Abstract
Aims/Introduction
We aimed to evaluate the metabolic status of pregnant women by assessing metabolic biomarkers of participants in the Japan Environment and Children’s Study, a nationwide, multicenter, pregnancy and birth cohort.
Materials and Methods
Pregnant women aged 14–50 years were studied in 15 centers across Japan. Clinical information was obtained using self‐administered questionnaires. Blood samples were taken during the first two trimesters to measure metabolic biomarkers. Samples were divided into seven groups according to the weeks of pregnancy.
Results
Among 82,972 pregnant women, 43 had only type 1 diabetes, 78 had only type 2 diabetes, 2,315 had only gestational diabetes and 354 had only dyslipidemia. Glycated hemoglobin, total cholesterol, low‐density lipoprotein cholesterol and triglyceride across all the percentiles increased as prepregnancy body mass index increased, whereas high‐density lipoprotein cholesterol levels across all the percentiles decreased as body mass index increased. Glycated hemoglobin was high in participants with type 1 diabetes or type 2 diabetes only, but not in those with gestational diabetes or hyperlipidemia only. Participants with type 2 diabetes or dyslipidemia only had high triglyceride in the first trimester, which then decreased in the second trimester. Participants with type 2 diabetes only also showed low high‐density lipoprotein cholesterol, whereas participants with dyslipidemia only showed high total cholesterol and low‐density lipoprotein cholesterol throughout.
Conclusions
Metabolic biomarkers were affected by blood sample timing and underlying metabolic disease. The Japan Environment and Children’s Study will clarify the influences of metabolic status during pregnancy on the health and development of the offspring in future studies.
Keywords: Cohort, Diabetes, Pregnancy
This is the first study to describe the metabolic status of a large cohort of pregnant women aged 14–50 years and living in Japan.
Introduction
The glucose and lipid metabolism of women is known to change during pregnancy. Insulin resistance increases during pregnancy because of an increased secretion of hormones, such as placental growth hormone, that promote the transplacental transport of glyconutrients to the fetus1. Because pregnant women use lipids as an energy source, the plasma levels of cholesterol and triglyceride in pregnant women are relatively high2. In contrast, earlier research has suggested that the timing of blood collection during different stages of pregnancy is an important point to consider. Because of the changes in lipid profile during the second and third trimesters, other factors including pregnancy‐related complications and/or placenta dysfunction might impede interpretation regarding cause or consequence3. Pregnant women with obesity are at risk of many complications, including stillbirth, large‐for‐gestational‐age infants and associated complications at birth4. Children born to women with higher maternal prepregnancy Body mass index (BMI) and excess gestational weight gain could themselves be at risk of childhood obesity5. Identifying women at risk allows initiation of risk‐specific treatment and tailored care during pregnancy.
In the present study, we analyzed data collected from pregnant women who participated in a nationwide birth cohort study (the Japan Environment and Children’s Study [JECS]), using a questionnaire and blood samples taken during the first or second trimester of pregnancy, to evaluate their metabolic status. This is the first study to describe the metabolic status of a large cohort of pregnant women aged 14–50 years and living in Japan.
Methods
Overview
The JECS is a prospective nationwide birth cohort study that was launched by the Japanese Ministry of the Environment. The JECS covers a wide geographical area of Japan and comprises 15 regional centers (Hokkaido, Miyagi, Fukushima, Chiba, Kanagawa, Koshin, Toyama, Aichi, Kyoto, Osaka, Hyogo, Tottori, Kochi, Fukuoka and South Kyushu/Okinawa). Participants were pregnant women and their partners who were recruited during their early pregnancy from hospitals or local government offices when the maternal and child health handbook was provided. The main aim of the JECS was to evaluate whether environmental factors, such as chemicals, physical activity and lifestyle, influence childhood health. The health of mothers reflects genetic factors and lifestyle, and one of the important themes of the study is to establish how the health of mothers during pregnancy affects the subsequent health of their child. Recruitment began in January 2011, and the number of pregnant women enrolled reached 100,000 in March 20146. Participating children are expected to remain in the study until they reach 13 years of age.
Ethical approval
The JECS was carried out based on the Ethical Guidelines for Epidemiological Research published by the Japanese Ministry of Health and Welfare (now the Ministry of Health, Labor and Welfare). The JECS protocol was reviewed and approved by the Japanese Ministry of the Environment’s Institutional Review Board on Epidemiological Studies and the Ethics Committees of all the participating institutions. Written informed consent was obtained from all participants.
