) | Model 8 (92/1888) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
| VTDR (+) | 9/114 | 5.6 | 1.49 (0.71–3.13) | 0.29 | 1.61 (0.75–3.46) | 0.22 | ||||
| Advanced DKD (+) | 11/274 | 2.8 | 0.81 (0.42–1.54) | 0.51 | 0.74 (0.38–1.45) | 0.38 | ||||
| Categorization into 4 groups | ||||||||||
| VTDR (–) Advanced DKD (–) | 75/1,471 | 3.1 | 1 | |||||||
| VTDR (–) Advanced DKD (+) | 8/211 | 2.5 | 0.78 (0.37–1.64) | 0.50 | ||||||
| VTDR (+) Advanced DKD (–) | 6/51 | 7.6 | 1.70 (0.70–4.14) | 0.24 | ||||||
| VTDR (+) Advanced DKD (+) | 3/63 | 3.7 | 1.10 (0.33–3.63) | 0.87 | ||||||
| Vascular mortality | n with/without outcome | Incidence rate (1,000 person‐years) | Model 5 (78/1888) | Model 6 (78/1888) | Model 7 (78/1888) | Model 8 (78/1888) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
| VTDR (+) | 14/109 | 8.8 | 2.93 (1.53–5.62) | 0.001 | 2.49 (1.29–4.82) | 0.007 | ||||
| Advanced DKD (+) | 27/258 | 6.8 | 2.18 (1.31–3.61) | 0.003 | 1.93 (1.15–3.23) | 0.013 | ||||
| Categorization into 4 groups | ||||||||||
| VTDR (–) Advanced DKD (–) | 46/1,500 | 1.9 | 1 | |||||||
| VTDR (–) Advanced DKD (+) | 18/201 | 5.7 | 1.85 (1.03–3.32) | 0.041 | ||||||
| VTDR (+) Advanced DKD (–) | 5/52 | 6.3 | 2.23 (0.84–5.97) | 0.11 | ||||||
| VTDR (+) Advanced DKD (+) ‡ | 9/57 | 11.2 | 5.01 (2.28–11.0) | <0.0001 | ||||||
| Non‐cancer non‐vascular mortality | n with/without outcome | Incidence rate (1,000 person‐years) | Model 5 (82/1888) | Model 6 (82/1888) | Model 7 (82/1888) | Model 8 (82/1888) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
| VTDR (+) | 16/107 | 10.0 | 3.67 (2.00–6.74) | <0.0001 | 2.88 (1.54–5.36) | 0.0009 | ||||
| Advanced DKD (+) | 26/259 | 6.6 | 3.08 (1.87–5.08) | <0.0001 | 2.59 (1.55–4.31) | 0.0003 | ||||
| Categorization into 4 groups | ||||||||||
| VTDR (–) Advanced DKD (–) | 50/1,496 | 2.1 | 1 | |||||||
| VTDR (–) Advanced DKD (+) | 16/203 | 5.1 | 2.40 (1.31–4.38) | 0.005 | ||||||
| VTDR (+) Advanced DKD (–) | 6/51 | 7.6 | 2.47 (1.01–6.05) | 0.047 | ||||||
| VTDR (+) Advanced DKD (+) § | 10/56 | 12.4 | 7.97 (3.81–16.68) | <0.0001 | ||||||
All models were adjusted for age, sex, duration of diabetes, body mass index, glycated hemoglobin, systolic blood pressure, total cholesterol, high‐density lipoprotein cholesterol, smoking status and alcohol intake at baseline.
CI, confidence interval; DKD, diabetic kidney disease; HR, hazard ratio; VTDR, vision‐threatening diabetic retinopathy.
Biological interaction: relative excess risk as a result of interaction 1.263 (−0.613–3.140); attributable proportion due to interaction 0.345 (−0.052–0.742); synergy index 1.904 (0.735–4.935).
Biological interaction: relative excess risk as a result of interaction 1.929 (−2.175–6.032); attributable proportion due to interaction 0.385 (−0.225–0.995); synergy index 1.928 (0.487–7.631).
Biological interaction: relative excess risk as a result of interaction 4.106 (−1.721–9.933); attributable proportion due to interaction 0.515 (0.080–0.950); synergy index 2.432 (0.791–7.481).
