Abstract
A growing number of environmental pollutants are known to adversely affect the thyroid hormone system, and major gaps have been identified in the tools available for the identification, and the hazard and risk assessment of these thyroid hormone disrupting chemicals. We provide an example of how the adverse outcome pathway (AOP) framework and associated data generation can address current testing challenges in the context of fish early-life stage tests, and fish tests in general. We demonstrate how a suite of assays covering all the essential biological processes involved in the underlying toxicological pathways can be implemented in a tiered screening and testing approach for thyroid hormone disruption, using the levels of assessment of the OECD’s Conceptual Framework for the Testing and Assessment of Endocrine Disrupting Chemicals as a guide.
Keywords: thyroid hormone disruption, tiered testing, adverse outcome pathway, fish, early-life tages
Graphical Abstract

1. Background
Screening and testing programs for the assessment of endocrine-active chemicals are being implemented throughout the world (Matthiessen et al., 2017). Endocrine disruption by chemicals is not restricted to the sex hormone and reproductive systems, but also includes thyroid hormone disruption. Thyroid hormones (TH) play a crucial role in the regulation of vertebrate development and homeostatic processes related to growth and energy metabolism, and a growing number of high-profile environmental pollutants has been shown to adversely affect the hypothalamic-pituitary-thyroid (HPT) axis (Crofton, 2008; Murk et al., 2013). While there are many models and assays available for detecting chemicals that impact the hypothalamic-pituitary-gonadal axis, such as estrogen and androgen receptor agonists and antagonists, major gaps have been identified in the tools available for the hazard and risk assessment of HPT-active substances (Bopp et al., 2017; Kortenkamp et al., 2017). The scientific community is therefore challenged with developing new or improved testing approaches to evaluate TH disruption. A substance is considered as having endocrine-disrupting properties if (1) it shows an adverse effect, (2) it has an endocrine mode of action, and (3) the adverse effect is a consequence of the endocrine mode of action (EFSA, 2013; WHO/UNEP, 2013; OECD, 2018). A high level of uncertainty relative to the causal relationship between mechanistic responses and apical, adverse outcomes is one of the main limitations of the current test systems for the identification of endocrine-disrupting chemicals and for evaluating endocrine hazard and risk (Coady et al., 2017). This is of particular importance in the case of TH disruption since the adverse effects associated with disruption of the HPT-axis are often associated with general biological processes (e.g., embryonic development, energy metabolism) that can be affected by many different toxicological pathways, including mechanisms unrelated to the thyroid system. A second limitation of current test methods is that they are costly, time-consuming, and animal intensive (Burden et al., 2016). To address these various challenges, tiered testing approaches for the assessment of endocrine-active chemicals have been developed by different countries and international organisations, in which lower tier data (e.g., in silico, in vitro or short-term in vivo data) are used to decide whether more elaborate, resource-intensive higher tier in vivo tests are needed to demonstrate adverse apical effects. The U.S. Environmental Protection Agency’s (USEPA) Endocrine Disruptor Screening Program (EDSP) and the Organisation for Economic Cooperation and Development (OECD) Conceptual Framework (CF) for the Testing and Assessment of Endocrine Disrupting Chemicals (OECD, 2012, 2018) are among the most important examples of well-established tiered testing approaches (Browne et al., 2017; Coady et al., 2017).
The adverse outcome pathway (AOP) framework (Ankley et al., 2010; Ankley and Edwards, 2018) is, by design, well suited to directly support the development of tiered testing approaches by providing evidence for the association between a toxicological pathway perturbation and downstream responses (Coady et al., 2017). An AOP summarizes available empirical evidence demonstrating the mechanistic, causal linkages leading from a molecular initiating event (e.g., inhibition of an enzyme involved in TH synthesis) to an adverse apical outcome (e.g., reduced growth). The AOP framework can thus provide the critical scientific support for the link between an endocrine-active mechanism detected using in vitro or lower tier in vivo assays, and potential apical effects measured in higher tier in vivo tests (Coady et al., 2017; Matthiessen et al., 2017). The present paper provides an example of how the AOP framework and associated data generation can address current TH disruption testing challenges in the context of fish early-life stage assays, and fish assays in general. Although standardized and validated fish assays are routinely used in environmental hazard and risk assessment, the current fish test guidelines lack endpoints that are informative of TH disruption (OECD, 2018). Here, we build upon a recently developed AOP network linking disruption of the HPT-axis in fish to impaired inflation of the swim bladder, leading to reduced swimming performance and ultimately survival (Knapen et al., 2018; Villeneuve et al., 2018). We demonstrate how different assays covering all the essential biological processes along the continuum of the AOP network can be implemented in a tiered screening and testing approach for TH disruption in fish. The levels of assessment as established by the OECD CF are used as the primary guide for structuring our discussion.
