Abstract
Frailty is defined as diminished physiological reserve predisposing one to adverse outcomes when exposed to stressors. Currently, there is no standardized Frail assessment tool used perioperatively. Edmonton Frail Scale (EFS), which is validated for use by non-geriatricians and in selected surgical populations, is a candidate for this role. However, little evaluation of its use has been carried out in the Asian populations so far. This is a prospective observational study done among patients aged 70 years and above attended Preoperative Assessment Clinic (PAC) in Singapore General Hospital prior to major abdominal surgery from December 2017 to September 2018. The Comprehensive Complication Index (CCI) and Postoperative Morbidity Survey (POMS) were used to assess their postoperative morbidity respectively. Patient’s acceptability of EFS was measured using the QQ-10 questionnaire and the inter-rater reliability of EFS was assessed by Kappa statistics and Bland Altman plot. The primary aim of this study is to assess if frailty measured by EFS is predictive of postoperative complications in elderly patients undergoing elective major abdominal surgery. We also aim to assess the feasibility of implementing EFS as a standard tool in the outpatient preoperative assessment clinic setting. EFS score was found to be a significant predictor of postoperative morbidity. (OR 1.35, p < 0.001) Each point increase in EFS score was associated with a 3 point increase in CCI score. (Coefficient b 2.944, p < 0.001) EFS score more than 4 has a fair predictability of both early and 30-day postoperative complications. Feasibility study demonstrated an overall acceptance of the EFS among our patients with good inter-rater agreement.
Subject terms: Risk factors, Geriatrics, Rehabilitation
Introduction
Frailty is defined as the biologic syndrome of a decreased physiological reserve, resulting from the cumulative declines of multiple organ systems, which predisposes one to adverse outcomes when exposed to stressors such as surgery1,2. As frailty is the composite effect of multiple organ deficiencies, it has been shown to be better in predicting hospitalization stay, complications and morbidity among the elderly patients when compared to individual assessment of medical comorbidity or functional status1,3.
There is an increasing number of non-cardiac surgeries done among elderly people worldwide4. The prevalence of frailty is estimated to be at 10–15% among those between 65 to 75 years old. This frequency increases to more than 40% among those beyond 85 years old3,5–7. However, despite several frailty assessment tools being available, routine assessment is not commonly done prior to elective surgery8–10. Furthermore, a patient's preoperative optimization strategy rarely incorporates the formal diagnosis of frailty8,11,12. This is an important observation as frailty is associated with poorer outcomes among surgical patients, such as the increased risk of infection, postoperative delirium, postoperative mortality, prolonged hospital stay and healthcare-related cost4,13–16. While multiple implementation barriers may contribute to this, the choice of the assessment tool is an important consideration8. In the perioperative setting, a frailty assessment tool should be highly predictive of postoperative outcomes, and able to highlight areas amenable for optimization before surgery. Moreover, it should be both feasible and simple to administer by the non-geriatricians in the busy preoperative assessment setting.
The current frailty assessment tools can be divided into the phenotype model such as frailty phenotype which focuses on functional assessment or the cumulative deficit model such as frailty index giving deficits in the domains of comorbidities14. However, both tests are time-consuming when incorporated into routine pre-operative assessment and they provide clinicians little information regarding areas for modification2,17. The Edmonton Frail Scale (EFS), being a performance-based multidimensional frailty assessment tool, is designed to aid in the assessment and screening of frail elderly patients in the primary care setting, as well as at bedside, by non-geriatricians in the western population18.
EFS consists of 11 questions over nine different domains such as cognition, social support, mood, nutrition, and functional performance. The EFS was found to be a valid measure of frailty as the result was well correlated with the clinical impression of geriatricians through detailed history and physical examination18,19. EFS has also been shown to be able to predict operative risk when used as a screening tool in the Caucasian population11. However, little evaluation of its use has been carried out in the Asian population so far, which has one of the fastest aging populations in the world.
The primary aim of this study is to assess if frailty measured by EFS is predictive of postoperative complications among elderly patients who are undergoing elective major abdominal surgery in an Asian healthcare system. Furthermore, we aim to assess the feasibility of implementing EFS as a standard tool in the outpatient preoperative assessment clinic setting.
Methods
Study design
This is a single-center, prospective observational study conducted in Singapore General Hospital (SGH), which is the largest tertiary public hospital in Singapore with over 1,700 beds. This study consisted of two separate parts, namely assessment of predictive validity of EFS for postoperative complications and assessment of the feasibility of EFS in the preoperative outpatient setting. Informed consent was obtained from all patients and the study protocol was approved by the Institutional Review Board (SingHealth CIRB 2018/2548) prior to the start of the study. The study was performed in accordance with relevant guidelines and regulations.
Patients aged 70 years and above, who attended SGH Preoperative Assessment Clinic (PAC) prior to elective major abdominal surgery between December 2017 and September 2018 were enrolled in this study. Major abdominal surgery is defined as an intra-peritoneal surgery, expected to last more than 2 h, or with an anticipated blood loss greater than 500 mL including surgeries in the disciplines of general surgery (upper gastrointestinal and hepatobiliary surgery), colorectal surgery, urological and gynecological surgery.