With regard to biological interaction between DR and DKD, estimates of RERI, AP and S were − 0.225 (95% CI −1.191–0.740), −0.104 (95% CI −0.567–0.360) and 0.838 (95% CI 0.397–1.773) for all‐cause mortality, −1.869 (95% CI −4.890–1.152), −0.525 (95% CI −1.515–0.465) and 0.578 (95% CI 0.243–1.373) for vascular mortality, and 1.673 (95% CI −0.562–3.908), 0.432 (95% CI 0.012–0.852) and 2.394 (95% CI 0.666–8.605) for non‐cancer non‐vascular mortality, respectively. Similar to the biological interaction between DR and DKD, for that between VTDR and advanced DKD, estimates of RERI, AP and S were 1.263 (95% CI −0.613–3.140), 0.345 (95% CI −0.052–0.742) and 1.904 (95% CI 0.735–4.935) for all‐cause mortality, 1.929 (95% CI −2.175–6.032), 0.385 (95% CI −0.225–0.995) and 1.928 (95% CI 0.487–7.631) for vascular mortality, and 4.106 (95% CI −1.721–9.933), 0.515 (95% CI 0.080–0.950) and 2.432 (95% CI 0.791–7.481) for non‐cancer non‐vascular mortality, respectively. As a result, biological interactions between DR and DKD for all‐cause and vascular mortality were not significant, because the lower limits of the 95% CIs of RERI and AP were <0, and that of S was <1. Similarly, the biological interactions between VTDR and advanced DKD for all‐cause and vascular mortality were not significant. However, the interaction for only non‐cancer non‐vascular mortality between DR and DKD, and that between VTDR and advanced DKD appeared to be significant, because the lower limit of the 95% CI of AP was >0.
DISCUSSION
In the present study, DR and DKD predicted all‐cause, vascular and non‐cancer non‐vascular mortality, but not cancer mortality, independently of each other in real‐world patients with type 2 diabetes. VTDR and advanced DKD were more powerful predictors of all‐cause, vascular and non‐cancer non‐vascular mortality, but not cancer mortality, than DR and DKD. The combined effect of DR and DKD for all‐cause, vascular and non‐cancer non‐vascular mortality may increase depending on the severity of these microvascular complications, with a potential synergistic effect for non‐cancer non‐vascular mortality.
The National Health and Nutrition Examination Surveys 1988–1994 and 2005–2008 showed that DR and chronic kidney disease significantly increased the risk for all‐cause mortality 23 , 24 , which supports the present findings. In a previous study, the causes of death among 45,708 Japanese patients with diabetes (29,801 men and 15,907 women) who died at 241 hospitals nationwide from 2001–2010 were examined on the basis of hospital records 13 . This previous study showed that cancer (38.3%) was the most common cause of death, followed by infections (17.0%) and vascular disease (14.9%). Our study showed that DR and DKD or VTDR and advanced DKD were associated with vascular and non‐cancer non‐vascular mortality, but not cancer mortality. Therefore, these diabetic microvascular complications may not be relevant to a shared biology of cancer and CVD. Japanese patients with type 2 diabetes who develop microvascular complications may have impaired insulin secretion rather than hyperinsulinemia with insulin resistance that can contribute to the risk of cancer. Diabetes is also associated with non‐cancer non‐vascular death due to infectious diseases, external causes, intentional self‐harm and degenerative disorders, independent of several major risk factors 6 . To the best of our knowledge, this is the first study to show that DR and DKD may be jointly, as well as independently, associated with non‐cancer non‐vascular mortality according to their severity. Further research is warranted to verify the present results.
Two epidemiological studies assessed the effect of the biological interaction between DR and DKD/micro‐ or macroalbuminuria on mortality 25 , 26 . One study was from Hong Kong in which the biological interaction between DR and macroalbuminuria significantly affected development of renal events in patients with type 2 diabetes. This is because the lower limit of the 95% CIs of RERI and AP were >0, and that of S was >1. Additionally, the authors found that this interaction significantly affected composite end‐points, including cardiorenal end‐points and all‐cause mortality, because the lower limit of the 95% CI of only AP was >0. Therefore, the authors of the previous study concluded that the coexistence of DR and macroalbuminuria was associated with an increased risk of composite cardiorenal end‐points and death 25 . The other study was from Singapore in which the biological interaction between DR and DKD was not significant for all‐cause and cardiovascular mortality, because the lower limit of the 95% CI of RERI was <0. In sensitivity analysis, there was no significant interaction between severity categories of DR and DKD for all‐cause mortality 26 . The results of these two studies are essentially consistent with the results of the present study. In the present study, the interactions between DR and DKD, and between VTDR and advanced DKD for non‐cancer non‐vascular mortality appeared to be potentially significant, because the lower limit of the 95% CI of AP was >0. Therefore, the coexistence of DR and DKD might have a synergistic effect on non‐cancer non‐vascular mortality, depending on their severity.