2. Brief description of the AOP network
The AOP network used in this case example links TH disruption to impaired swim bladder inflation in fish and is mainly based on experimental evidence from studies on zebrafish and fathead minnow (Knapen et al., 2018; Villeneuve et al., 2018). The swim bladder is an internal gas-filled organ found in many bony fish species and typically consists of two gas-filled chambers. The posterior chamber inflates during early development and contributes to the ability of fish to control their buoyancy, while the anterior chamber inflates during late development and has an additional role as a resonating chamber to produce or receive sound (Robertson et al., 2007). A large body of evidence is available demonstrating the role of THs in swim bladder development and inflation. The AOP network describes how decreased synthesis and/or decreased biological activation of THs leads to incomplete or improper inflation of the swim bladder, leading to reduced swimming performance and ultimately to reduced survival.
Specifically, the AOP network includes two distinct molecular initiating events, corresponding to the inhibition of enzymes involved in the TH metabolism (Figure 1). Thyroperoxidase (Tpo) is the main enzyme involved in TH synthesis in the thyroid gland, and deiodinase (Dio) 1 and 2 are mainly involved in the activation of thyroxin (T4) to triiodothyronine (T3), the most biologically active form of TH. Inhibition of Dio directly results in reduced serum T3 levels, while inhibition of Tpo leads to decreased T4 levels and thus to lower availability of T4 for activation to T3, also resulting in decreased serum T3 levels. As such, reduced T3 levels are a point in the AOP network where different TH disrupting mechanisms converge (Knapen et al., 2018) and which is essential for the progression to different adverse outcomes, depending on life-stage.
Figure 1.
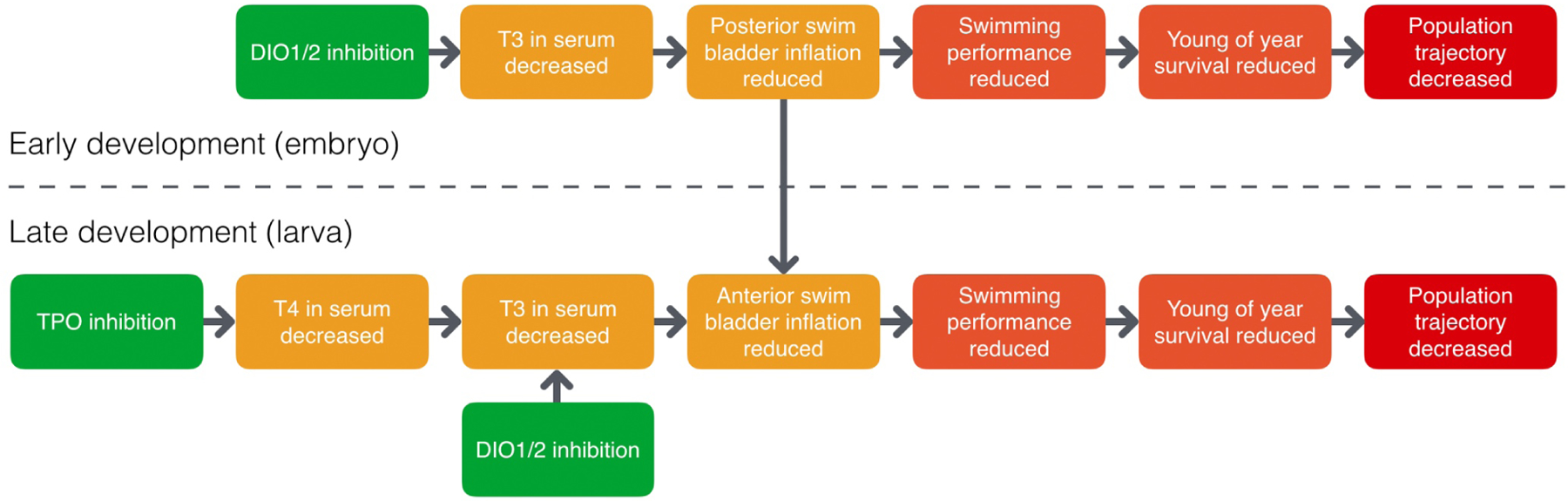
Graphical overview of an adverse outcome pathway (AOPs) network linking thyroperoxidase (TPO) inhibition and inhibition of deiodinase (DIO) 1/2 to reduced swim bladder inflation in fish and subsequent impacts on young of year survival. The AOPs relevant during different life stages are depicted above and below the dashed line (https://aopwiki.org/aops/155-159).