Assessment of predictive validity of EFS
Five trained nurses in the PAC who are blinded to other details related to the patient’s planned surgery (type of surgery, patient’s medical comorbidities, surgical plan etc.) administered the questionnaire to patients who met the recruitment criteria in English, Chinese, Malay or Tamil in the same standard manner. The total EFS score was calculated by the nurses at the end of the assessment. Patient’s demographic data, clinical laboratory data, comorbidities as well as 30-day postoperative complications were searched from the institution’s clinical information system (Sunrise Clinical Manager, Allscripts, Illinois, USA) by two assessors (HYK and LWL).
Assessment of the feasibility of EFS in the preoperative setting
We define the feasibility of EFS based on patients’ acceptability, duration of time taken to complete the questionnaire and inter-rater variability. Patients’ acceptability of EFS was evaluated using the QQ-10 questionnaire. The time taken for those patients to complete EFS was also recorded.
Measurement
Assessment of frailty
Edmonton Frail Scale (EFS)
The EFS is an 11-question questionnaire which analyses nine domains of frailty (cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence, functional performance), with the maximum score being 17, representing the highest level of frailty. The patients were then classified into five categories based on their EFS scores as suggested on the official EFS website (Edmontonfrailscale.org), ranging from Fit (0–3), Vulnerable (4–5), Mildly frail (6–7), Moderately Frail (8–9) and Severely frail (≥ 10).
Assessment of predictive validity of EFS
Postoperative outcome data were entered into the electronic medical system by the primary surgical team. Postoperative morbidity data were analyzed using the Comprehensive Complication Index (CCI)20 and Postoperative Morbidity Survey (POMS)21 to assess the predictive validity of EFS in the preoperative setting.
Comprehensive Complication Index (CCI)
The Comprehensive Complication Index (CCI) is a measure currently widely adopted to assess postoperative complications20. Based on the widely established Clavien–Dindo classification (CDC), it integrates into a single formula all complications of varying severity, ranging from 0 (uneventful) to 100 (death). Compared to CDC, which includes only the most severe complications, it captures all postoperative morbidity by severity over time, producing a continuous scoring variable22. 1-month follow-up was selected to allow better capture of all postoperative complications.
Postoperative Morbidity Survey (POMS)
Postoperative Morbidity Survey (POMS) is a method used to describe short-term morbidity after major surgery. It is a nine domain tool based on organ systems. POMS was assessed on postoperative days 3, 5, 7. For each domain, the presence or absence of morbidity is recorded based on objective criteria. A score of 0 will be given if no complication is present and 1 for any complication present under each domain on that particular postoperative day21.
Assessment of the feasibility of EFS in the perioperative setting
QQ-10 questionnaire
QQ-10 is a 10-item questionnaire, developed to assess the face validity, feasibility and utility of other healthcare questionnaires23,24. 6 out of 10 questions assess how patients value the healthcare questionnaire (helped me communicate about my condition, relevant to my condition, easy to complete, included all the aspects of my condition I am concerned about, was enjoyable, would be happy to complete as part of routine care), while 4 out of 10 assess the burden of the questionnaire to the patient (too long, embarrassing, complicated, upset me). The patient scores each question from a scale of 0 to 4, with 0 being “strongly disagree” and 4 being “strongly agree”. The mean value score is calculated as an average score of the 6 questions assessing the value of the questionnaire from all patients, while the mean burden score is calculated from the four burden questions.
Time taken to complete EFS
Time taken to complete the EFS (in seconds) in PAC was recorded for patients recruited in the feasibility study by five clinic nurses.
Inter-rater variability of EFS
Inter-rater reliability was assessed using weighted Kappa and Bland Altman plot25.
Sample size calculation
Assessment of predictive validity of EFS
The mean difference of CCI across the groups of frailty (non-frail, vulnerable and frail) was about 11–12 in our pilot study. Based on the effect size obtained, an estimated sample size of at least 14 patients in each group will allow us to significantly detect the mean difference of CCI across the groups of frailty with Type 1 error of 0.05 and Type II error of 0.2 using ANOVA analysis, assuming a dropout rate of around 20%.
Assessment of the feasibility of EFS in the perioperative setting
Based on our prior clinical experience, we estimated the mean value score obtained from QQ-10 questionnaire to be around 80%. 30 patients’ data will allow us to have an interval estimate (confidence level 95%) of mean value score with precision of 15%.
Statistical analyses
Data collected were analyzed using R 3.5.1 (R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/). Descriptive data are expressed as mean with standard deviations for continuous variables and frequencies (percentages) for categorical variables.
One-way ANOVA was performed to assess the differences in continuous variables including age, BMI, length of stay as well as CCI and POMS scores among the five categories of frailty groups. Chi-square /Fisher’s exact test was performed to investigate the correlation between level of frailty and categorical variables, e.g. gender, race, surgical disciplines, types of surgery.
A multivariable linear regression model was applied to assess the correlation of EFS score with CCI adjusted by patients’ demography and surgical characteristics. A multivariable generalized linear mix model was performed to estimate adjusted odds ratio of EFS score in predicting the presence of 30-day postoperative complications (measured by total POMS score more than 0). The area under the receiver operating characteristic (ROC) curve which is plotted by the true positive rate (sensitivity) against the false positive rate (1-specificity) was used to evaluate performance of using EFS to predict the presence of early and 30-day complication and quantify the cut-off EFS score using Youden’s index. Weighted Kappa and Bland Altman plot for 2 raters were used to assess inter-rater reliability.