Recruitment
The eligibility criteria for participants in the JECS were as follows: (i) the participant should reside in the study area at the time of recruitment and expect to continue to reside in Japan for the foreseeable future; (ii) their expected delivery date should be between 1 August 2011 and mid‐2014; and (iii) the participant should be capable of participating in the study without difficulty; that is, they must be able to understand the Japanese language and complete the self‐administered questionnaire.
Either or both of the following two recruitment protocols were applied: (i) recruitment at the time of first prenatal examination at cooperating health care providers such as obstetric facilities, and/or (ii) recruitment at local government offices issuing pregnancy journals, namely Mother‐Child Health Handbooks (the Mother‐Child Health Handbook is an official booklet that all expecting mothers in Japan are given complimentary when they become pregnant in order to receive municipal services for pregnancy, delivery and childcare)6. The study population contained 104,102 fetal records.
Assessment of clinical information
Information about the week of pregnancy was obtained from pregnant participants using an initial self‐administered questionnaire during the second or third trimester of pregnancy. The height and weight of each mother before pregnancy and the maternal age at registration were obtained either from the participant’s doctors or from a self‐administered questionnaire. The lifetime prevalence of diabetes and other endocrine disorders, including complications of type 1 diabetes, type 2 diabetes, gestational diabetes (GDM) and dyslipidemia, was also assessed based on the diagnoses made by the participant’s doctors and the initial self‐administered questionnaire.
Screening tests for all pregnant women for GDM and “overt diabetes in pregnancy” are carried out in Japan, using the following stepwise method7:
Measure random blood glucose level in the first trimester (each hospital should determine its own cut‐off value). Before planning a 75‐g oral glucose tolerance test for women with a random blood glucose level of 200 mg/dL, check: (i) fasting plasma glucose 126 mg/dL; (ii) glycated hemoglobin (HbA1c) 6.5%, expressed as National Glycohemoglobin Standardization Program value; and (iii) definite diabetic retinopathy for differential diagnosis of “overt diabetes in pregnancy.”
Give the pregnant woman a 50‐g glucose challenge test (cut‐off value 140 mg/dL) or measure the random blood glucose level a second time (cut‐off value 100 mg/dL) between 24 and 28 gestational weeks in women not diagnosed as having GDM or “overt diabetes in pregnancy.”
A 75‐g oral glucose tolerance test is given to all women with a positive screening test result, except women diagnosed as having “overt diabetes in pregnancy” and GDM is diagnosed with International Association of Diabetes and Pregnancy Study Groups criteria8. “Overt diabetes in pregnancy” is classified as type 2 diabetes.
The screening method for GDM described in the guideline7, 8 was also used at the time the present study was carried out. It is reported that the prevalence of GDM was approximately 8.5% when the 75‐g oral glucose tolerance test was given to all pregnant women9.
Biomarker assays
Non‐fasting blood samples were obtained from pregnant women during their second or third trimester of pregnancy. The following biomarkers were assayed by a contract clinical laboratory (SRL, Inc., a commercial laboratory in Tokyo, Japan): HbA1c (National Glycohemoglobin Standardization Program) was measured using a high‐performance liquid chromatographic method; serum total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol (HDL‐C) and triglyceride (TG) were analyzed enzymatically using a 7700 clinical chemistry/immunoassay hybrid analyzer (Hitachi High‐Technologies Co., Ltd, Tokyo, Japan).
Exclusion of study participants
We prespecified that pregnant participants whose data for HbA1c, Hb, TC, LDL‐C, HDL‐C or TG were missing or below the quantifiable range in the transcripts of medical records would be excluded. Participants who had missing data or results outside the reference range for other variables, or those who had other endocrine disorders, were also excluded from subsequent analyses.