Indeed, specific parts of the AOP network are relevant to different life stages (see Figure 1). The earliest life stages of teleost fish rely on maternally transferred THs to regulate certain developmental processes until embryonic TH synthesis is active (Power et al., 2001). As a result, early developmental processes that are dependent on THs, such as posterior swim bladder chamber inflation, appear to be less sensitive to inhibition of TH synthesis. On the other hand, when maternally derived THs are depleted during late development (larval stage), endogenous TH synthesis becomes more important and inhibition of Tpo interferes with proper inflation of the anterior swim bladder chamber (Stinckens et al. submitted; Nelson et al., 2016; Stinckens et al., 2016; Godfrey et al., 2017). In all life stages however, the conversion of T4 into T3 is essential. Inhibition of Dio therefore impacts swim bladder inflation in both early and late developmental life stages (Stinckens et al. submitted; Jomaa et al., 2014; Cavallin et al., 2017; Godfrey et al., 2017; Stinckens et al., 2018). The anterior chamber develops by budding out of the posterior chamber and thus failure to properly inflate the posterior chamber during early development directly impacts anterior chamber inflation during late development. Impaired swim bladder inflation results in reduced swimming performance (Stinckens et al. submitted; Hagenaars et al., 2014; Stinckens et al., 2016; Stinckens et al., 2018), an adverse outcome that can affect feeding behavior and predator avoidance, ultimately leading to lower survival probability and population trajectory decline (Villeneuve et al., 2014).
3. Toward an AOP network-based tiered testing strategy
The OECD is an international organization promoting global cooperation to face modern day challenges in various areas including human and environmental health. In 2002 (updated in 2012 and 2018), the OECD released the Conceptual Framework (CF) for Testing and Assessment of Endocrine Disrupters that organizes current methods for screening and testing of endocrine-active substances into 5 levels (OECD, 2012). Level 1 of the CF relies on existing data and quantitative structure–activity relationship (QSAR) or non-test information to predict the endocrine-active potential of chemicals (Figure 2). Level 2 (in vitro) and Level 3 (short-term in vivo) assays directly inform whether or not a substance can interact with endocrine pathways. These assays can be used to screen for possible endocrine activity but are typically limited in their coverage of endocrine mechanisms and in the observation of adverse apical effects. Level 4 is comprised of longer-term in vivo assays that provide data on endocrine-relevant adverse apical effects and are typically responsive to more than one endocrine mode of action. Finally, Level 5 assays include full life-cycle tests and multigenerational studies providing more comprehensive data on adverse effects over more extensive parts of the life cycle. Level 4 and Level 5 assays are focused on observing adverse effects and can be used for evaluating both the actual endocrine disrupting properties of substances and their potential risk. It should be noted that within the context of the CF, entering and exiting at all levels is possible and depends on the nature of existing information and needs for testing and assessment (OECD, 2018). The USEPA EDSP uses a two-tiered approach in which Tier 1 screening data, corresponding to CF Levels 1–3, are used to identify substances that have the potential to interact with endocrine systems and Tier 2 identifies and characterizes any adverse endocrine-related apical effects, corresponding to CF Levels 4–5.
Figure 2.

Overview of assays aligned with the thyroid hormone (TH) disruption AOP network and how they could be used in a tiered testing strategy based on the Organisation for Economic Cooperation and Development (OECD) Conceptual Framework (CF). Only test guidelines that are directly relevant to zebrafish and/or fathead minnow early-life stages, on which the current AOP network is based, are mentioned. Level 1 is mentioned for completeness.
In 2018, the OECD published an updated version of Guidance Document 150, Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption (OECD, 2018). This document, originally published in 2012, is intended to provide guidance for evaluating chemicals using standardised test guidelines within the context of the OECD CF. The guidance document provides advice on how to use and interpret the outcome of individual tests/assays and attempts to address the need for a causal linkage between likely mechanisms of endocrine action and resulting apical effects. It also provides a list of assays, including those for the assessment of TH disruption, that could be valuable additions to existing test guidelines but for which currently no formal test guidelines are available. The AOP network described here provides a mechanistic basis for adding a suite of TH disruption-specific assays and relevant additional endpoints for a number of existing fish test guidelines (Figure 2).
3.1. In vitro assays for thyroid activity screening
There are currently no internationally validated test guidelines for in vitro assays to screen for thyroid-active substances at Level 2 of the CF (Figure 2). Several international efforts have, however, assessed the availability and readiness of in vitro screening assays for thyroid-active chemicals (Murk et al., 2013; OECD, 2014). Important progress has recently been made on the development of assays to evaluate reduced TH synthesis via inhibition of Tpo activity (Paul Friedman et al., 2016) and iodide uptake (Wang et al., 2018), reduced TH (in)activation by inhibition of deiodinase activity (Olker et al., 2019), and inhibition of cellular TH uptake (Dong and Wade, 2017). Several of these assays have been applied to large chemical libraries and are currently being added to the USEPA Toxicity Forecaster (ToxCast™) program, a chemical prioritization effort that uses a suite of high-throughput screening assays to rank and prioritize chemicals for future testing, thereby aiding in efficient management and regulation of environmental contaminants. Recently, the extent to which available high throughput screening assays cover the known molecular targets for TH disruption across vertebrates was evaluated, and these molecular interactions were linked to downstream events and adverse outcomes based on a cross-species TH disruption AOP network (Noyes et al., 2019). In July 2017, the Joint Research Centre EU Reference Laboratory for alternatives to animal testing (JRC EURL ECVAM) launched a validation study to assess a battery of 17 in vitro screening methods covering a series of TH disrupting modes of action. Consequently, the scope of assays available for Level 2 of the CF is expected to continue to grow in the near future.