The strengths of the present study include the use of real‐world, long‐term, follow‐up data and accurate diagnosis of DR by ophthalmologists who subspecialize in diabetic eye diseases, and timely and appropriate photocoagulation treatment by them. However, the present study had some possible limitations. First, the design of our study was retrospective. Changes in laboratory measurement methods can lead to possible information bias. However, data from different measurement methods were transformed by linear regression equations that were obtained from duplicate assays. Second, the follow‐up rate for survival was relatively low. When we compared baseline characteristics between patients who completed follow up and those who did not complete follow up, sex, alcohol intake, the proportions of patients with DKD and those with advanced DKD, and BMI were significantly different. Third, almost half of the events were based on questionnaire responses. A complete review of medical records, which were written by the attending physician, showed 130 (48.9%) deaths, and questionnaire responses from family members confirmed 136 (51.1%) deaths, including 14 (5.3%) unknown causes of death. Therefore, the causes of death were determined by a thorough review of medical records for 130 (51.6%) patients, and were confirmed based on questionnaire responses for 122 (48.4%) patients. The causes of death were identified using unified criteria. We add the fact that we unintentionally received a direct call from some families or received a copy of the death certificate or the certificate identifying the cause of death attached to the questionnaire responses. Although effort was made to avoid misclassification as much as possible, the risk of misclassification cannot be completely ruled out. Fourth, interobserver variability among several ophthalmologists on the diagnosis of DR could not be evaluated, because this was a real‐world observational study. However, interobserver variability among these ophthalmologists could be regarded as small, because a subspecialty of all of the ophthalmologists was diabetic eye diseases. Finally, the participants of our study were recruited from a single clinic in Japan. Therefore, generalizability of the present findings to other ethnic populations is limited.
In conclusion, DR and DKD may be jointly, as well as independently, associated with all‐cause, vascular and non‐cancer non‐vascular mortality, but not cancer mortality, according to the severity of these microvascular complications in real‐world patients with type 2 diabetes. These findings suggest that periodic screening for DR and DKD, and assessment of their severity are important for identifying high‐risk patients for vascular and non‐cancer non‐vascular death. However, different management and monitoring programs may be required to reduce the risk of cancer death.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Figure S1 | Kaplan–Meier curves according to vision‐threatening diabetic retinopathy (VTDR) and advanced diabetic kidney disease (DKD) status for all‐cause, cancer, vascular, and non‐cancer non‐vascular mortality. (a) All‐cause mortality. (b) Cancer mortality. (c) Vascular mortality. (d) Non‐cancer non‐vascular mortality.
Table S1 | Baseline clinical characteristics of the patients who completed and those who did not complete follow up.
Acknowledgments
The authors express appreciation to all of the study participants for their cooperation in this research. The authors thank Ms Kumiko Kimura and the staff of the General Affairs Department at the Institute for Adult Diseases, Asahi Life Foundation, Tokyo, Japan, for their support for data collection and questionnaire surveys. The authors also thank Ellen Knapp, PhD, from Edanz Group (www.edanzediting.com/ac) for English proofreading of this manuscript. No specific funding or grant was received for this work.
