Abstract
Objective
To summarize and evaluate current reports on community-onset severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in young infants.
Study design
We performed a systematic review to identify reports published from November 1, 2019, until June 15, 2020, on laboratory-confirmed community-onset SARS-CoV-2 infection in infants younger than 3 months of age. We excluded studies reporting neonates with perinatal coronavirus disease 2019 (COVID-19) exposure and diagnosis before hospital discharge and hospital-onset disease, as well as clinically diagnosed cases without confirmation. Two independent reviewers performed study screening, data abstraction, and risk of bias assessment. Variables of interest included patient age, exposure to COVID-19, medical history, clinical symptoms, SARS-CoV-2 testing, laboratory findings, clinical course, and disposition.
Results
In total, 38 publications met inclusion criteria, including 23 single case reports, 14 case series, and 1 cohort study, describing 63 infants younger than 3 months of age with laboratory-confirmed SARS-CoV-2 infection. Most cases were mild to moderate. Fever, respiratory, gastrointestinal, cardiac, and neurologic findings were reported. Laboratory abnormalities included neutropenia, lymphopenia, and elevated serum levels of inflammatory markers and aminotransferases. Fifty-eight (92%) infants were hospitalized, 13 (21%) were admitted to the intensive care unit, and 2 (3%) required mechanical ventilation. No death was reported.
Conclusions
Among young infants with laboratory-confirmed SARS-CoV-2 infection, most cases were mild to moderate and improved with supportive care. Our results demonstrate a need for a high index of suspicion for SARS-CoV-2 infection in young infants presenting with generalized symptoms such as fever or decreased feeding, even in the absence of respiratory symptoms.
Keywords: neonate, pediatric, severe acute respiratory syndrome coronavirus 2
Abbreviations: COVID-19, Coronavirus disease 2019; ICU, Intensive care unit; PCR, Polymerase chain reaction; RSV, Respiratory syncytial virus; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Since its identification in December 2019, severe acute respiratory coronavirus 2 (SARS-CoV-2) has proven highly contagious and globally devastating, causing 728 013 fatalities as of August 10, 2020.1 Although coronavirus disease 2019 (COVID-19) is generally milder in children than in adults, infants have disproportionate risk of serious infection. In a retrospective study of 728 laboratory-confirmed pediatric cases in China, 97% presented with asymptomatic-to-moderate illness. Of confirmed severe or critically ill pediatric patients, however, one-third occurred in infants younger than 1 year of age.
As in adults, the most common pediatric symptoms of COVID-19 are fever and cough, with gastrointestinal and neurologic involvement in some patients.2 , 3 In a retrospective study of 74 pediatric patients in China, Wu et al4 noted a 51% prevalence of coinfection with other pathogens, including Mycoplasma pneumoniae (n = 16), respiratory syncytial virus (RSV) (n = 3), cytomegalovirus (n = 3), Epstein–Barr virus (n = 3), and influenza (n = 1). Moreover, recent evidence has emerged linking COVID-19 to multisystem inflammatory syndrome in children, including those as young as 6 months.5 Published reports from the US and Italy describe increasing cases of SARS-CoV-2 infection in children manifesting with inflammatory symptoms similar to Kawasaki disease or toxic shock syndrome, frequently coming to medical attention with nonspecific gastrointestinal symptoms and elevation of cardiac biomarkers.6 , 7
SARS-CoV-2 spreads primarily through droplet and contact transmission, with a high incidence of familial clustering and significant proportion of asymptomatic infection.8 Infants may be at greater risk of exposure to SARS-CoV-2 infection due to frequent contact with healthcare workers in the first few weeks of life, dependency on caretakers, and necessity for close contact during feeding and care that may increase exposure to respiratory secretions of infected persons. At present, studies of community-onset SARS-CoV-2 infection in young infants are primarily limited to case reports and small case series. This systematic review aims to consolidate reports of laboratory-confirmed, community-onset cases in infants younger than 3 months of age.