The AOP network links molecular interactions measured in in vitro Tpo and Dio inhibition assays with altered TH levels and downstream adverse in vivo effects in fish. Overall, the level of confidence in the different linkages in the TH disruption AOP network is high (https://aopwiki.org/aops/155-159), allowing it to be used in a tiered testing strategy to guide the selection of suitable assays and endpoints for the evaluation of adverse in vivo effects. Specifically, a positive result in the in vitro Tpo and/or the Dio inhibition assay could trigger fish in vivo testing to confirm the occurrence of downstream events along the AOP network including altered TH levels, impaired swim bladder inflation, and altered swimming performance (see sections 3.2 and 3.3). An initial validation case study showed that adverse swim bladder effects could be predicted along the AOP network based on in vitro Dio inhibition data (Stinckens et al., 2018). The further development of such predictive approaches, including expanding the quantitative understanding of the relationships depicted in the AOPs where possible (Hassan et al., 2017), would significantly reduce the need for in vivo testing in the future.
3.2. In vivo assays for thyroid activity screening
Currently, the only non-mammalian in vivo assays assessing thyroid-specific endpoints at Level 3 of the CF use amphibians. The Amphibian Metamorphosis Assay (AMA, OECD TG 231, US EPA OPPTS 890.1100) is the most widely-used assay for detecting HPT-active substances, but was recently complemented with the Xenopus Eleutheroembryonic Thyroid Assay (XETA, TG 248). None of the current CF Level 3 fish assays include thyroid-specific endpoints.
Fish and amphibian embryo assays have added value for screening purposes compared to in vitro assays due to the increased biological relevance gained from using a model organism with an intact HPT axis and ongoing, complex development. In this context, the Fish Embryo Acute Toxicity (FET) test (OECD TG 236) with the addition of TH measurements as thyroid-specific endpoints could be a valuable Level 3 screening assay for TH activity. Similarly, TH measurements could be carried out as part of the the “EASZY Assay” (Detection of Endocrine Active Substance, acting through estrogen receptors, using transgenic Zebrafish embrYos), for which an OECD test guideline was recently drafted. The importance and relevance of determining altered TH levels as an indicator of endocrine activity in in vivo assays for TH disrupter screening and testing has already been acknowledged (Kortenkamp et al., 2017; OECD, 2018). The AOP network further highlights the critical nature of altered TH levels as a point of convergence for several TH disruption mechanisms and essential step in the progression towards an adverse outcome. The ZETA (Zebrafish Eleutheroembryo Thyroid Assay), which quantifies intrafollicular T4 content as an indirect measurement of TH synthesis in 5 day old zebrafish embryos, is a first example of a thyroid-specific fish test that has been proposed and is currently being explored as part of the JRC EURL ECVAM validation effort (Thienpont et al., 2011; OECD, 2014). Viable methods for directly measuring altered whole body TH levels (T4, T3) in fish embryos have recently been developed (Stinckens et al. submitted; Nelson et al., 2016; Stinckens et al., 2016; Cavallin et al., 2017). Today, the addition of TH measurements to the FET test, and possibly the ZETA and EASZY Assay, for detecting TH disruption screening has therefore become both sensible and achievable. An accurate assessment of posterior chamber inflation and swimming performance however cannot be reliably carried out within the context of the FET test, which has a duration of 96 hours, since the posterior chamber of zebrafish inflates around 5 d post-fertilisation and many endocrine and non-endocrine mechanisms negatively impact growth rate, thereby potentially further delaying posterior chamber inflation. Therefore, we only suggest the addition of TH measurements, and not assessment of swim bladder inflation, to existing fish embryo tests for TH activity screening.
Importantly, assays using fish or amphibian embryos are considered non-animal methods in many parts of the world. For example, non-mammalian vertebrate embryos are not protected until the stage of free-feeding under the current EU legislation on the use of laboratory animals (EC, 2010). In a tiered testing approach, in vitro and non-animal assays (e.g., fish and amphibian embryo assays) could reduce the need for in vivo testing. Naturally, the limitations that have been considered in the debate on the regulatory acceptance of the FET test within the context of the REACH legislation should be taken into account, including the presence of a chorion during the first few days of the test which may function as a barrier to some chemicals, and the limited xenobiotic metabolism capacity compared to later life stages (Sobanska et al., 2018).