A p value of < 0.05 was used to determine statistical significance.
Ethical approval and consent to participate
The study protocol and written informed consent were approved by the Institutional Review Board (SingHealth Centralized Institutional Review Board 2018/2548, Singapore health service group).
Consent for publication
Written informed consent was obtained from all patients.
Results
Demographics
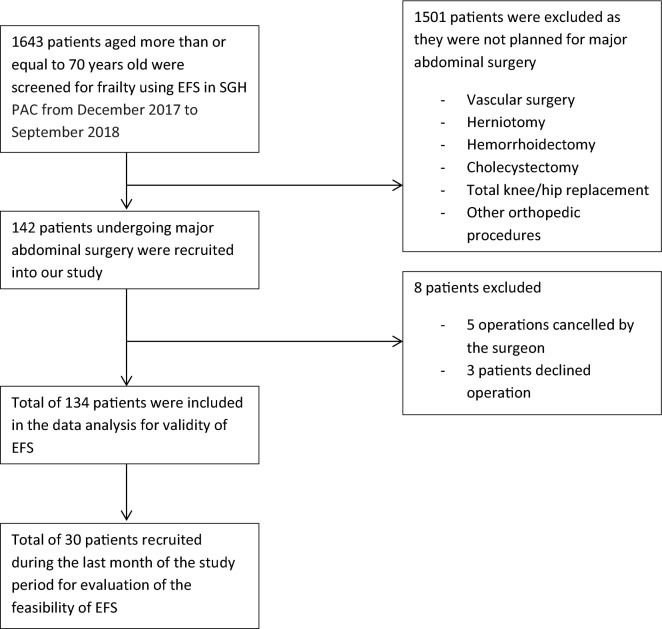
A total of 142 patients who met our inclusion criteria were recruited into the validity study. Eight of them were excluded from the data analysis as the operation was either cancelled by the surgeon or by the patients themselves (Fig. 1). The remaining 134 patients undergoing major abdominal surgery were included in the analysis of the validity study and 30 patients out of this cohort into the feasibility study, as none of them refused to participate. Based on their EFS scores, 62 of them are fit (EFS ≤ 3) (46.3%), 33 of them are vulnerable (EFS 4–5) (10.4%). 14 patients (10.4%) are mildly frail (EFS 6–7), 12 are moderately frail (9%) and the remaining 13 are severely frail (9.7%) (Table 1). Patients in the frail group are older but there is no significant difference in terms of BMI, gender, race as well as surgical discipline they belonged to among the different groups of patients. In addition, a higher proportion of frail patients underwent open surgery compared to the non-frail group who had more laparoscopic procedures done for similar surgical conditions (p = 0.013) (Table 1).
Figure 1.
Study flowchart.
Table 1.
Comparison of demographics among patients with different degrees of frailty.
| Fit | Vulnerable | Mildly frail | Moderately frail | Severely frail | p value | |
|---|---|---|---|---|---|---|
| Number of patients | 62 (46.3) | 33 (24.6) | 14 (10.4) | 12 (9) | 13 (9.7) | |
| Age mean (SD) | 74.2 (4.2) | 76.8 (4.9) | 77.5 (6.2) | 77.7 (5.7) | 78.3 (6.9) | 0.029 |
| Gender (freq (percent)) | 0.077 | |||||
| Male | 39 (58.2) | 14 (20.9) | 6 (9) | 4 (6) | 4 (6) | |
| Female | 23 (34.3) | 19 (28.4) | 8 (11.9) | 8 (11.9) | 9 (13.4) | |
| Race (freq (percent)) | 0.454 | |||||
| Chinese | 57 (46.3) | 31 (25.2) | 12 (9.8) | 11 (8.9) | 12 (9.8) | |
| Malay | 3 (60) | 1 (20) | 0 (0) | 0 (0) | 1 (20) | |
| Indian | 0 (0) | 1 (50) | 1 (50) | 0 (0) | 0 (0) | |
| Others | 2 (50) | 0 (0) | 1 (25) | 1 (25) | 0 (0) | |
| BMI (kg/m2) (mean(sd)) | 23.2 (5.3) | 24.3 (4.7) | 23.7 (3.7) | 24.7 (4.2) | 25.3 (4.2) | 0.545 |
| Surgical discipline (freq (percent)) | 0.066 | |||||
| Urology/gynaecology | 30 (63.8) | 10 (21.3) | 4 (8.5) | 1 (2.1) | 2 (4.3) | |
| Colorectal | 16 (41) | 12 (30.8) | 4 (10.3) | 4 (10.3) | 3 (7.7) | |
| General surgery | 16 (33.3) | 11 (22.9) | 6 (12.5) | 7 (14.6) | 8 (16.7) | |
| Type of surgery (freq (percent)) | 0.013 | |||||
| Open | 15 (29.4) | 13 (25.5) | 8 (15.7) | 7 (13.7) | 8 (15.7) | |
| Laparoscopic | 47 (56.6) | 20 (24.1) | 6 (7.2) | 5 (6) | 5 (6) | |
Validity of EFS to predict postoperative risk
Both CCI scores and POMS were used to study short-term postoperative morbidity. A statistically significant difference in mean CCI score was found between fit, vulnerable, mildly frail, moderately frail and severely frail group. (p < 0.001) (Table 2). A similar result was demonstrated when POMS analysis was performed at postoperative day 3, 5 and 7. (p < 0.05) A step-wise increase in mean CCI and POMS score was also observed across the five groups of patients. Furthermore, frail patients have a longer length of hospital stay compared to fit or vulnerable elderly patients going through similar operations (p = 0.034) (Table 2).
