Abstract
Type A insulin resistance (IR) syndrome is a severe IR form caused by insulin receptor (INSR) gene defects. Antidiabetic drugs, including high‐dose insulin and insulin‐sensitizing agents, often fail to control associated hyperglycemia. Therapy with recombinant human insulin‐like growth factor 1 can be more effective, but it is expensive. We report a case of type A IR syndrome with an in‐frame INSR heterozygous deletion (ΔLeu999) that was treated with a combination of conventional therapy and ipragliflozin, a sodium–glucose cotransporter 2 inhibitor. Treatment reduced hemoglobin A1c levels (10.0–7.5%) and induced weight loss (54.4–52.0 kg) within 2 months, and the effects were sustained for >3 years. Sodium–glucose cotransporter 2 inhibitors might be useful to normalize blood glucose in type A IR syndrome by reducing bodyweight and ameliorating glucotoxicity.
Keywords: Ipragliflozin, Sodium–glucose cotransporter 2 inhibitor, Type A insulin resistance syndrome
This is the first article to report on the long‐term efficacy of ipragliflozin (a sodium–glucose cotransporter 2 inhibitor) in a patient with insulin receptor mutation. Our findings can be helpful in the development of more effective and affordable treatment strategies for patients with severe insulin resistance syndrome.

Introduction
Insulin resistance (IR), a major cause of type 2 diabetes, is closely associated with chronic morbidities, including atherosclerosis, hepatic steatosis and polycystic ovary syndrome. Its prevalence is rapidly increasing and becoming a worldwide public health concern. Whereas IR is commonly caused by obesity, it can also occur in non‐obese patients with rare genetic defects in insulin signaling or adipocyte development1.
There are two types of IR syndrome, type A and type B. The former is caused by insulin receptor (INSR) gene defects, whereas the latter is characterized by anti‐INSR antibodies1. Disease severity is inversely correlated with residual INSR activity, and both types can result in hyperglycemia when pancreatic β‐cell hyperplasia fails to compensate for extreme IR. Treating genetically‐induced IR is challenging and often unsuccessful. Herein, we report the long‐term efficacy of a newer oral antidiabetic drug, ipragliflozin, in combination with conventional therapy, in a patient with type A IR syndrome.
Case Report
The patient was a non‐obese Japanese woman diagnosed with diabetes mellitus in June 2009, at the age of 31 years (6 weeks’ gestation during first pregnancy). The clinical course of her successful pregnancy and delivery was reported previously2. Before delivery, large insulin doses (up to 480 units/day) were required to sufficiently control blood glucose levels. Genetic analysis showed an in‐frame heterozygous INSR deletion mutation (ΔLeu999). During her second pregnancy, we improved her glycemic control by combining metformin (2250 mg/day) with lower doses of insulin (up to 174 units/day). Both infants, harboring the same INSR mutation, had no abnormalities except being slightly small for their gestational age and having transient hypoglycemia at birth2.
Figure 1 shows the patient’s clinical course after the second delivery in May 2013. As metformin can enter breast milk3, its administration was discontinued during breast‐feeding. The patient’s diabetes, which was treated with 120 units of insulin per day, became progressively worse. After the cessation of breast‐feeding, metformin therapy was re‐started and acarbose was initiated. However, hemoglobin A1c (HbA1c) levels decreased only slightly. She declined therapy with recombinant human insulin‐like growth factor 1 (IGF‐1) or higher doses of insulin because of treatment costs.
Figure 1.
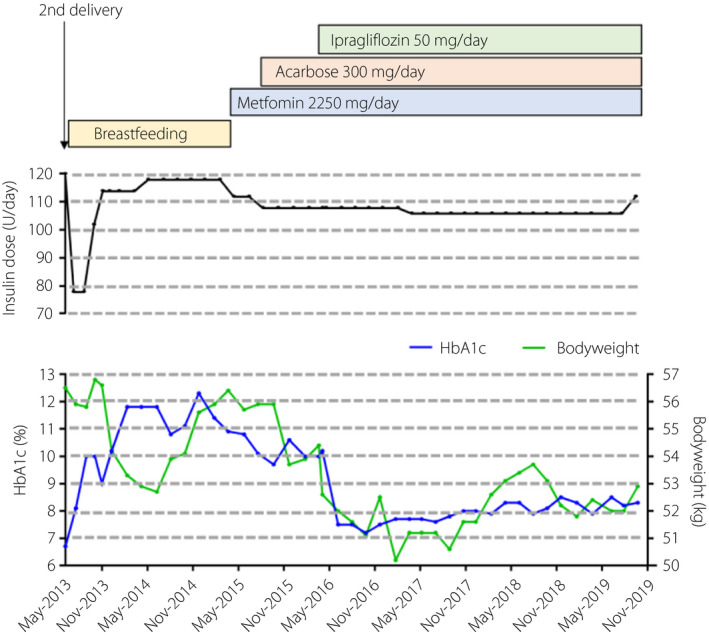
Clinical course of the patient before and after ipragliflozin treatment. HbA1c, hemoglobin A1c.
Therefore, in March 2016, we administered ipragliflozin. Before administration, the patient’s bodyweight, height, body mass index and HbA1c were 54.4 kg, 150.8 cm, 23.9 kg/m2 and 10.0%, respectively. After 1 month, her bodyweight and HbA1c were 52.6 kg and 10.2%, respectively. After 2 months, they were 52.0 kg and 7.2%, respectively. HbA1c content was maintained at approximately 8.0% for >3 years after first administration. Ketoacidosis and other adverse effects were not observed. The only complication was simple diabetic retinopathy.
