Clinical Implications.
-
•
A previously healthy 13-month-old boy admitted for pneumonia and presumed multisystem inflammatory syndrome in children and other possible inflammatory conditions was ultimately found to have Mendelian susceptibility to mycobacterial disease caused by a homozygous deletion in IFN-γ receptor 2.
A previously healthy 13-month-old Hispanic boy born to consanguineous parents from Dominican Republic was referred by his pediatrician to the emergency room for 7 days of fever and marked leukocytosis of 40 × 103/μL. Two months earlier his parents experienced upper respiratory symptoms with anosmia but were not tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The patient was subsequently admitted from the emergency room with tachypnea and mild intercostal retractions, normal saturation, leukocytosis, elevated inflammatory markers and D-dimer, and negative SARS-CoV-2 RT-PCR result from a nasopharyngeal swab (Table I ). Blood and urine cultures were negative. Chest radiograph showed a patchy consolidation in the right lower lobe. An echocardiogram revealed mild pericardial effusion and hyperdynamic contractility. He failed to respond to 3-day intravenous ceftriaxone, and developed a cough and abdominal distention, for which he was transferred to our hospital because of the concern for decompensated pneumonia and possible multisystem inflammatory syndrome in children (MIS-C).
Table I.
Laboratory values
| CBC | Normal | OSH | Admit | Peak | Discharge |
|---|---|---|---|---|---|
| WBC | 6.2-15.5 × 103/μL | 49 (6.5% bands) | 35.5 | 45.3 | 17.9 |
| Neutrophil % | 21.3%-69.3% | 64 | 80 | 50.5 | |
| Absolute neutrophil count | 1.9-8.0 × 103/μL | 23.78 | 23.78 | 9.71 | |
| Lymphocyte % | 17%-63.7% | 21 | 39.7 | 39.4 | |
| Absolute lymphocyte count | 1.2-7.0 × 103/μL | 7.46 | 12.9 | 7.1 | |
| HGB | 10.3-13.2 g/dL | 8 | 11.2 | 9.9 | |
| PLTS | 150-500 × 103/μL | 287 | 689 | 500 | |
| Inflammatory markers | |||||
| CRP | 0.0-5.0 mg/L | 26.7 | 279.2 | 314.2 | 44.7 |
| ESR | 0-10 mm/h | 81 | 103 | — | |
| LDH | 170-450 U/L | 1774 | 450 | 628 | — |
| Ferritin | 20-200 ng/mL | 230 | 236 | 484 | — |
| Procalcitonin | <0.49 ng/mL | 33.66 | 7.9 | 249.21 | — |
| Uric acid | 2.2-6.0 mg/dL | 5.5 | 4.7 | 6.8 | — |
| Albumin | 3.5-4.9 g/dL | 2.2 | 3.7 | — | |
| IL-1β | 0-5.0 pg/mL | 1.1 | — | — | |
| IL-6 | 0-5.0 pg/mL | 395 | — | — | |
| IL-8 | 0-5.0 pg/mL | 38.9 | — | — | |
| TNF-α | 0-22.0 pg/mL | 95.2 | — | — | |
| Coagulation/cardiac studies | |||||
| D-Dimer | 0.00-0.50 μg/mL | 7.72 | 7.65 | 9.46 | — |
| Troponin I | <0.03 ng/mL | Negative | <0.01 | 0.02 | — |
| BNP | 0-100 pg/mL | 29.47 | 47.10 | — | |
| Microbiology | |||||
| Respiratory PCR | Negative | Negative | Negative | — | — |
| Respiratory PCR method | FilmArray Respiratory Panel 2 | — | — | ||
| SARS-CoV-2 PCR | Negative | Negative | Negative | Negative | — |
| SARS-CoV-2 PCR method | Roche Cobas 6800 | Roche Cobas 6800 | Simplexa | — |
BNP, Brain naturetic peptide; CBC, complete blood cell count; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HGB, hemoglobin; LDH, lactate dehydrogenase; OSH, outside hospital; PLTS, platelets; WBC, white blood cell count.
Pediatric ranges for patients aged 13 mo within our hospital are provided.
Bolded numbers are abnormal lab values.