Methods
We conducted a systematic review to identify studies published from November 1, 2019, until June 15, 2020, on laboratory-confirmed community-onset SARS-CoV-2 infection in infants younger than 3 months of age. We searched PubMed and Embase and only included peer-reviewed publications for which full-text articles could be retrieved (search strategy, Table I [available at www.jpeds.com]). Additional articles were identified via snowball search. Inclusion criteria were infant aged younger than 3 months, community onset of illness, and laboratory-based confirmation by at least 1 positive SARS-CoV-2 polymerase chain reaction (PCR) test. Exclusion criteria were perinatal COVID-19 exposure with positive PCR testing in infants before hospital discharge, nosocomial infection, and presentation of aggregate pediatric data that included age groups greater than 3 months.
Two independent reviewers performed screening of articles in Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia) and data abstraction in Microsoft Excel (Microsoft Corporation, Redmond, Washington). Risk of bias assessment was performed by 2 independent reviewers for all studies meeting inclusion criteria using The Joanna Briggs Institute Critical Appraisal Checklists (Figure 1; available at www.jpeds.com).9, 10, 11 Discrepancies were resolved by discussion among the 2 reviewers with available third reviewer adjudication if needed. Descriptive analysis was performed to summarize infant demographic and clinical characteristics, laboratory findings, imaging studies, and clinical course, including hospital admission, intensive care unit (ICU) admission, need for respiratory support, antibiotic administration, COVID-specific therapy, and disposition. Categorical variables were expressed as numbers of cases (n), and percentages (%) and continuous variables were expressed as the median with IQR. Statistical analyses were performed using Microsoft Excel.