Table 2.
Comparison of postoperative complications among patients with different degrees of frailty.
| Fit | Vulnerable | Mildly frail | Moderately frail | Severely frail | p value | |
|---|---|---|---|---|---|---|
| Number of patients | 62 (46.3) | 33 (24.6) | 14 (10.4) | 12 (9) | 13 (9.7) | |
| CCI (mean(SD)) | 11.1 (12) | 16.1 (12.1) | 24.5 (16.4) | 28.6 (17.1) | 42.1 (27.4) | < 0.001 |
| POMS day 3 (mean(SD)) | 1.3 (1.5) | 1.9 (1.7) | 1.9 (1.3) | 2.3 (1.5) | 3.2 (2) | 0.012 |
| POMS day 5 (mean(SD)) | 0.5 (0.9) | 0.8 (1.1) | 1.2 (1.4) | 1.4 (1.3) | 3.1 (2.3) | 0.003 |
| POMS day 7 (mean(SD)) | 0.2 (0.4) | 0.4 (0.7) | 0.8 (1.4) | 0.9 (1.3) | 2.5 (2.4) | 0.005 |
| LOS (days) (mean(SD)) | 7.4 (6.5) | 10 (11.4) | 15.1 (12.8) | 12.7 (8.4) | 17.9 (17.8) | 0.034 |
Regression models were then constructed to assess the performance of EFS in predicting postoperative complications as measured by both CCI and POMS. Total POMS score more than 0 was used as a surrogate marker for the presence of any postoperative complications within 30 days. EFS score was found to be a significant predictor of postoperative morbidity in both models after adjusting for other confounders including age, gender, race, surgical disciplines and surgical technique (open or laparoscopic surgery). (OR 1.35, p < 0.001) (Table 3). Each point increase in EFS score was associated with a 3 point increase in CCI score (Coefficient b 2.944, p < 0.001) (Table 4).
Table 3.
Variables associated with presence of 30-day postoperative complications (measured by TOTAL POMS score > 0 within 30 postoperative days) using generalized linear mix model (age was excluded due to small number range resulting in convergence issue in model fitting).
| Odds.ratio (95% CI) | p value | |
|---|---|---|
| EFS.SCORE | 1.35 (1.18, 1.57) | < 0.001 |
| Gender | ||
| Male | Ref | |
| Female | 0.42 (0.2, 0.83) | 0.015 |
| Race | ||
| Chinese | Ref | |
| Non-Chinese | 4.21 (1.2, 17.6) | 0.031 |
| BMI | 0.95 (0.88, 1.01) | 0.121 |
| Surgical discipline | ||
| General surgery | Ref | |
| Urology/gynaecology | 0.31 (0.13, 0.7) | 0.006 |
| Colorectal | 0.45 (0.19, 1.03) | 0.061 |
| Type of surgery | ||
| Lap | Ref | |
| Open | 1.63 (0.77, 3.57) | 0.18 |
Table 4.
Variables associated with presence of 30-day postoperative complications (measured by CCI score) using linear regression model.
| Coefficient b (95% CI) | p value | |
|---|---|---|
| EFS.SCORE | 2.944 (2.012, 3.877) | < 0.001 |
| Age | − 0.047 (− 0.541, 0.448) | 0.852 |
| Gender | ||
| Male | Ref | |
| Female | − 9.53 (− 14.471, − 4.588) | < 0.001 |
| Race | ||
| Chinese | Ref | |
| Non-Chinese | 0.053 (− 8.864, 8.971) | 0.991 |
| BMI | − 0.179 (− 0.686, 0.328) | 0.485 |
| Surgical discipline | ||
| General surgery | Ref | |
| Urology/gynaecology | − 12.268 (− 18.268, − 6.267) | < 0.001 |
| Colorectal | − 8.229 (− 14.231, − 2.228) | 0.008 |
| Type of surgery | ||
| Lap | Ref | |
| Open | 3.98 (− 1.463, 9.422) | 0.15 |
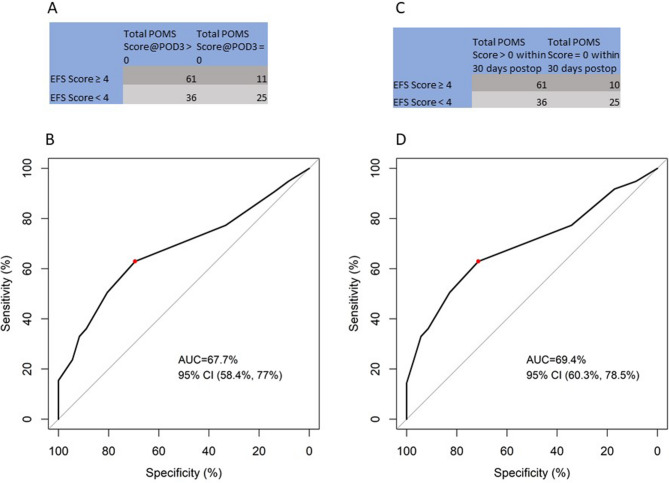
ROC curve was plotted to find an optimal cut-off value that can help to predict both early (measured by POMS score > 0 at postoperative day 3) and 30-day (measured by total POMS score > 0) postoperative complication. In both ROC curves, frailty defined as EFS score more than 4 was found to have a fair predictability demonstrated by the area under the curve (AUC) of 67.7% and 69.4% respectively (Fig. 2).