Upon transfer on day 12, he was afebrile, with blood pressure of 113/89 mm Hg and tachycardia, but had normal oxygen saturation and physical examination. Chest radiograph demonstrated right middle and lower lobe infiltrates. Laboratory studies revealed leukocytosis, neutrophilia and lymphophilia, microcytic anemia, hypoalbuminemia, and elevated inflammatory markers and D-dimer (Table I). Repeat nasopharyngeal PCR test results for respiratory pathogens and SARS-CoV-2 were negative, whereas coronavirus disease 2019 (COVID-19) antibodies were positive at a titer of 1:960.
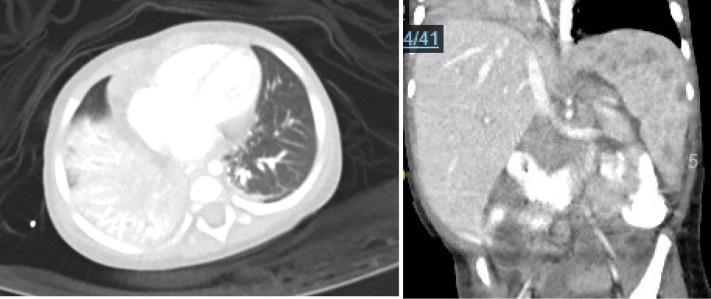
His initial presentation of pneumonia had a differential including malignancy, HIV, Kawasaki disease, MIS-C, and/or mycobacterial infections. There were no rashes, edema, conjunctival injection, or mucosal changes to suggest Kawasaki, flow cytometry was not consistent with leukemia, and his HIV test result was negative, though the possibility of lymphoma remained. His presentation coincided with the peak of MIS-C cases in New York City.1 Children with COVID-19 generally do well, with a mortality rate of 0.1%.2 Worldwide, more than 1000 children who previously appeared healthy were hospitalized for MIS-C.1 , 3 , 4 This child demonstrated the characteristics of MIS-C, including fever with positive SARS-CoV-2 antibodies, elevated inflammatory markers, elevated D-dimer, and multiorgan involvements.5 The patient was treated with intravenous antibiotics for presumed bacterial pneumonia, and enoxaparin and intravenous immune globulin for presumed MIS-C. However, he did not respond and continued to have tachypnea, abdominal distention, and elevated inflammatory markers. Marked leukocytosis and lymphophilia were inconsistent with MIS-C. On day 14, a QuantiFERON-TB Gold Plus, which had been submitted, was reported to be positive. An abdominal ultrasound (day 16) revealed innumerable small splenic hypoechoic foci, mild hepatomegaly, and lymphadenopathy in the portal region. He was sedated and intubated to obtain a chest angiography and abdominal computerized tomography, which revealed dense consolidation of the right lower and middle lobes, hepatosplenomegaly with many hypodense splenic lesions, and portacaval adenopathy (see Figure E1 in this article's Online Repository at www.jaci-inpractice.org). Following difficult intubation and the imaging studies, he was transferred to intensive care and remained intubated for 7 additional days. Day 17 bronchoscopy revealed an endobronchial mass obstructing 80% of the bronchus. Tuberculous meningitis was excluded with cerebrospinal fluid studies. Because of positive IFN-γ release assay, splenic lesions, and lymphadenopathy, the patient was started on treatment for presumed miliary tuberculosis with rifampin, isoniazid, pyrazinamide, and ethambutol, and with corticosteroids to relieve the endobronchial obstruction on day 18. A skin test PPD was read as negative 2 days later while PCR results for tuberculosis and repeat QuantiFERRON-TB Gold Plus were also negative. Immunologic workup showed elevated immunoglobulin levels, normal lymphocyte subsets, and a normal dihydrorhodamine test result.
Figure E1.
Imaging from day 16 of illness shows consolidation in the right middle and lower lobes. Computed tomography (CT) angiography of the chest (left) and hypodense splenic lesions and porta hepatic and portacaval adenopathy. CT of the abdomen (right).