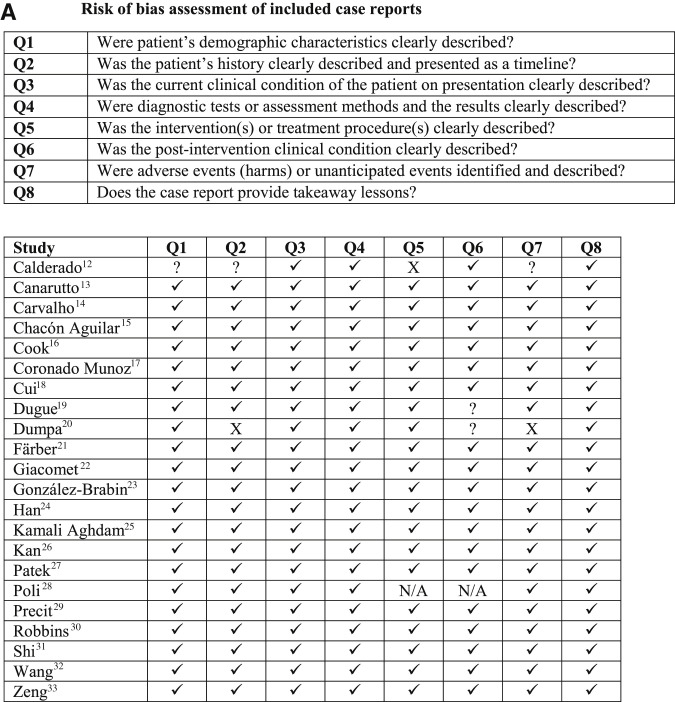
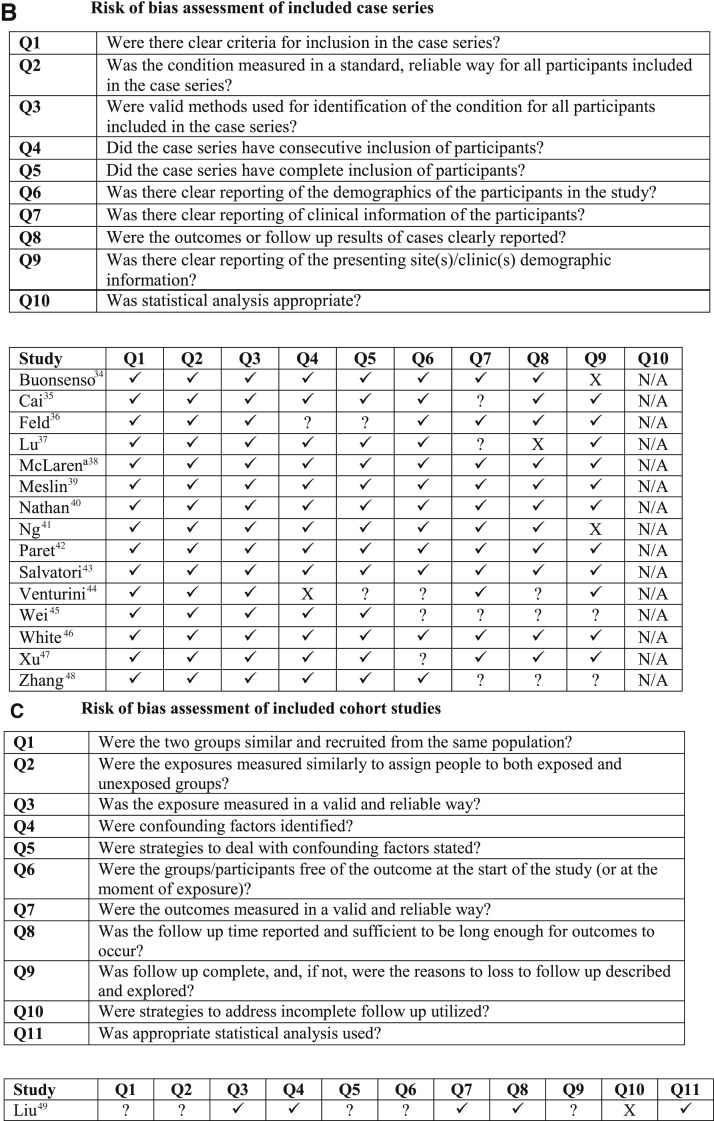
Figure 1.
Risk of bias assessment of studies reporting community-onset COVID-19 among infants younger than 3 months of age. A, Risk of bias assessment of included case reports. B, Risk of bias assessment of included case series. C, Risk of bias assessment of included cohort studies. Risk of bias assessment of case reports using The Joanna Briggs Institute (JBI) Critical Appraisal Tools: A, Checklist for Case Reports; B, Checklist for Case Series; and C, Checklist for Cohort Studies. Original study design was used for determining the appropriate critical appraisal tool. If a single case of a case series met inclusion criteria for the review, the Checklist for Case Series was used to assess risk of bias. aMcLaren et al38 described the overarching study design as a mixed retrospective/prospective cohort study but stated that the preliminary data presented was a case series. The Checklist for Case Series was used for risk of bias assessment. Key: ✓ indicates yes; ? indicates unclear; X indicates no; N/A, not applicable.
Results
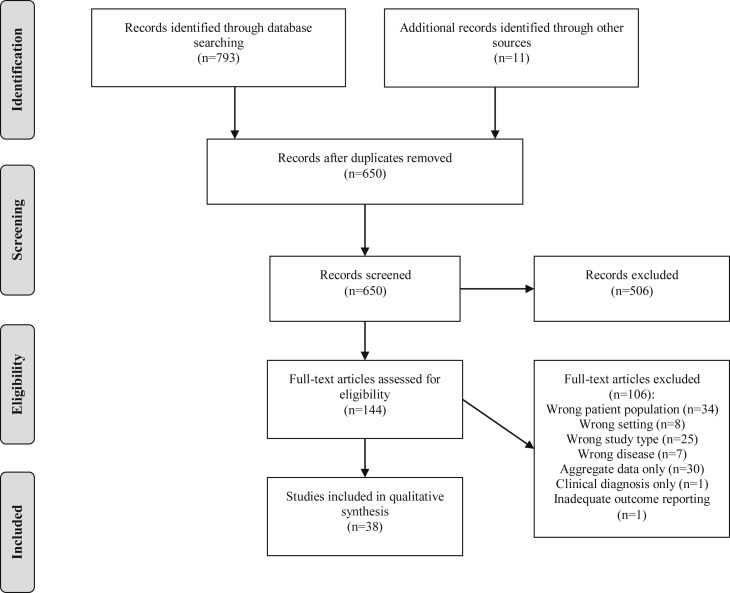
Our search strategy identified 793 articles published between November 1, 2019, and June 15, 2020, along with 11 additional articles identified via snowball search (Figure 2 ). After exclusion of duplicates, 650 records were screened; 506 were excluded after title and abstract screening and 106 were excluded after full-text review. We identified 38 studies for qualitative analysis, describing 63 infants younger than 3 months of age with community-onset SARS-CoV-2 infection. Among included studies, 23 were single case reports, 14 were case series, and 1 was a cohort study, with the majority of publications from the US (n = 11), China (n = 10), and Italy (n = 7) (Table II ).12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48
Figure 2.