Figure 2.
Cutoff of EFS score for predicting presence of postoperative complications (A) Table showing choice of EFS cut off value to predict early postoperative complications (measured by POMS > 0 @ postoperative day 3) (B) ROC curve of choice of EFS cut off value to predict early postoperative complications (measured by POMS > 0 @ postoperative day3) (C) Table showing choice of EFS cut off value to predict 30-day postoperative complications (measured by Total POMS Score > 0 in within 30 postoperative days) (D) ROC curve of choice of EFS cut off value to predict 30-day postoperative complications (measured by Total POMS Score > 0 in within 30 postoperative days).
Feasibility analysis of EFS in the outpatient preoperative clinic setting.
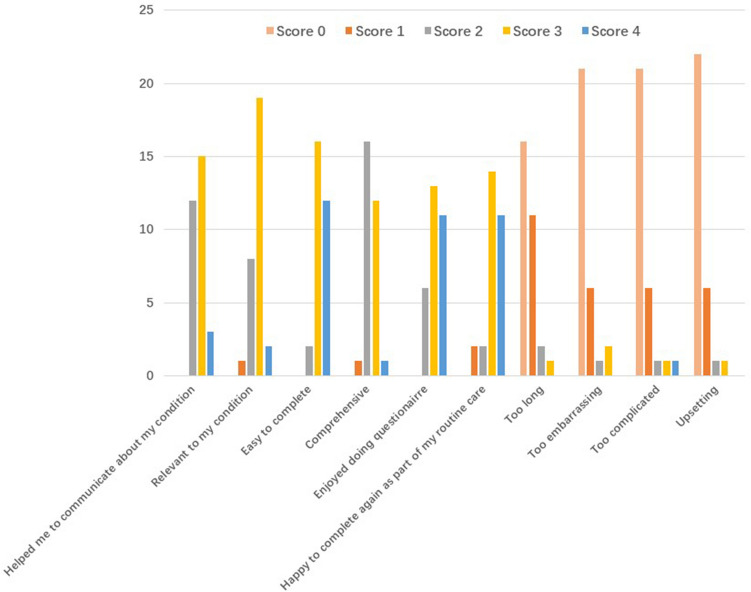
30 patients with a mean age of 76 years old took part in the QQ-10 questionnaire to understand their acceptability of EFS. The overall mean value score was 72% (SD 11.9) with a mean burden score of 12% (SD 17.1). The exact distribution of patients’ answers to each question is shown in Fig. 3. The high value score and low burden score show an overall acceptance of the EFS among our patients.
Figure 3.
QQ-10 questionnaire result. X axis shows the content of each question (value questions in blue colour and burden questions in red colour) and Y axis shows the number of patients giving a particular score for that question. (0-strongly disagree, 1-mostly disagree, 2-neither agree nor disagree, 3-mostly agree, 4-strongly agree).
The average time taken for those 30 patients to complete EFS was 225 s (SD 57). Weighted Kappa for 2 raters was 0.973, which demonstrated almost perfect inter-observer reliability. Bland Altman plot between 2 nurses assessing the same patient also demonstrated good agreement with a mean difference of 0.07 (95% CI − 1.1, 0.97) (Fig. 4).
Figure 4.
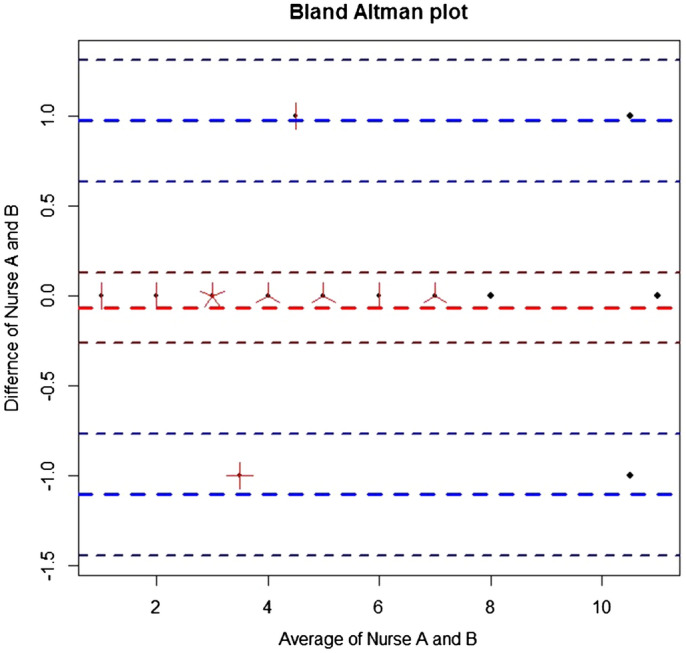
Bland Altman plot showing inter-rater agreement of EFS.