3.3. In vivo assays for thyroid hormone disruption testing
There are two non-mammalian assays, one with an amphibian and one with an avian species, at Levels 4 and 5 of the CF and Tier 2 of the EDSP that have thyroid-specific endpoints (OECD TG 241, US EPA OCSPP 890.2100). Several fish assays with zebrafish and/or fathead minnow early-life stages are also listed as Level 4 and 5 tests in the CF (Figure 2): the Fish Early Life Stage Toxicity (FELS) Test (OECD TG 210, Level 4), Fish Sexual Development Test (FSDT, OECD TG 234, Level 4), Zebrafish Extended One-Generation Reproduction Test (ZEOGRT, draft OECD TG, Level 5), and Fish Life Cycle Toxicity Test (FLCTT, US EPA OPPTS 850.1500, Level 5). These assays all assess endpoints that are potentially sensitive to, but not necessarily diagnostic of TH disruption (i.e., general adverse effects such as reduced growth that might respond to TH disruption but can also be affected by other toxicological pathways). It has been suggested that new, specific endpoints could be added to these existing test guidelines to increase their diagnostic value for the assessment of TH disruption (Kortenkamp et al., 2017). Recently, addition of thyroid-related endpoints in OECD fish test guidelines such as the FET and FSDT was included in the OECD’s Work Plan for the Test Guidelines Programme (project 2.64).
Neither swim bladder inflation nor swimming performance are in themselves endpoints specific to TH disruption since they can be affected through various mechanisms. The strength of an AOP-based approach, however, lies in linking these adverse outcomes to an endocrine mechanism. In the case of TH disruption in fish, the strong evidence for the relationship between reduced TH levels and impaired swim bladder inflation is crucial in this respect (Stinckens et al. submitted; Stinckens et al., 2016). Measurements of altered TH levels thus increase the diagnostic value of general endpoints such as growth and swim bladder inflation by placing these endpoints in a TH disruption context based on the causal linkages in the AOP network. Specifically, the combination of whole-body TH measurements (T4, T3) and the assessment of swim bladder inflation (both chambers) and swimming performance could be included as an AOP-based suite of endpoints in any test guideline using zebrafish or fathead minnow early-life stages. This includes the FELS test, FSDT, ZEOGRT and FLCT. In the European Union, the FELS test is the most important standard ecotoxicological data requirement for industrial chemicals (REACH), and active substances in biocides (528/2012) and plant protection products (283/2013). Increasing the diagnostic value of the FELS test for the detection of TH disrupters may therefore significantly increase the efficiency of chemical safety evaluation in terms of cost and use of animals. Future development of new AOPs linking TH disruption to adverse effects that are already being assessed as a part of these test guidelines (e.g., growth) may further improve the significance of these endpoints.
4. Expanding the domain of applicability
The TH disruption AOP network to which the assays discussed in this case example are aligned (Figure 2) is included in the OECD AOP development programme workplan as Project 1.35 (The AOP on thyroperoxidase and/or deiodinase inhibition leading to impaired swim bladder inflation in fish during early-life stages) and is mainly based on studies using zebrafish and fathead minnow. A first logical step in expanding the applicability of the AOP network is to assess its relevance to other species that are frequently used in existing fish test guidelines, such as the Japanese rice fish (also known as the medaka), three-spined stickleback and rainbow trout. Further, several other endpoints and biomarkers that have been shown to respond to impaired thyroid function and/or altered TH levels are not yet addressed as part of the OECD AOP workplan, including gene expression, eye development (e.g., size, pigmentation, retina histology), skin pigmentation, thyroid histopathology, scale development and impaired fin development (van der Ven et al., 2006; Walpita et al., 2009; Baumann et al., 2016). The development of AOPs covering these adverse effects would facilitate the assessment of their specificity and sensitivity in the context of TH disruption. In addition, linking these AOPs to the existing AOP network would help expand the life stage applicability from early-life stages to juveniles and reproductively active, adult fish for a range of species. Such efforts would make the AOP network relevant to a number of additional fish test guidelines, including the fish short-term reproduction assay (OECD TG 229), the 21-day fish assay (OECD TG 230), the androgenised female stickleback screen (OECD GD 148), the juvenile medaka anti-androgen screening assay (draft OECD GD) and the rapid androgen disruption adverse outcome reporter assay (draft OECD TG) at Level 3 of the CF, and the medaka extended one-generation reproduction test (OECD TG 240) at Level 5.
Tiered testing strategies for the evaluation of TH disrupting properties are being developed in parallel for human and environmental health (Murk et al., 2013; OECD, 2018). Evaluation of the hazards and risks of chemicals derived from tests for human health effects and environmental effects are largely separate processes, and sharing of data is uncommon. While human toxicology and ecotoxicology have historically used different models, terminologies and interpretation approaches, the AOP framework facilitates the application of similar strategies for developing assays, and using them in a unified weight of evidence analysis, effectively bridging the gap between these two disciplines (Perkins et al., 2013). A relatively large number of AOPs related to TH disruption is currently being developed in the AOP-Wiki (www.aopwiki.org), involving a variety of species and taxonomic groups. Based on the fact that well-known targets along the HPT-axis are highly conserved among vertebrate classes (Sachs and Buchholz, 2017; LaLone et al., 2018), a cross-species TH disruption AOP network covering mammals, fish and amphibians is emerging from these datasets (conceptually visualized in Figure 3) (Knapen et al., 2018; Noyes et al., 2019). The underlying AOPs and their interrelationships were recently described in detail to support the use of in vitro assays for the evaluation of TH disruption (Noyes et al., 2019). Further development and biological validation of this larger AOP network can form the basis of a harmonized, integrated approach to testing and assessment of TH disrupters addressing both human and environmental health. Finally, while we presently focus on TH disrupting activity and fish, tiered testing strategies for other modes of endocrine disruption can in principle also be informed by emerging AOPs and AOP networks (Knapen et al., 2015).