PRISMA study flow diagram. Flow diagram of study identification, screening, eligibility, and included studies.
Table II.
Studies reporting community-onset COVID-19 among infants younger than 3 months of age by country, author, and study type
| Countries | Authors | Date | Study type | Infants, n | SARS-CoV-2 PCR testing by source, n positive/total n tested (%) |
|---|---|---|---|---|---|
| Brazil | Carvalho et al14 | 6/3/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) |
| China | Cai et al35 | 5/12/2020 | Case series | 1 | Nasopharyngeal, 1/1 (100) Oropharyngeal, 1/1 (100) |
| China | Cui et al18 | 3/17/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) Oropharyngeal, 0/1 Anal swab/stool sample, 1/1 (100) |
| China | Liu et al49 | 3/21/2020 | Cohort study | 1 | Oropharyngeal, 1/1 (100) |
| China | Lu et al37 | 5/1/2020 | Case report | 1 | Oropharyngeal, 1/1 Anal swab/stool sample, 0/1 Urine, 0/1 |
| China | Shi et al31 | 4/15/2020 | Case report | 1 | Oropharyngeal, 1/1 (100) |
| China | Wang et al32 | 3/25/2020 | Case report | 1 | Oropharyngeal, 1/1 (100) Anal swab/stool sample, 1/1 (100) |
| China | Wei et al45 | 2/14/2020 | Case series | 1 | Nasopharyngeal, 1/1 (100) |
| China | Xu et al47 | 3/13/2020 | Case series | 1 | Nasopharyngeal, 1/1 (100) Anal swab/stool sample, 1/1 (100) |
| China | Zeng et al33 | 4/2/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) Oropharyngeal, 1/1 (100) Anal swab/stool sample, 1/1 (100) |
| China | Zhang et al48 | 4/8/2020 | Case series | 2 | Anal swab/stool sample, 2/2 (100) |
| France | Meslin et al39 | 5/2020 | Case series | 6 | Nasopharyngeal, 6/6 (100) |
| France | Nathan et al40 | 4/27/2020 | Case series | 5 | Nasopharyngeal, 5/5 (100) CSF, 0/4 |
| Germany | Färber et al21 | 6/3/2020 | Case report | 1 | Oropharyngeal, 1/1 (100) CSF, 1/1 (100) |
| Iran | Kamali Aghdam et al25 | 4/1/2020 | Case report | 1 | Oropharyngeal, 1/1 (100) |
| Italy | Buonsenso et al34 | 5/2/2020 | Case series | 1 | Nasopharyngeal, 1/1 (100) Oropharyngeal, 0/1 |
| Italy | Calderaro et al12 | 5/14/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) |
| Italy | Canarutto et al13 | 4/6/2020 | Case report | 1 | Oropharyngeal, 1/1 (100) |
| Italy | Giacomet et al22 | 5/19/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) |
| Italy | Poli et al28 | 4/13/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) |
| Italy | Salvatori et al43 | 4/21/2020 | Case series | 2 | Nasopharyngeal, 2/2 (100) |
| Italy | Venturini et al44 | 5/19/2020 | Case series | 2 | Nasopharyngeal, 2/2 (100) |
| South Korea | Han et al24 | 4/16/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) Oropharyngeal, 1/1 (100) Saliva, 1/1 (100) Anal swab/stool sample, 1/1 (100) Urine, 1/1 (100) Blood, 1/1 (100) |
| Spain | Chacón-Aguilar et al15 | 4/17/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) |
| Spain | González-Brabin et al23 | Case report | 1 | Nasopharyngeal, 1/1 (100) | |