Statistical analysis
We used the data from individuals in the first and second trimesters with no missing values. The distribution of each biomarker was summarized according to the prepregnancy BMI and maternal age at registration, according to the week of pregnancy (the first trimester was divided into 4–7, 8–11 and 12–13 weeks, and the second trimester into 14–15, 16–19, 20–23 and 24–27 weeks), and according to the presence or absence of diabetes or other endocrine disorders. Each woman was evaluated only once during pregnancy. Each biomarker was summarized using the 2.5th, 25th, 50th, 75th and 97.5th percentiles. The presence or absence of diabetes and other endocrine disorders was also summarized according to the prepregnancy BMI. Descriptive analysis was carried out using SAS version 9.4 (SAS Institute, Cary, NC, USA) and SPSS statistics 25.0 (IBM, Chicago, IL, USA) 10.
Results
The present study contained 80,636 pregnant women, including 4,611 women with diabetes, dyslipidemia or endocrine disorders, while excluding women with missing data. A summary of the 80,636 participants is shown in Figure 1. There were 43 participants with type 1 diabetes only, 78 with type 2 diabetes only, 2,315 with GDM only, 354 with dyslipidemia only and 1,821 with other endocrine disorders (including complications of type 1 diabetes, type 2 diabetes, GDM or dyslipidemia). Blood samples were obtained from 32,132 healthy participants during the first trimester of pregnancy and from 43,893 healthy participants during the second trimester of pregnancy.
Figure 1.
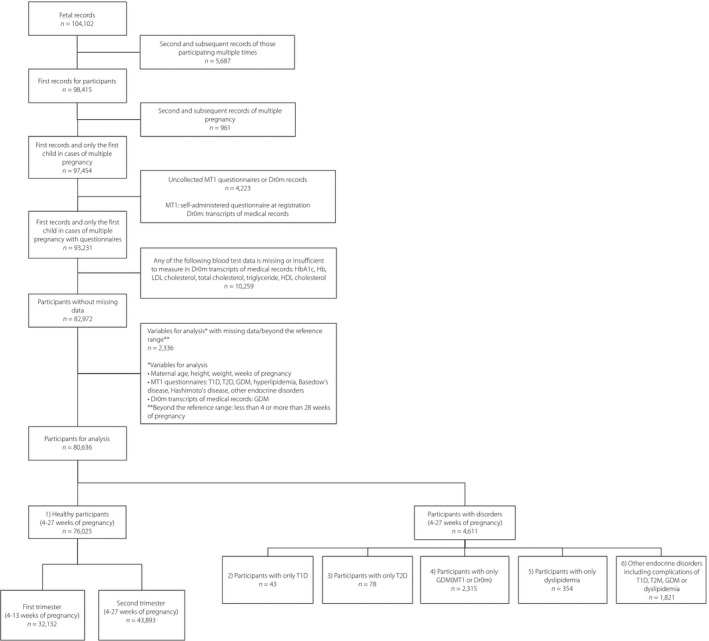
Flow chart characterizing the study participants and reasons for their exclusion. GDM, gestational diabetes; T1D, type 1 diabetes; T2D, type 2 diabetes.
Table 1 describes the distribution of metabolic biomarkers in healthy participants according to their prepregnancy BMI and age at registration. Across all the percentiles, the levels of HbA1c, TC, LDL‐C and TG elevated as BMI increased, whereas that of HDL‐C decreased as BMI increased. The levels of HbA1c and HDL‐C across all the percentiles slightly increased with the advance of age. The levels of TC, LDL‐C and TG across all the percentiles increased with the advance of age, except among participants aged <20 years. Table 2 shows the distribution of metabolic biomarkers in healthy participants according to the week of pregnancy during which blood samples were collected. HbA1c levels did not differ substantially between the first and second trimesters. The serum levels of all lipid biomarkers (TC, LDL‐C, HDL‐C and TG) across all the percentiles increased as pregnancy progressed.
Table 1.