Discussion
Our data, from a single-center study, support that EFS is able to predict operative risk in elderly patients undergoing major abdominal surgery. We demonstrated that frail patients measured by higher EFS scores have greater postoperative complications risk as measured by both higher POMS and CCI scores (OR 1.35). Secondly, we propose to define frailty in Asian surgical population using EFS cut-off score of 4 and above to predict postoperative morbidity. Our ROC curve using cut-off score of 4 shows a fair performance in predicting the presence of both early and 30-days postoperative complications. Furthermore, we have demonstrated that the use of EFS in the outpatient preoperative assessment clinic is feasible and well accepted by elderly patients. It can be completed quickly and has minimal inter-rater variability. These features suggest that it is feasible and appropriate for EFS to be incorporated into the routine workflow of a busy preoperative assessment clinic.
Frailty is recognized as one of the strongest preoperative predictors of postoperative complications in one of meta-analysis study recently26. Having a clear understanding of postoperative recovery trajectories is essential to help clinicians conduct appropriate discussions about treatment plans with patients and family27. The value of Comprehensive Geriatric Assessment (CGA), the current gold standard for frailty assessment among geriatricians, in predicting frailty in colorectal cancer patients was shown by a study conducted by Krishnan et al., but its applicability in the busy preoperative clinic setting is questionable28. In another retrospective observational study, Saxton et al. demonstrated the predictive role of frailty measured by Frailty Index in identifying high risk patients planning for abdominal surgery29. However, as the items in Frailty Index were obtained through either history or physical examination, it provides little information on the specific deficit domain which the clinicians need to work on. The absence of a universally accepted measurement tool prevents frailty assessment from being incorporated into routine preoperative practice.
An assessment tool for use in the clinical setting should consider the two main purposes of preoperative identification of frailty: risk stratification, and identification of factors for potential modification10. In the acute care hospital population, multi-dimensional frailty assessment tools like EFS provide more information on the severity and the types of deficits experienced by a patient10. Previous studies have shown that EFS captures appropriately every area of frailty, with a high degree of correlation between EFS scores and CGA as well as other frailty scales18,30. Our results in the preoperative setting of patients planning for major abdominal surgery are consistent with findings by Dasgupta et al. that increasing EFS scores were associated with increased length of hospital stay and postoperative complications independent of age in orthopedic patients11. Similar results have also been reported in urological, cardiac as well as colorectal surgery patients in North America and Europe30–32.
Previous work on the perioperative use of EFS has been focused on its predictability of postoperative mortality and length of hospital stay, but little attention has been given to the exact EFS score that could help with risk stratification in the surgical population11,33,34. Currently, there is no consensus on the use of EFS cut-off scores to define non-frail, vulnerable and frail among the clinicians globally. The most widely used classifications include a two cut-off point system dividing the elderly patients into non-frail (EFS < 5), vulnerable (EFS 6–7) and frail (EFS > 8)31,35 and a four cut-off point system which separate the population into five categories like our study11,36. By having a more delicate classification system, we felt that the five-category system allows clinicians to risk stratify the patients with higher specificity based on their degree of frailty. This will facilitate clinical decision making and proper risk counselling when encountering frail patients in the preoperative clinic. In our study, the estimated prevalence of frailty in elderly surgical population is around 29% (EFS score more than 6). Study conducted by Hoover et al. estimated the prevalence of frailty in the Canadian community to be 22% based on community health survey37. The difference in prevalence can be possibly due to the larger number of frail elderly in the surgical population compared to the general public. Our ROC analysis demonstrated for the first time that frailty defined as EFS score of 4 and above in the surgical population could give perioperative clinicians fair predictability of increased postoperative complication risk. This is consistent with the result described by Dasgupta et al., who has demonstrated that EFS score more than 4 is associated with a significantly higher positive likelihood ratio when predicting postoperative complications11.
A good assessment tool in a clinical setting should be acceptable to the elderly surgical patients, easy to use by non-geriatricians and not burdensome to the healthcare system. Compared to other multidimensional tools such as CGA and Comprehensive Assessment of Frailty (CAF) score which are either too complex or too lengthy restricted for research use, EFS can be completed within 5 min and easily followed by non-geriatricians. The study by McIsaac et al. have suggested that almost all elderly patients are willing to participate in a frailty assessment before going for major surgery34. The high value score and low burden score obtained from the QQ-10 data suggested that our patients had a pleasant experience with EFS questionnaire during their preoperative visit and found it as making a positive impact on their health care. This makes EFS an ideal tool to be incorporated into routine preoperative frailty assessment.
The strength of the study includes the prospective study design with a complete follow up. The 1-month follow up period includes some of the subacute post-operative complications, allowing us to better validate the use of EFS in the preoperative setting. The use of two different postoperative morbidity measurements (CCI and POMS) with different grading systems helped to reduce the potential assessor bias making the conclusions more objective. The use of a separate patient’s acceptability measurement tool (QQ-10) further enhanced our knowledge about the clinical usefulness of EFS.
This study has several limitations. The single-center study design and the relatively small number of sample size may restrict its generalizability. Secondly, the outcome assessors were not blinded to the EFS score of the recruited patients. However, since the postoperative outcome data were entered into the electronic medical system by the surgical team who were unaware of the EFS score, this is unlikely to result in any observer bias. Further studies should be conducted to validate the ability of the EFS cut-off score proposed in predicting postoperative complications in larger patient populations, as well investigating the frailty domains that are particularly associated with poor outcomes to facilitate preoperative rehabilitation programs.