Figure 3.

Graphical representation of a cross-species AOP network, present in the AOP-Wiki, that links molecular initiating events (green circles) through impacts on circulating thyroid hormone levels, to adverse outcomes (red circles) in mammals, fish, and/or amphibians.
Acknowledgements
This work was funded by the Cefic Long-range Research Initiative (http://www.cefic-lri.org/) project LRI-ECO20.2-UA (Development of an alternative testing strategy for the fish early life-stage test for predicting chronic toxicity: assay validation) with support of ECETOC. This work was further supported by the Society of Environmental Toxicology and Chemistry (SETAC) / Procter & Gamble Company Global Fellowship for Doctoral Research in Environmental Science 2016, sponsored by The Procter & Gamble Company, and a Small Research Grant financed by the University of Antwerp (ID 3880). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 825753 (ERGO). The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. The European Union cannot be held responsible for any use that may be made of the information contained in this paper. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- Ankley G, Edwards S, 2018. The adverse outcome pathway: A multifaceted framework supporting 21st century toxicology. Current Opinion in Toxicology 9, 1.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL, 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry 29, 730.-. [DOI] [PubMed] [Google Scholar]
- Baumann L, Ros A, Rehberger K, Neuhauss SCF, Segner H, 2016. Thyroid disruption in zebrafish (Danio rerio) larvae: Different molecular response patterns lead to impaired eye development and visual functions. Aquatic Toxicology 172, 44.-. [DOI] [PubMed] [Google Scholar]
- Bopp S, Nepelska M, Halder M, Munn S, 2017. Expert survey on identification of gaps in available test methods for evaluation of endocrine disruptors. JRC Technical Reports. European Union, Luxembourg. [Google Scholar]
- Browne P, Noyes PD, Casey WM, Dix DJ, 2017. Application of Adverse Outcome Pathways to US EPA’s Endocrine Disruptor Screening Program. Environmental Health Perspectives 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden N, Benstead R, Clook M, Doyle I, Edwards P, Maynard SK, Ryder K, Sheahan D, Whale G, van Egmond R, Wheeler JR, Hutchinson TH, 2016. Advancing the 3Rs in regulatory ecotoxicology: A pragmatic cross-sector approach. Integrated Environmental Assessment and Management 12, 417.-. [DOI] [PubMed] [Google Scholar]
- Cavallin JE, Ankley GT, Blackwell BR, Blanksma CA, Fay KA, Jensen KM, Kahl MD, Knapen D, Kosian PA, Poole ST, Randolph EC, Schroeder AL, Vergauwen L, Villeneuve DL, 2017. Impaired swim bladder inflation in early life stage fathead minnows exposed to a deiodinase inhibitor, iopanoic acid. Environmental Toxicology and Chemistry 36, 2942.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coady KK, Biever RC, Denslow ND, Gross M, Guiney PD, Holbech H, Karouna-Renier NK, Katsiadaki I, Krueger H, Levine SL, Maack G, Williams M, Wolf JC, Ankley GT, 2017. Current Limitations and Recommendations to Improve Testing for the Environmental Assessment of Endocrine Active Substances. Integrated Environmental Assessment and Management 13, 302.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM, 2008. Thyroid disrupting chemicals: mechanisms and mixtures. International Journal of Andrology 31, 209–222. [DOI] [PubMed] [Google Scholar]
- Dong HY, Wade MG, 2017. Application of a nonradioactive assay for high throughput screening for inhibition of thyroid hormone uptake via the transmembrane transporter MCT8. Toxicology in Vitro 40, 234.-. [DOI] [PubMed] [Google Scholar]
- EC, 2010. EU Parliament and Council Directive 2010/63/EU of 22 September 2010 on the protection of animals used for scientific purposes. [Google Scholar]
- EFSA, 2013. Scientific opinion on the hazard assessment of endocrine disruptors: Scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the environment. EFSA Journal, p. 3132. [Google Scholar]
- Godfrey A, Hooser B, Abdelmoneim A, Horzmann KA, Freemanc JL, Sepulveda MS, 2017. Thyroid disrupting effects of halogenated and next generation chemicals on the swim bladder development of zebrafish. Aquatic Toxicology 193, 228.-. [DOI] [PubMed] [Google Scholar]
- Hagenaars A, Stinckens E, Vergauwen L, Bervoets L, Knapen D, 2014. PFOS affects posterior swim bladder chamber inflation and swimming performance of zebrafish larvae. Aquatic Toxicology 157, 225.-. [DOI] [PubMed] [Google Scholar]