J Diabetes Investig 2020; 11: 1170–1180
References
- 1. Koene RJ, Prizment AE, Blaes A, et al Shared risk factors in cardiovascular disease and cancer. Circulation 2016; 133: 1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Masoudkabir F, Sarrafzadegan N, Gotay C, et al Cardiovascular disease and cancer: Evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis 2017; 263: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson CB, Davis MK, Law A, et al Shared risk factors for cardiovascular disease and cancer: Implications for preventive health and clinical care in oncology patients. Can J Cardiol 2016; 32: 900–907. [DOI] [PubMed] [Google Scholar]
- 4. Giovannucci E, Harlan DM, Archer MC, et al Diabetes and cancer: A consensus report. Diabetes Care 2010; 33: 1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Renehan A, Smith U, Kirkman MS. Linking diabetes and cancer: a consensus on complexity. Lancet 2010; 375: 2201–2202. [DOI] [PubMed] [Google Scholar]
- 6. The Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause‐specific death. N Engl J Med 2011; 364: 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tuttle KR, Bakris GL, Bilous RW, et al Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care 2014; 37: 2864–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salinero‐Fort MÁ, San Andrés‐Rebollo FJ, de Burgos‐Lunar C , et al Cardiovascular and all‐cause mortality in patients with type 2 diabetes mellitus in the MADIABETES Cohort Study: association with chronic kidney disease. J Diabetes Complications 2016; 30: 227–236. [DOI] [PubMed] [Google Scholar]
- 9. Nichols GA, Déruaz‐Luyet A, Hauske SJ, et al The association between estimated glomerular filtration rate, albuminuria, and risk of cardiovascular hospitalizations and all‐cause mortality among patients with type 2 diabetes. J Diabetes Complications 2018; 32: 291–297. [DOI] [PubMed] [Google Scholar]
- 10. Kramer CK, Rodrigues TC, Canani LH, et al Diabetic retinopathy predicts all‐cause mortality and cardiovascular events in both type 1 and 2 diabetes. Meta‐analysis of observational studies. Diabetes Care 2011; 34: 1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu X‐R, Zhang Y‐P, Bai L, et al Prediction of risk of diabetic retinopathy for all‐cause mortality, stroke and heart failure. Evidence from epidemiological observational studies. Medicine 2017; 96: e5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pearce I, Simó R, Lövestam‐Adrian M, et al Association between diabetic eye disease and other complications of diabetes: Implications for care. A systematic review. Diabetes Obes Metab 2019; 21: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakamura J, Kamiya H, Haneda M, et al Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001–2010: Report of the Committee on Causes of Death in Diabetes Mellitus. J Diabetes Investig 2017; 8: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takao T, Takahashi K, Suka M, et al Association between postprandial hyperglycemia at clinic visits and all‐cause and cancer mortality in patients with type 2 diabetes: A long‐term historical cohort study in Japan. Diabetes Res Clin Pract 2019; 148: 152–159. [DOI] [PubMed] [Google Scholar]
- 15. Goto A, Takao T, Yoshida Y, et al Causes of death and estimated life expectancy among people with diabetes: A retrospective cohort study in a diabetes clinic. J Diabetes Investig 2020; 11: 52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahlbom A, Alfredsson L. Interaction: A word with two meanings creates confusion. Eur J Epidemiol 2005; 20: 563–564. [DOI] [PubMed] [Google Scholar]
- 17. Andersson T, Alfredsson L, Källberg H, et al Calculating measures of biological interaction. Eur J Epidemiol 2005; 20: 575–579. [DOI] [PubMed] [Google Scholar]
- 18. Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol 2012; 41: 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology 1992; 3: 452–456. [DOI] [PubMed] [Google Scholar]
- 20. Wilkinson CP, Ferris FLIII, Klein RE, et al Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003; 110: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 21. Matsuo S, Imai E, Horio M, et al Revised equations for estimated GFR fromserum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 22. Takao T, Matsuyama Y, Suka M, et al Analysis of the duration and extent of the legacy effect in patients with type 2 diabetes: A real‐world longitudinal study. J Diabetes Complications 2019; 33: 516–522. [DOI] [PubMed] [Google Scholar]
- 23. Ricardo AC, Grunwald JE, Parvathaneni S, et al Retinopathy and CKD as predictors of all‐cause and cardiovascular mortality: National Health and Nutrition Examination Survey (NHANES) 1988–1994. Am J Kidney Dis 2014; 64: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pavkov ME, Harding JL, Chou CF, et al Prevalence of diabetic retinopathy and associated mortality among diabetic adults with and without chronic kidney disease. Am J Ophthalmol 2019; 198: 200–208. [DOI] [PubMed] [Google Scholar]
- 25. Tong PCY, Kong AP, So WY, et al Interactive effect of retinopathy and macroalbuminuria on all‐cause mortality, cardiovascular and renal end points in Chinese patients with type 2 diabetes mellitus. Diabet Med 2007; 24: 741–746. [DOI] [PubMed] [Google Scholar]
- 26. Sabanayagam C, Chee ML, Banu R, et al Association of diabetic retinopathy and diabetic kidney disease with all‐cause and cardiovascular mortality in a multiethnic Asian population. JAMA Network Open 2019; 2: e191540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Kaplan–Meier curves according to vision‐threatening diabetic retinopathy (VTDR) and advanced diabetic kidney disease (DKD) status for all‐cause, cancer, vascular, and non‐cancer non‐vascular mortality. (a) All‐cause mortality. (b) Cancer mortality. (c) Vascular mortality. (d) Non‐cancer non‐vascular mortality.
Table S1 | Baseline clinical characteristics of the patients who completed and those who did not complete follow up.