On day 21, splenic biopsy showed acute inflammation and ill-defined minute nonnecrotizing granulomas. Bronchoalveolar lavage and gastric aspirate cultures were positive for Mycobacterium avium complex on day 33, and the treatment was optimized to cover for this with rifampin, ethambutol, and clarithromycin, in addition to moxifloxacin for latent tuberculosis infection. These new findings increased the index of suspicion for Mendelian susceptibility to mycobacterial disease (MSMD), and further investigation was conducted to identify an underlying genetic defect.
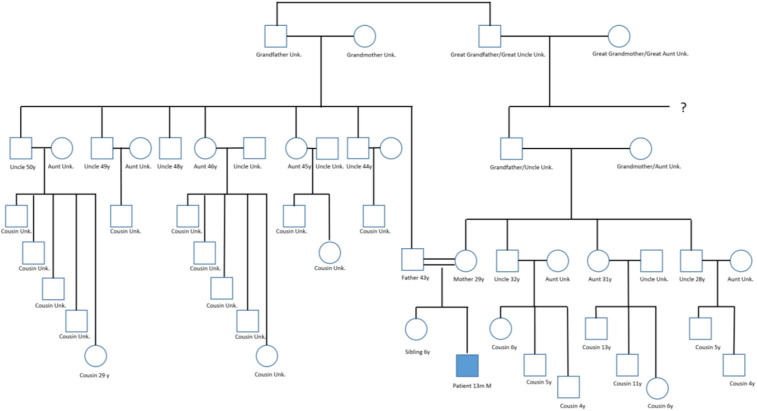
Family history indicated that the parents were second-degree cousins who had no apparent family history of primary immunodeficiency disease, as shown in the family pedigree (see Figure E2 in this article's Online Repository at www.jaci-inpractice.org). MSMD was confirmed, with immunogenetic studies revealing a homozygous deletion mutation in IFN-γ receptor 2 (IFNGR2), at position c.503_504del (p.Thr168Ilefs∗33), a previously reported pathogenic mutation in a child with autosomal-recessive MSMD.6
Figure E2.
Pedigree chart. The mother is the daughter of one the patient’s father’s cousins (a second cousin to the father).
He showed clinical improvement after 3 weeks of aggressive antimycobacterial therapy. He was taken off ventilator and supplemental oxygen on day 23. Repeated bronchoscopy showed resolution of bronchoalveolar mass on day 21, and subsequent serial chest radiographs showed resolution of right-sided consolidation. Weekly ultrasounds showed decreased hepatosplenomegaly and lymphadenopathy by hospital discharge on day 55. The patient was discharged home on antimycobacterial treatment and referred for hematopoietic stem cell transplant.
Mendelian susceptibility to mycobacterial disease is a rare primary immunodeficiency characteristic of severe and disseminated infections with weakly virulent mycobacteria, such as Bacillus Calmette-Guerin–BCG vaccine strain and mycobacterium avium complex, and in 50% of cases Salmonella species. MSMD can be caused by 1 of the 15 genetic mutations in the macrophage and lymphocyte loop primarily involving molecules in the IL-12/IFN-γ signaling pathways including IFNGR2.7 To date there have been fewer than 30 cases of IFNGR2 reported in the literature. More than half the cases had poor survival. Hematopoietic stem cell transplant is considered curative.8 , 9
At the time of the patient's presentation, MIS-C was increasingly recognized in New York City. He had an initial clinical picture mimicking MIS-C, which is a diagnosis of exclusion. Through microbiology, pathology, radiology, and crucial genetic studies, MSMD was discovered.
This case emphasizes overlapping and distinct features of MIS-C and MSMD, which is outlined in Table II . Both diseases present with prolonged fever, cough, and elevated inflammatory markers, particularly TNF-α and IL-6. MIS-C is associated with lymphopenia, abnormal coagulation, multisystem involvement, serologic evidence of COVID-19, but unlikely to have lymphadenopathy and positive bacterial cultures. In comparison, MSMD characteristically presents with lymphophilia, disseminated multiorgan lesions, lymphadenopathy, and positive mycobacterial cultures, and is less likely to have abnormal coagulation and thrombotic events.
Table II.