Biomarkers classified by percentile in healthy pregnant participants according to body mass index before pregnancy and maternal age
| n | Adjusted HbA1c* (%) | Total cholesterol (mg/dL) | LDL cholesterol (mg/dL) | HDL cholesterol (mg/dL) | Triglyceride (mg/dL) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | ||
| BMI before pregnancy | ||||||||||||||||||||||||||
| <18.5 | 12,148 | 4.6 | 5.0 | 5.1 | 5.4 | 5.7 | 137 | 171 | 191 | 216 | 271 | 58 | 83 | 99 | 118 | 166 | 54 | 69 | 78 | 87 | 107 | 54 | 87 | 112 | 144 | 238 |
| 18.5–24 | 56,156 | 4.6 | 5.0 | 5.2 | 5.4 | 5.7 | 140 | 175 | 196 | 220 | 273 | 61 | 88 | 104 | 123 | 168 | 52 | 68 | 76 | 86 | 106 | 56 | 92 | 118 | 154 | 263 |
| 25–29 | 5,817 | 4.7 | 5.1 | 5.2 | 5.5 | 5.8 | 148 | 182 | 204 | 226 | 278 | 68 | 97 | 114 | 132 | 178 | 48 | 63 | 72 | 81 | 100 | 65 | 109 | 143 | 185 | 326 |
| ≥30 | 1,634 | 4.8 | 5.1 | 5.4 | 5.6 | 6.0 | 148 | 185 | 205 | 228 | 280 | 70 | 102 | 119 | 136 | 180 | 46 | 60 | 69 | 77 | 97 | 72 | 118 | 151 | 197 | 352 |
| Maternal age at registration (years) | ||||||||||||||||||||||||||
| <20 | 786 | 4.5 | 4.8 | 5.0 | 5.2 | 5.5 | 139 | 175 | 194 | 217 | 266 | 66 | 92 | 108 | 126 | 168 | 48 | 64 | 72 | 80 | 102 | 62 | 97 | 127 | 166 | 293 |
| 21–24 | 7,590 | 4.6 | 4.9 | 5.1 | 5.2 | 5.6 | 136 | 171 | 193 | 216 | 270 | 60 | 86 | 103 | 122 | 169 | 51 | 66 | 75 | 84 | 104 | 54 | 90 | 117 | 153 | 258 |
| 25–29 | 22,680 | 4.6 | 5.0 | 5.1 | 5.3 | 5.6 | 140 | 173 | 194 | 217 | 271 | 61 | 87 | 103 | 122 | 167 | 52 | 67 | 76 | 85 | 105 | 55 | 89 | 115 | 150 | 260 |
| 30–34 | 26,771 | 4.7 | 5.0 | 5.2 | 5.4 | 5.7 | 141 | 175 | 197 | 221 | 274 | 61 | 88 | 104 | 124 | 170 | 52 | 68 | 76 | 86 | 106 | 57 | 93 | 119 | 156 | 270 |
| 35–39 | 15,607 | 4.7 | 5.1 | 5.2 | 5.4 | 5.8 | 142 | 178 | 199 | 224 | 276 | 61 | 90 | 106 | 126 | 171 | 52 | 68 | 77 | 86 | 107 | 59 | 96 | 124 | 162 | 276 |
| ≥40 | 2,591 | 4.7 | 5.1 | 5.3 | 5.5 | 5.8 | 145 | 182 | 203 | 227 | 278 | 66 | 93 | 110 | 128 | 175 | 52 | 68 | 76 | 86 | 106 | 60 | 100 | 129 | 168 | 288 |
*Adjusted glycated hemoglobin (HbA1c) is defined as HbA1c (National Glycohemoglobin Standardization Program) or calculated HbA1c = 1.02 × HbA1c (Japan Diabetes Society) + 0.25. BMI, body mass index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Table 2.