- Hassan I, El-Masri H, Kosian PA, Ford J, Degitz SJ, Gilbert ME, 2017. Neurodevelopment and Thyroid Hormone Synthesis Inhibition in the Rat: Quantitative Understanding Within the Adverse Outcome Pathway Framework. Toxicological Sciences 160, 57.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomaa B, Hermsen SAB, Kessels MY, van den Berg JHJ, Peijnenburg AACM, Aarts JMMJG, Piersma AH, Rietjens IMCM, 2014. Developmental Toxicity of Thyroid-Active Compounds in a Zebrafish Embryotoxicity Test. Altex-Alternatives to Animal Experimentation 31, 303.-. [DOI] [PubMed] [Google Scholar]
- Knapen D, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, Munn S, O’Brien JM, Pollesch N, Smith LC, Zhang XW, Villeneuve DL, 2018. Adverse outcome pathway networks I: Development and applications. Environmental Toxicology and Chemistry 37, 1723.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen D, Vergauwen L, Villeneuve DL, Ankley GT, 2015. The potential of AOP networks for reproductive and developmental toxicity assay development. Reproductive Toxicology 56, 52.-. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Martin O, Baynes A, Silva E, Axelstad M, Hass U, 2017. Supporting the organisation of a workshop on thyroid disruption – Final Report Framework Contract ENV.A.3/FRA/2014/0029 on implementation of the Community strategy on Endocrine Disrupters. Publications Office of the European Union, Luxembourg. [Google Scholar]
- LaLone C, Villeneuve DL, Doering JA, Blackwell BR, Transue TR, Simmons CW, Swintek J, Degitz S, Williams AJ, Ankley GT, 2018. Evidence for Cross Species Extrapolation of Mammalian-Based High-Throughput Screening Assay Results. Environmental Science & Technology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthiessen P, Ankley GT, Biever RC, Bjerregaard P, Borgert C, Brugger K, Blankinship A, Chambers J, Coady KK, Constantine L, Dang Z, Denslow ND, Dreier DA, Dungey S, Gray LE, Gross M, Guiney PD, Hecker M, Holbech H, Iguchi T, Kadlec S, Karouna-Renier NK, Katsiadaki I, Kawashima Y, Kloas W, Krueger H, Kumar A, Lagadic L, Leopold A, Levine SL, Maack G, Marty S, Meador J, Mihaich E, Odum J, Ortego L, Parrott J, Pickford D, Roberts M, Schaefers C, Schwarz T, Solomon K, Verslycke T, Weltje L, Wheeler JR, Williams M, Wolf JC, Yamazaki K, 2017. Recommended approaches to the scientific evaluation of ecotoxicological hazards and risks of endocrine-active substances. Integr Environ Assess Manag 9999, 1.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murk AJ, Rijntjes E, Blaauboer BJ, Clewell R, Crofton KM, Dingemans MML, Furlow JD, Kavlock R, Kohrle J, Opitz R, Traas T, Visser TJ, Xia MH, Gutleb AC, 2013. Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicology in Vitro 27, 1320.-. [DOI] [PubMed] [Google Scholar]
- Nelson K, Schroeder A, Ankley G, Blackwell B, Blanksma C, Degitz S, Flynn K, Jensen K, Johnson R, Kahl M, Knapen D, Kosian P, Milsk R, Randolph E, Saari T, Stinckens E, Vergauwen L, Villeneuve D, 2016. Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part I: Fathead minnow. Aquatic Toxicology 173, 192.-. [DOI] [PubMed] [Google Scholar]
- Noyes PD, Friedman KP, Browne P, Haselman JT, Gilbert ME, Hornung MW, Barone S, Crofton KM, Laws SC, Stoker TE, Simmons SO, Tietge JE, Degitz SJ, 2019. Evaluating Chemicals for Thyroid Disruption: Opportunities and Challenges with in Vitro Testing and Adverse Outcome Pathway Approaches. Environmental Health Perspectives 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD, 2012. OECD conceptual framework for testing and assessment of endocrine disruptors. OECD, Paris (FR). [Google Scholar]
- OECD, 2014. New scoping document on in vitro and ex vivo assays for the identification of modulators of thyroid hormone signalling Series on Testing and Assessment No. 207 OECD, Paris. [Google Scholar]
- OECD, 2018. Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption OECD Series on Testing and Assessment. OECD Publishing, Paris. [Google Scholar]