Comparing MIS-C related to COVID-19 (MIS-C) and MSMD
| Parameter | CDC MIS-C5 | MSMD |
|---|---|---|
| Age | <21 y | Early in childhood and rarely in adulthood |
| Fever | ≥38.0°C for ≥24 h, or report of subjective fever lasting ≥24 h | Likely and can occur with weight loss |
| Hospitalization | Required | Likely |
| Laboratory | Evidence of inflammation: Lymphopenia, neutrophilia, elevated inflammatory markers (CRP, ESR, IL-6, procalcitonin, ferritin, LDH) Abnormal coagulation (elevated fibrinogen and D-dimer) Hypoalbuminemia |
Lymphophilia Elevated inflammatory markers: CRP, ESR, TNF-α, IL-6 Normal coagulation May have hypoalbuminemia |
| SARS-CoV-2 presence | At least 1 required: Positive by RT-PCR for RNA Positive serological assay for antibodies Positive COVID-19 antigen by antigen assay Exposure to a known case within 4 wk before onset of symptoms |
Unlikely except for current COVID-19 pandemic |
| Blood and tissue cultures | Negative—must exclude other diagnoses | Positive blood and/or tissue cultures Nontypical mycobacteria, Salmonella, Listeria, histoplasmosis, etc |
| Chest radiography | Not required for diagnosis but may include opacities (ground glass), peribronchial thickening, and/or pleural effusions | Disseminated pulmonary lesions are common |
| Multisystem involvement | At least 2 organ systems involved (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurological) | Not required but often lymphadenopathy present and multisystem involvement likely with disseminated infections |
| Genetics | Not found at present | Confirmed by immunodeficiency screen for mutations in IKBKG, IFNGR1, IFNGR2, STAT1, IL12B, IL12RB1, IL12RB2, IL23R, ISG15, IRF8, TYK2, CYBB, RORC, JAK1, and SPPL2A |
CDC, Centers for Disease Control and Prevention; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase.
MIS-C can be diagnosed if no other diagnosis is possible. Some patients with MIS-C may have overlapping symptoms with complete or incomplete Kawasaki disease.
Acknowledgments
We thank all frontline providers, consultants, nurses, and staff of the Kravis Children's Hospital and Mount Sinai Hospital for their care of the patient. We extend a special thanks to Samantha Ganz, MD, and Erik Sanchez, MD, for their assistance in obtaining immunogenetic testing for the patient, as well as Dr Julie Teruya-feldstein, MD, and Dr Christian Salib for the pathology study.
Footnotes
Conflicts of interest: D. Bogunovic has given lectures for Genentech, Inc, owns stock in Lab11 Therapeutics, LLC, and acknowledges support from the National Institutes of Health (grant nos. AI127372 and AI148963) as well as the March of Dimes. C. Cunningham-Rundles has received consulting fees from CSL Behring, Momenta, Atara, Pharming, and UBC; served on boards for CSL Behring and Takeda Pharmaceutical Company Limited; serves on the Scientific Advisory Board of the Immune Deficiency Foundation; and received support from the National Institutes of Health (grant nos. AI 101093, AI-086037, and AI-48693) and the David S. Gottesman Immunology Chair.
Online Repository.
References
- 1.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:1–6. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C., Shah P. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Centers for Disease Control and Prevention . U.S. Centers for Disease Control and Prevention, Health Advisory Network; Atlanta, GA: 2020. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) [Google Scholar]
- 6.Kamoun C., Morsheimer M., Sullivan K.E., Holland S.M., Cunningham-Rundles C., Bunin N. Successful unrelated cord blood transplant for complete IFN-gamma receptor 2 deficiency. J Allergy Clin Immunol. 2016;138:1489–1491. doi: 10.1016/j.jaci.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Tangye S.G., Al-Herz W., Bousfiha A., Chatila T., Cunningham-Rundles C., Etzioni A. Human inborn errors of immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40:24–64. doi: 10.1007/s10875-019-00737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosain J., Kong X.F., Martinez-Barricarte R., Oleaga-Quintas C., Ramirez-Alejo N., Markle J. Mendelian susceptibility to mycobacterial disease: 2014-2018 update. Immunol Cell Biol. 2019;97:360–367. doi: 10.1111/imcb.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bustamante J., Boisson-Dupuis S., Abel L., Casanova J.L. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol. 2014;26:454–470. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]