Biomarkers classified by percentile in healthy participants according to the week of pregnancy
| Weeks of pregnancy | n | Adjusted HbA1c* (%) | Total cholesterol (mg/dL) | LDL cholesterol (mg/dL) | HDL cholesterol (mg/dL) | Triglyceride (mg/dL) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | ||
| First trimester of pregnancy | ||||||||||||||||||||||||||
| 4–7 weeks | 897 | 4.7 | 5.0 | 5.2 | 5.4 | 5.7 | 127 | 162 | 182 | 202 | 249 | 54 | 80 | 95 | 112 | 154 | 48 | 63 | 72 | 82 | 101 | 49 | 80 | 106 | 146 | 255 |
| 8–11 weeks | 16,660 | 4.7 | 5.0 | 5.2 | 5.4 | 5.7 | 135 | 167 | 186 | 209 | 260 | 57 | 83 | 98 | 116 | 157 | 50 | 66 | 74 | 83 | 103 | 52 | 84 | 109 | 144 | 248 |
| 12–13 weeks | 14,575 | 4.7 | 5.0 | 5.2 | 5.4 | 5.7 | 137 | 171 | 190 | 212 | 262 | 59 | 85 | 100 | 118 | 160 | 51 | 66 | 75 | 84 | 104 | 55 | 88 | 113 | 147 | 258 |
| Second trimester of pregnancy | ||||||||||||||||||||||||||
| 14–15 weeks | 13,607 | 4.7 | 5.0 | 5.2 | 5.4 | 5.7 | 142 | 175 | 196 | 218 | 269 | 62 | 88 | 104 | 123 | 166 | 52 | 67 | 76 | 85 | 105 | 58 | 93 | 119 | 154 | 263 |
| 16–19 weeks | 19,725 | 4.6 | 5.0 | 5.1 | 5.4 | 5.7 | 147 | 182 | 203 | 227 | 278 | 65 | 93 | 109 | 128 | 173 | 53 | 69 | 78 | 87 | 108 | 62 | 98 | 126 | 162 | 275 |
| 20–23 weeks | 7,883 | 4.6 | 4.9 | 5.1 | 5.3 | 5.7 | 148 | 189 | 211 | 235 | 288 | 67 | 97 | 115 | 135 | 181 | 54 | 70 | 79 | 89 | 108 | 64 | 103 | 133 | 171 | 294 |
| 24–27 weeks | 2,678 | 4.6 | 4.9 | 5.1 | 5.3 | 5.7 | 147 | 188 | 211 | 239 | 303 | 67 | 96 | 115 | 138 | 192 | 53 | 70 | 79 | 88 | 110 | 61 | 103 | 133 | 176 | 314 |
| Overall | 76,025 | 4.7 | 5.0 | 5.2 | 5.4 | 5.7 | 135 | 168 | 188 | 210 | 261 | 58 | 84 | 99 | 117 | 158 | 51 | 66 | 74 | 83 | 103 | 53 | 86 | 111 | 146 | 253 |
*Adjusted glycated hemoglobin (HbA1c) is defined as HbA1c (National Glycohemoglobin Standardization Program) or calculated HbA1c = 1.02 × HbA1c (Japan Diabetes Society) + 0.25. HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Table 3 shows a comparison of the distribution of metabolic biomarkers between participants with and without diabetes or other endocrine disorders. HbA1c was higher in participants with type 1 diabetes, type 2 diabetes or GDM only than in healthy participants. Furthermore, HbA1c was higher than the recommended target of 6.5%1 for glycemic control during pregnancy in 39.5% of those with type 1 diabetes only and 24.3% of those with type 2 diabetes only.
Table 3.
Distribution of biomarkers in pregnant participants with or without diabetes/endocrine disorders
| Variables | Trimester of pregnancy | Healthy participants | Participants with type 1 diabetes only | Participants with type 2 diabetes only | Participants with gestational diabetes only | Participants with dyslipidemia only | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 32,136 (1st), 43,899 (2nd) | n = 28 (1st), 15 (2nd) | n = 35 (1st), 43 (2nd) | n = 1,002 (1st), 1,313 (2nd) | n = 149 (1st), 205 (2nd) | ||||||||||||||||||||||
| 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | 2.5th | 25th | 50th | 75th | 97.5th | ||
| Adjusted HbA1c* (%) | First | 4.7 | 5.0 | 5.2 | 5.4 | 5.7 | 5.2 | 5.9 | 6.4 | 7.0 | 9.1 | 5.0 | 5.6 | 6.0 | 6.7 | 10.7 | 4.8 | 5.2 | 5.4 | 5.6 | 6.4 | 4.7 | 5.1 | 5.2 | 5.5 | 5.9 |
| Second | 4.6 | 5.0 | 5.1 | 5.4 | 5.7 | 5.2 | 5.6 | 6.1 | 6.7 | 7.7 | 4.8 | 5.4 | 6.0 | 6.4 | 7.6 | 4.7 | 5.1 | 5.4 | 5.6 | 6.2 | 4.6 | 5.1 | 5.2 | 5.5 | 5.8 | |
| TC (mg/dL) | First | 135 | 168 | 188 | 210 | 261 | 122 | 170 | 183 | 210 | 257 | 142 | 166 | 184 | 202 | 234 | 145 | 174 | 195 | 217 | 263 | 158 | 204 | 225 | 251 | 328 |
| Second | 145 | 181 | 203 | 227 | 279 | 164 | 172 | 191 | 214 | 231 | 149 | 182 | 198 | 223 | 289 | 148 | 184 | 206 | 230 | 289 | 172 | 224 | 245 | 278 | 362 | |
| LDL‐C (mg/dL) | First | 58 | 84 | 99 | 117 | 158 | 50 | 75 | 93 | 104 | 142 | 61 | 84 | 95 | 124 | 145 | 64 | 89 | 107 | 124 | 166 | 83 | 113 | 124 | 156 | 232 |
| Second | 64 | 92 | 109 | 128 | 174 | 68 | 90 | 91 | 117 | 148 | 57 | 95 | 105 | 128 | 176 | 66 | 96 | 112 | 132 | 181 | 79 | 126 | 144 | 174 | 261 | |