- Olker JH, Korte JJ, Denny JS, Hartig PC, Cardon MC, Knutsen CN, Kent PM, Christensen JP, Degitz SJ, Hornung MW, 2019. Screening the ToxCast Phase 1, Phase 2, and e1k Chemical Libraries for Inhibitors of Iodothyronine Deiodinases. Toxicological Sciences 168, 430.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul Friedman K, Watt ED, Hornung MW, Hedge JM, Judson RS, Crofton KM, Houck KA, Simmons SO, 2016. Tiered High-Throughput Screening Approach to Identify Thyroperoxidase Inhibitors Within the ToxCast Phase I and II Chemical Libraries. Toxicological Sciences 151, 160.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins EJ, Ankley GT, Crofton KM, Garcia-Reyero N, LaLone CA, Johnson MS, Tietge JE, Villeneuve DL, 2013. Current Perspectives on the Use of Alternative Species in Human Health and Ecological Hazard Assessments. Environmental Health Perspectives 121, 1002.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power DM, Llewellyn L, Faustino M, Nowell MA, Bjornsson BT, Einarsdottir IE, Canario AV, Sweeney GE, 2001. Thyroid hormones in growth and development of fish. Comp Biochem Physiol C Toxicol Pharmacol 130, 447.-. [DOI] [PubMed] [Google Scholar]
- Robertson GN, McGee CAS, Dumbarton TC, Croll RP, Smith FM, 2007. Development of the swimbladder and its innervation in the zebrafish, Danio rerio. Journal of Morphology 268, 967.-. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Buchholz DR, 2017. Frogs model man: In vivo thyroid hormone signaling during development. Genesis 55, 10. [DOI] [PubMed] [Google Scholar]
- Sobanska M, Scholz S, Nyman AM, Cesnaitis R, Alonso SG, Kluver N, Kuhne R, Tyle H, de Knecht J, Dang ZC, Lundbergh I, Carlon C, De Coen W, 2018. Applicability of the fish embryo acute toxicity (FET) test (OECD 236) in the regulatory context of Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH). Environmental Toxicology and Chemistry 37, 657.-. [DOI] [PubMed] [Google Scholar]
- Stinckens E, Vergauwen L, Ankley GT, Blust R, Darras VM, Villeneuve DL, Witters H, Volz DC, Knapen D, 2018. An AOP-based alternative testing strategy to predict the impact of thyroid hormone disruption on swim bladder inflation in zebrafish. Aquatic Toxicology 200, 1.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinckens E, Vergauwen L, Blackwell BR, Ankley GT, Villeneuve DL, Knapen D, The effect of thyroperoxidase and deiodinase inhibition on anterior swim bladder inflation in the zebrafish. Environmental Science & Technology submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinckens E, Vergauwen L, Schroeder A, Maho W, Blackwell B, Witters H, Blust R, Ankley G, Covaci A, Villeneuve D, Knapen D, 2016. Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part II: Zebrafish. Aquatic Toxicology 173, 204.-. [DOI] [PubMed] [Google Scholar]
- Thienpont B, Tingaud-Sequeira A, Prats E, Barata C, Babin PJ, Raldua D, 2011. Zebrafish Eleutheroembryos Provide a Suitable Vertebrate Model for Screening Chemicals that Impair Thyroid Hormone Synthesis. Environmental Science & Technology 45, 7525.-. [DOI] [PubMed] [Google Scholar]
- van der Ven LTM, van den Brandhof EJ, Vos JH, Power DM, Wester PW, 2006. Effects of the antithyroid agent propylthiouracil in a partial life cycle assay with zebrafish. Environmental Science & Technology 40, 74.-. [DOI] [PubMed] [Google Scholar]
- Villeneuve D, Angrish M, Fortin M, Katsiadaki I, Leonard M, Margiotta-Casaluci L, Munn S, O’Brien J, Pollesch N, Smith L, Zhang X, Knapen D, 2018. Adverse Outcome Pathway Networks II: Network Analytics. Environ Toxicol Chem doi: 10.1002/etc.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve D, Volz DC, Embry MR, Ankley GT, Belanger SE, Leonard M, Schirmer K, Tanguay R, Truong L, Wehmas L, 2014. Investigating alternatives to the fish early-life stage test: a strategy for discovering and annotating adverse outcome pathways for early fish development. Environmental Toxicology and Chemistry 33, 158.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walpita CN, Crawford AD, Janssens ED, Van der Geyten S, Darras VM, 2009. Type 2 iodothyronine deiodinase is essential for thyroid hormone-dependent embryonic development and pigmentation in zebrafish. Endocrinology 150, 530.-. [DOI] [PubMed] [Google Scholar]
- Wang J, Hallinger DR, Murr AS, Buckalew AR, Simmons SO, Laws SC, Stoker TE, 2018. High-Throughput Screening and Quantitative Chemical Ranking for Sodium-Iodide Symporter Inhibitors in ToxCast Phase I Chemical Library. Environmental Science & Technology 52, 5417.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/UNEP, 2013. State of the science of endocrine disrupting chemicals - 2012. in: Bergman Å,HJ, Jobling S, Kidd KA, Zoeller RT (Ed.). ISBN: 978 92 4 150503 1 WHO and UNEP, Geneva (CH). [Google Scholar]