Discussion
We describe a patient with type A IR syndrome who responded well to ipragliflozin, which significantly improved glycemic control and induced bodyweight loss. SGLT2 inhibitors comprise a newer class of antidiabetics that reduce blood glucose by inhibiting renal glucose reabsorption independently of insulin action. Their use is rapidly increasing, driven by favorable results of cardiovascular trials of type 2 diabetes mellitus4. Recently, a nationwide survey of IR syndrome was reported in Japan, including individuals with type A, type B and type A‐like clinical features without INSR mutations and Rabson–Mendenhall/Donohue syndrome with biallelic INSR mutations5. In that study, SGLT2 inhibitors were used for seven of 71 study participants. However, SGLT2 inhibitor efficacy data for patients with genetically‐determined severe IR are limited6, 7. To our knowledge, we are the first to report the long‐term efficacy of an SGLT2 inhibitor for a patient with an INSR mutation.
Treating type A IR syndrome is challenging. Abundant insulin or recombinant human IGF‐1 can activate the IGF‐1 receptor and can be effective for type A IR syndrome, because the IGF‐1 receptor shares structural homology and downstream signaling pathways with INSR8. However, recombinant human IGF‐1 therapy is expensive, and adults with type A IR syndrome do not receive sufficient financial support in Japan. Insulin‐sensitizing agents are also important to manage type A IR syndrome. Metformin is often effective, as observed with the present patient during her second pregnancy2; thiazolidinediones can also exert beneficial effects1. The present patient’s hyperglycemia became resistant to metformin after restarting treatment, which might be partly explained by the discontinuation of dietary restriction and/or decreased physical activity during parenting.
Just two articles have reported the efficacy of SGLT2 inhibitors in genetically‐induced severe IR6, 7. Combining dapagliflozin with insulin and metformin therapy lowered bodyweight and HbA1c levels in a patient with a heterozygous mutation in the phosphatidylinositol 3‐kinase, regulatory subunit 1 gene, which is involved in post‐INSR signaling6. Furthermore, combining ipragliflozin with other oral antidiabetic drugs significantly improved severe IR in a patient with a homozygous nonsense mutation of Berardinelli–Seip congenital lipodystrophy 2 gene, which is responsible for adipocyte differentiation, as assessed by a homeostasis model7.
Generally, type A IR syndrome is characterized by the absence of obesity. However, we previously reported that obesity is a risk factor for worsening glycemic control in a family with an INSR mutation9. Although the patient was not obese (body mass index 22.08–23.92 kg/m2), Pearson’s correlation analysis showed that HbA1c was positively correlated with bodyweight (data from Figure 1; n = 44, r = 0.255, P = 0.0005). Therefore, even if type A IR syndrome patients are not obese, adiposity can be an exacerbating factor for glycemic control.
Sodium–glucose cotransporter 2 inhibitors might act by enhancing urinary glucose excretion and shifting energy substrate utilization from carbohydrates to lipids10, which is expected to eventually reduce adiposity. Here, weight loss preceded ipragliflozin‐mediated HbA1c reductions. SGLT2 inhibitor‐induced weight loss might explain why glycemic control is improved in patients with defective INSR or post‐INSR signaling.
Two‐week dapagliflozin therapy significantly increases insulin‐mediated glucose disposal, of which ~80% occurs in skeletal muscle11. SGLT2 inhibitors were speculated to indirectly improve muscle insulin sensitivity by ameliorating glucotoxicity. This indicates that SGLT2 inhibitors can even improve glycemic control in patients with lipodystrophy.
Thus, long‐term ipragliflozin achieved effective glycemic control in a patient with an INSR mutation. This might be explained primarily by weight loss and secondarily by alleviated glucotoxicity. This will be helpful to develop more effective and affordable treatment strategies for severe IR.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2020; 11: 1363–1365
References
- 1. Semple RK, Savage DB, Cochran EK, et al Genetic syndromes of severe insulin resistance. Endocr Rev 2011; 32: 498–514. [DOI] [PubMed] [Google Scholar]
- 2. Enkhtuvshin B, Nagashima S, Saito N, et al Successful pregnancy outcomes in a patient with type A insulin resistance syndrome. Diabet Med 2015; 32: e16–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hale TW, Kristensen JH, Hackett LP, et al Transfer of metformin into human milk. Diabetologia 2002; 45: 1509–1514. [DOI] [PubMed] [Google Scholar]
- 4. Zelniker TA, Wiviott SD, Raz I, et al SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet 2019; 393: 31–39. [DOI] [PubMed] [Google Scholar]
- 5. Takeuchi T, Ishigaki Y, Hirota Y, et al Clinical characteristics of insulin resistance syndromes: a nationwide survey in Japan. J Diabetes Investig 2020; 11: 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamaguchi T, Hirota Y, Takeuchi T, et al Treatment of a case of severe insulin resistance as a result of a PIK3R1 mutation with a sodium‐glucose cotransporter 2 inhibitor. J Diabetes Investig 2018; 9: 1224–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawana Y, Imai J, Sawada S, et al Sodium‐glucose cotransporter 2 inhibitor improves complications of lipodystrophy: a case report. Ann Intern Med 2017; 166: 450–451. [DOI] [PubMed] [Google Scholar]
- 8. McDonald A, Williams RM, Regan FM, et al IGF‐1 treatment of insulin resistance. Eur J Endocrinol 2007; 157: S51–56. [DOI] [PubMed] [Google Scholar]
- 9. Ando A, Yatagai T, Rokkaku K, et al Obesity is a critical risk factor for worsening of glucose tolerance in a family with the mutant insulin receptor. Diabetes Care 2002; 25: 1484–1485. [DOI] [PubMed] [Google Scholar]
- 10. Ferrannini E, Muscelli E, Frascerra S, et al Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Investig 2014; 124: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merovci A, Solis‐Herrera C, Daniele G, et al Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Investig 2014; 124: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]