| HDL‐C (mg/dL) | First | 51 | 66 | 74 | 83 | 103 | 56 | 68 | 78 | 99 | 123 | 33 | 57 | 64 | 73 | 107 | 49 | 63 | 73 | 83 | 101 | 48 | 65 | 75 | 84 | 108 |
| Second | 53 | 69 | 77 | 87 | 107 | 48 | 68 | 72 | 84 | 102 | 43 | 59 | 66 | 81 | 91 | 50 | 66 | 75 | 85 | 104 | 52 | 71 | 80 | 91 | 116 | |
| TG (mg/dL) | First | 53 | 86 | 111 | 146 | 253 | 45 | 73 | 91 | 126 | 232 | 44 | 112 | 151 | 196 | 295 | 63 | 97 | 127 | 169 | 321 | 64 | 108 | 144 | 200 | 383 |
| Second | 61 | 98 | 125 | 163 | 280 | 46 | 79 | 124 | 180 | 281 | 72 | 123 | 159 | 232 | 434 | 65 | 108 | 142 | 190 | 332 | 72 | 114 | 143 | 195 | 329 | |
Adjusted glycated hemoglobin (HbA1c) is defined as HbA1c (National Glycohemoglobin Standardization Program) or calculated HbA1c = 1.02 × HbA1c (Japan Diabetes Society) + 0.25. HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Our evaluation of lipid biomarkers showed that participants with type 1 diabetes had slightly better lipid profiles than healthy participants. Lipid profiles in participants with type 2 diabetes were characterized by higher TG levels and lower HDL‐C during the first trimester of pregnancy, and a slight increase in lipid levels between the first and second trimesters. TG levels were higher in participants with GDM than in healthy participants, but no differences in the distribution of cholesterol levels, including HDL‐C, were observed between these groups. In participants with dyslipidemia, the distribution of HDL‐C was similar to those of healthy participants, whereas TC, LDL‐C and TG levels were higher than those of healthy participants.
Participants with higher prepregnancy BMI were more likely to have diabetes or endocrine disorders (Table S1).
Discussion
This is the first report of the metabolic profiles of pregnant women, which was based on big data extracted from questionnaires and blood samples taken during pregnancy, obtained from a nationwide large cohort study across Japan. We report changes in the distribution of metabolic biomarkers with the progression of pregnancy, and differences in the distribution of those biomarkers between healthy pregnant women and pregnant women with diabetes or other metabolic disorders.
According to previous reports, HbA1c levels decrease during the second trimester of pregnancy and increase during the third trimester11, 12. The participants in the present study also showed a decrease in HbA1c between the first and second trimesters.
HbA1c was higher in participants with pregestational diabetes (type 1 diabetes or type 2 diabetes) or GDM than in healthy participants. Approximately half of the participants had HbA1c levels that were less than the recommended target of 6.5% for glycemic control during pregnancy13, whereas >5.0% had HbA1c levels that were higher than the generally recommended target of 7.0% as an indicator of glycemic control in the Japanese population14. This observation led us to speculate that our participants often conceived without planning and while their glycemic control was poor.
It is known that levels of cholesterol and TG increase during pregnancy2, and this study also showed that levels of all lipid biomarkers, including HDL‐C, across all percentiles, increased as pregnancy progressed. However, the present results also showed that the second‐highest TG level was found in the youngest age group (<20 years). This finding is inconsistent with another study that reported that TG levels were significantly higher in women of older ages3. Although the reason for the incongruent results between the present study and the previous study is uncertain, the fact remains that teenage pregnancy carries heightened risks for the mother and newborn. Despite the limitation that blood samples were not obtained after fasting, and were taken at any time, such samples should be informative, because the European Atherosclerosis Society recently recognized that it is not essential to use fasting blood samples to measure lipid profiles15. In addition, our sample size was sufficient to permit accurate statistical analysis.