Conclusion
Our study has demonstrated that frailty measured by EFS is able to predict postoperative complications in elderly patients undergoing elective major abdominal surgery. EFS scores are closely correlated with postoperative morbidity as measured by POMS and CCI scores. Frailty defined as EFS score of 4 and above gives clinicians a fair predictability of an increase in post-operative risk. We have also shown that EFS is a feasible tool to be incorporated as a standard assessment in the outpatient preoperative clinic setting, with high patients’ acceptance and low inter-rater variability.
Acknowledgements
We thank the assistance of all the nurses in PAC, SGH. Special thanks to research nurses Ms. Thin Thiri Naing and Ms. Brenda Tan Pei Yi for their assistance during patient recruitment.
Abbreviations
- EFS
Edmonton Frail Scale
- CCI
Comprehensive Complication Index
- POMS
Postoperative Morbidity Survey
- PAC
Preoperative Assessment Clinic
- CGA
Comprehensive Geriatric Assessment
- CAF
Comprehensive Assessment of Frailty
Author contributions
Y.K.H. and L.W.L. helped with patient recruitment, data collection, data analysis and manuscript preparation. R.L. and M.S.L. helped with patient recruitment and data collection. Y.H. helped with statistical analysis of the data. Y.H., E.Y.S., K.L.N. and R.P. helped to edit the manuscript. H.R.A. conceived the idea and critically revised the manuscript. All authors read and approved the final manuscript.
Funding
There is no funding to be declared.
Data availability
The dataset analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Griffiths R, Mehta M. Frailty and anaesthesia: what we need to know. Contin. Educ. Anaesth. Criti. Care Pain. 2014;14:273–277. doi: 10.1093/bjaceaccp/mkt069. [DOI] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Cardiovascular health study collaborative research group: frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 3.Lin H-S, McBride RL, Hubbard RE. Frailty and anesthesia—risks during and post-surgery. Local Reg. Anesth. 2018;11:61–73. doi: 10.2147/LRA.S142996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buigues C, Juarros-Folgado P, Fernández-Garrido J, Navarro-Martínez R, Cauli O. Frailty syndrome and pre-operative risk evaluation: a systematic review. Arch. Gerontol. Geriatr. 2015;61:309–321. doi: 10.1016/j.archger.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open. 2018;8:e018195. doi: 10.1136/bmjopen-2017-018195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee L, Heckman G, Molnar FJ. Frailty. Can. Fam. Phys. 2015;61:227–231. [PMC free article] [PubMed] [Google Scholar]
- 7.Chan SP, Ip KY, Irwin MG. Peri-operative optimisation of elderly and frail patients: a narrative review. Anaesthesia. 2019;74:80–89. doi: 10.1111/anae.14512. [DOI] [PubMed] [Google Scholar]
- 8.Eamer G, Gibson JA, Gillis C, Hsu AT, Krawczyk M, MacDonald E, Whitlock R, Khadaroo RG. Surgical frailty assessment: a missed opportunity. BMC Anesthesiol. 2017;17:99. doi: 10.1186/s12871-017-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eamer G, Al-Amoodi MJH, Holroyd-Leduc J, Rolfson DB, Warkentin LM, Khadaroo RG. Review of risk assessment tools to predict morbidity and mortality in elderly surgical patients. Am. J. Surg. 2018;216:585–594. doi: 10.1016/j.amjsurg.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Partridge JSL, Harari D, Dhesi JK. Frailty in the older surgical patient: a review. Age Ageing. 2012;41:142–147. doi: 10.1093/ageing/afr182. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch. Gerontol. Geriatr. 2009;48:78–83. doi: 10.1016/j.archger.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Li JL, Henderson MA, Revenig LM, Sweeney JF, Kooby DA, Maithel SK, Master VA, Ogan K. Frailty and one-year mortality in major intra-abdominal operations. J. Surg. Res. 2016;203(507–512):e1. doi: 10.1016/j.jss.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Robinson TN, Walston JD, Brummel NE, Deiner S, Brown CH, Kennedy M, Hurria A. Frailty for surgeons: review of a national institute on aging conference on frailty for specialists. J. Am. Coll. Surg. 2015;221:1083–1092. doi: 10.1016/j.jamcollsurg.2015.08.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoicea N, Baddigam R, Wajahn J, Sipes AC, Arias-Morales CE, Gastaldo N, Bergese SD. The gap between clinical research and standard of care: a review of frailty assessment scales in perioperative surgical settings. Front. Public Health. 2016;4:150. doi: 10.3389/fpubh.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am. J. Surg. 2011;202:511–514. doi: 10.1016/j.amjsurg.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perna S, Francis MD, Bologna C, Moncaglieri F, Riva A, Morazzoni P, Allegrini P, Isu A, Vigo B, Guerriero F, Rondanelli M. Performance of Edmonton Frail Scale on frailty assessment: its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. 2017;17:2. doi: 10.1186/s12877-016-0382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien P. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann. Surg. 2013;258:1–7. doi: 10.1097/SLA.0b013e318296c732. [DOI] [PubMed] [Google Scholar]