The levels of lipid biomarkers, excluding HDL‐C, in participants with dyslipidemia were higher than those of healthy participants, as expected. Interestingly, participants with pregestational diabetes, in contrast to those with dyslipidemia, did not show higher levels of lipid biomarkers during the second trimester of pregnancy than healthy participants. This observation implies that pregnant women with pregestational diabetes are less likely to rely on lipids as an energy source because of their higher blood glucose levels. Participants with GDM reportedly have high TG levels and low HDL‐C levels16. However, although the present study found that these participants had higher TG levels, their HDL‐C levels were not different from those of healthy participants. The reason for the inconsistency between studies is unclear.
The present results concurred with those of a previous multi‐institutional retrospective study of pregnancy outcomes of women with GDM according to pregestational BMI in Japan17. The previous study reported that GDM is more frequent among women with a higher prepregnancy BMI. Both maternal prepregnancy BMI and GDM have consistently been reported to be risk factors for obesity among offspring5, 18. There is also growing evidence that GDM leads to adverse long‐term maternal outcomes including type 2 diabetes and cardiovascular diseases19, 20. Effective management of maternal diet and lifestyle factors is required before conception and during pregnancy to reduce the risks of morbidity for mothers and offspring. However, the effectiveness of providing dietary advice interventions during pregnancy lacks high‐quality evidence21. We suggest that future trials should be designed to monitor adherence, consider women’s views and preferences, and evaluate effects on both short‐ and long‐term outcomes. More importantly, there is a need to measure and report on core outcomes for research on GDM22.
The present study had several limitations. First, non‐fasting blood samples could affect the evaluation of changes in lipid and other metabolic parameters during their second or third trimester of pregnancy. Second, blood samples were less frequently obtained from participants at 4–7 weeks of pregnancy and during the latter half of the second trimester (from 20 weeks of pregnancy) owing to a skewed distribution of the elapsed duration of pregnancy among the participants. However, our analyses were not adjusted for age or physique, as the purpose of this study was to describe the metabolic status of pregnant women. In further studies, we will examine associations between metabolic biomarkers, the elapsed duration of pregnancy, and the presence or absence of diabetic and endocrine disorders while adjusting for any confounding factors. Third, some of the clinical information used in this study was obtained from a self‐administered questionnaire and might therefore have a limited reliability, given the likely variation in the diagnostic criteria and/or management strategies used. Additionally, the number of pregnant women with disorders or complications who enrolled in the present study were limited and might be insufficient, as our study was not a disease‐specific study, and could therefore have resulted in the low prevalence of metabolic disorders overall.
In summary, we have reported the time course of glucose and lipid metabolic biomarkers during pregnancy in Japanese women enrolled in the JECS. The distribution of these biomarkers was affected by the time of blood sample collection, as well as the presence or absence of underlying diseases. Monitoring pregnant women with poor glycemic and lipid profile is crucial to control and optimize these levels in reducing the risks of adverse pregnancy outcomes. In the future, the JECS will elucidate the influences of metabolic status during pregnancy on the health and development of the children born during the study.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Distribution of participants with or without diabetes/endocrine disorders by body mass index before pregnancy
Acknowledgments
We are grateful to all the participants who took part in the JECS. We thank all the staff members of the JECS. We also thank Dr Tadayuki Ayabe for the first draft of the manuscript. Members of the Japan Environment and Children’s Study (JECS) as of 2019 (principal investigator, Michihiro Kamijima): Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Michihiro Kamijima (Nagoya City University, Nagoya, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Youichi Kurosawa (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan) and Takahiko Katoh (Kumamoto University, Kumamoto, Japan). This work was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors, and do not represent the official views of this government agency.
J Diabetes Investig 2020; 11: 1318–1325
Contributor Information
Hatoko Sasaki, Email: sasaki-ht@ncchd.go.jp.
Japan Environment and Children’s Study (JECS) Group:
Michihiro Kamijima, Shin Yamazaki, Reiko Kishi, Nobuo Yaegashi, Koichi Hashimoto, Chisato Mori, Shuichi Ito, Zentaro Yamagata, Hidekuni Inadera, Michihiro Kamijima, Takeo Nakayama, Hiroyasu Iso, Masayuki Shima, Youichi Kurosawa, Narufumi Suganuma, Koichi Kusuhara, and Takahiko Katoh
References
- 1. Newbern D, Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes 2011; 18: 409–416. [DOI] [PubMed] [Google Scholar]
- 2. Ghio A, Bertolotto A, Resi V, et al Triglyceride metabolism in pregnancy. Adv Clin Chem 2011; 55: 134. [DOI] [PubMed] [Google Scholar]
- 3. Vrijkotte TG, Krukziener N, Hutten BA, et al Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study. J Clin Endocrinol Metab 2012; 97: 3917–3925. [DOI] [PubMed] [Google Scholar]
- 4. Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update 2010; 16: 255–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baidal JAW, Locks LM, Cheng ER, et al Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med 2016; 50: 761–779. [DOI] [PubMed] [Google Scholar]
- 6. Kawamoto T, Nitta H, Murata K, et al Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014; 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minakami H, Maeda T, Fujii T, et al Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J Obstet Gynaecol Res 2014; 40: 1469–1499. [DOI] [PubMed] [Google Scholar]
- 8. Duran A, Sáenz S, Torrejón MJ, et al Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos Gestational Diabetes Study. Diabetes Care 2014; 37: 2442–2450. [DOI] [PubMed] [Google Scholar]
- 9. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) the 2017 edition. Available from http://www.jsog.or.jp/activity/pdf/gl_sanka_2017.pdf. Accessed November 2, 2019 (In Japanese).
- 10. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’for medical statistics. Bone Marrow Transplant 2013; 48: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Versantvoort A, Van Roosmalen J, Radder J. Course of HbA1c in non‐diabetic pregnancy related to birth weight. Neth J Med 2013; 71: 22–25. [PubMed] [Google Scholar]
- 12. Hiramatsu Y, Shimizu I, Omori Y, et al Determination of reference intervals of glycated albumin and hemoglobin A1c in healthy pregnant Japanese women and analysis of their time courses and influencing factors during pregnancy. Endocr J 2012; 59: 145–151. [DOI] [PubMed] [Google Scholar]
- 13. American Diabetes Association . 13. Management of diabetes in pregnancy. Diabetes Care 2017; 40(Supplement 1): S114–S119. [DOI] [PubMed] [Google Scholar]
- 14. Sanaka M. The Japanese Society of Diabetes and Pregnancy. Available from: http://www.dm‐net.co.jp/jsdp/qa/b/q01/ Accessed June 17, 2008 (Japanese).
- 15. Nordestgaard BG, Langsted A, Mora S, et al Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut‐points—a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J 2016; 37: 1944–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryckman K, Spracklen C, Smith C, et al Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta‐analysis. BJOG Int J Gynaecol Obstet 2015; 122: 643–651. [DOI] [PubMed] [Google Scholar]
- 17. Sugiyama T, Nagao K, Metoki H, et al Pregnancy outcomes of gestational diabetes mellitus according to pre‐gestational BMI in a retrospective multi‐institutional study in Japan. Endocr J 2014; 61: 373–380. [DOI] [PubMed] [Google Scholar]
- 18. American Diabetes Association . Gestational diabetes mellitus. Diabetes Care 2004; 27: S88. [DOI] [PubMed] [Google Scholar]
- 19. Kessous R, Shoham‐Vardi I, Pariente G, et al An association between gestational diabetes mellitus and long‐term maternal cardiovascular morbidity. Heart 2013; 99: 1118–1121. [DOI] [PubMed] [Google Scholar]
- 20. Noctor E, Dunne FP. Type 2 diabetes after gestational diabetes: the influence of changing diagnostic criteria. World J Diabetes 2015; 6: 234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tieu J, Crowther CA, Middleton P. Dietary advice in pregnancy for preventing gestational diabetes mellitus. Cochrane Database Syst Rev 2008; 16: CD006674. [DOI] [PubMed] [Google Scholar]
- 22. Shepherd E, Gomersall JC, Tieu J, et al Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev 2017; 11: CD010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Distribution of participants with or without diabetes/endocrine disorders by body mass index before pregnancy