- 21.Grocott MPW, Browne JP, Van der Meulen J, Matejowsky C, Mutch M, Hamilton MA, Levett DZH, Emberton M, Haddad FS, Mythen MG. The postoperative morbidity survey was validated and used to describe morbidity after major surgery. J. Clin. Epidemiol. 2007;60:919–928. doi: 10.1016/j.jclinepi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Kim T-H, Suh Y-S, Huh Y-J, Son Y-G, Park J-H, Yang J-Y, Kong S-H, Ahn HS, Lee H-J, Slankamenac K, Clavien PA, Yang H-K. The comprehensive complication index (CCI) is a more sensitive complication index than the conventional Clavien–Dindo classification in radical gastric cancer surgery. Gastric Cancer. 2018;21:171–181. doi: 10.1007/s10120-017-0728-3. [DOI] [PubMed] [Google Scholar]
- 23.Moores KL, Jones GL, Radley SC. Development of an instrument to measure face validity, feasibility and utility of patient questionnaire use during health care: the QQ-10. Int. J. Qual. Health Care. 2012;24:517–524. doi: 10.1093/intqhc/mzs051. [DOI] [PubMed] [Google Scholar]
- 24.Twohig H, Jones G, Mackie S, Mallen C, Mitchell C. Assessment of the face validity, feasibility and utility of a patient-completed questionnaire for polymyalgia rheumatica: a postal survey using the QQ-10 questionnaire. Pilot Feasibility Stud. 2018;4:7. doi: 10.1186/s40814-017-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHugh ML. Interrater reliability: the kappa statistic. Biochem. Med. 2012;22:276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watt J, Tricco AC, Talbot-Hamon C, Pham B, Rios P, Grudniewicz A, Wong C, Sinclair D, Straus SE. Identifying older adults at risk of harm following elective surgery: a systematic review and meta-analysis. BMC Med. 2018;16:2. doi: 10.1186/s12916-017-0986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Brooks AK, Groban L. Preoperative assessment of the older surgical patient: honing in on geriatric syndromes. Clin. Interv. Aging. 2015;10:13–27. doi: 10.2147/CIA.S75285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan M, Beck S, Havelock W, Eeles E, Hubbard RE, Johansen A. Predicting outcome after hip fracture: using a frailty index to integrate comprehensive geriatric assessment results. Age Ageing. 2014;43:122–126. doi: 10.1093/ageing/aft084. [DOI] [PubMed] [Google Scholar]
- 29.Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann. Surg. 2011;253:1223–1229. doi: 10.1097/SLA.0b013e318214bce7. [DOI] [PubMed] [Google Scholar]
- 30.Meyers BM, Al-Shamsi HO, Rask S, Yelamanchili R, Phillips CM, Papaioannou A, Pond GR, Jeyabalan N, Zbuk KM, Dhesy-Thind SK. Utility of the Edmonton Frail Scale in identifying frail elderly patients during treatment of colorectal cancer. J. Gastrointest. Oncol. 2017;8:32–38. doi: 10.21037/jgo.2016.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moro FD, Morlacco A, Motterle G, Barbieri L, Zattoni F. Frailty and elderly in urology: Is there an impact on post-operative complications? Cent. Eur. J. Urol. 2017;70:197–205. doi: 10.5173/ceju.2017.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koizia L, Khan S, Frame A, Mikhail GW, Sen S, Ruparelia N, Hadjiloizou N, Malik IS, Fertleman MB. Use of the reported Edmonton frail scale in the assessment of patients for transcatheter aortic valve replacement: A possible selection tool in very high-risk patients? J. Geriatr. Cardiol. 2018;15:463–466. doi: 10.11909/j.issn.1671-5411.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amabili P, Wozolek A, Noirot I, Roediger L, Senard M, Donneau A-F, Hubert MB, Brichant J-F, Hans GA. The Edmonton Frail Scale improves the prediction of 30-day mortality in elderly patients undergoing cardiac surgery: a prospective observational study. J. Cardiothorac. Vasc. Anesth. 2019;33:945–952. doi: 10.1053/j.jvca.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 34.McIsaac DI, Taljaard M, Bryson GL, Beaulé PE, Gagné S, Hamilton G, Hladkowicz E, Huang A, Joanisse JA, Lavallée LT, MacDonald D, Moloo H, Thavorn K, van Walraven C, Yang H, Forster AJ. Frailty as a predictor of death or new disability after surgery: a prospective cohort study. Ann. Surg. 2018;210:901–908. doi: 10.1097/SLA.0000000000002967. [DOI] [PubMed] [Google Scholar]
- 35.Lal S, Gray A, Kim E, Bunton RW, Davis P, Galvin IF, Williams MJA. Frailty in elderly patients undergoing cardiac surgery increases hospital stay and 12-month readmission rate. Heart Lung Circ. 2019 doi: 10.1016/j.hlc.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs J, Moraru L, Antal K, Cioc A, Voidazan S, Szabo A. Are frailty scales better than anesthesia or surgical scales to determine risk in cardiac surgery? Korean J. Anesthesiol. 2017;70:157. doi: 10.4097/kjae.2017.70.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep. 2013;24:10–17. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analysed during the current study are available from the corresponding author on reasonable request.