| United Kingdom | Cook et al16 | Case report | 1 | Nasopharyngeal, 1/1 (100) | |
| United Kingdom | Ng et al41 | 5/2020 | Case series | 3 | Unspecified Nasopharyngeal/oropharyngeal, 3/3 (100) |
| US | Coronado Munoz et al17 | 4/22/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) |
| US | Dugue et al19 | 4/23/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) Anal swab/stool sample, 1/1 (100) Blood, 0/1 CSF, 0/1 |
| US | Dumpa et al20 | 5/17/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) |
| US | Feld et al36 | 5/13/2020 | Case series | 3 | Nasopharyngeal, 3/3 (100) |
| US | Kan et al26 | 4/22/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) Oropharyngeal, 1/1 (100) |
| US | McLaren et al38 | 6/11/2020 | Case series (subset of cohort study) |
7 | Nasopharyngeal, 7/7 (100) |
| US | Paret et al42 | 4/17/2020 | Case series | 2 | Nasopharyngeal, 2/2 (100) |
| US | Patek et al27 | 4/15/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) |
| US | Precit et al29 | 5/22/2020 | Case report | 1 | Nasopharyngeal, 1/1 (100) Blood, 0/1 |
| US | Robbins et al30 | 6/2020 | Case report | 1 | Unspecified, 1/1 (100) |
| US | White et al46 | 6/4/2020 | Case series | 3 | Nasopharyngeal, 3/3 (100) |
| Summary by sample type | Infant samples Nasopharyngeal, 48/48 (100) Oropharyngeal, 12/14 (83) Nasopharyngeal/oropharyngeal swab, 60/60 (100) Saliva, 1/1 (100) Anal swab/stool sample, 8/10 (80) Urine, 1/2 (50) Blood, 1/3 (33) CSF, 1/6 (17) |
||||
Summary of studies reporting community-onset COVID-19 among infants younger than 3 months of age with positive SARS-CoV-2 PCR testing.
Infant ages ranged from 5 days to less than 3 months (Table III ). Among 59 infants with reported sex, 41 (69%) were male. Six (16%) infants with known gestational age were premature. Eight infants were reported as having a significant medical history, including extreme prematurity (n = 1), congenital heart disease (n = 3), cystic fibrosis (n = 1), and renal anomalies (n = 3). One neonate had a genetic syndrome associated with multiple congenital anomalies. Among the cases with a known contact history, 41 infants (69%) were exposed to a symptomatic person or person positive for COVID-19.
Table III.
Clinical and demographic characteristics of infants younger than 3 months of age with community-onset SARS-CoV-2 infection
| Characteristics | Total N = 63 |
|---|---|
| Age, range | 5 d to <3 mo |
| Male, n/total (%) | 42/61 (69) |
| Gestational age at birth in completed weeks, median (IQR) | 39 (37-39) (n = 26) |
| History of prematurity, n/total (%) | 6/37 (16) |
| Significant medical history, n/total (%) | 8/42 (19) |
| Contact with individual symptomatic or positive for COVID-19, n/total (%) | 41/59 (69) |
| Clinical presentation | |
| Fever, n (%) | 46 (73) |
| Cough, n (%) | 23 (38) |
| Rhinitis, n (%) | 22 (36) |
| Respiratory distress, n (%) | 16 (26) |
| Poor feeding, n (%) | 15 (24) |
| Emesis, n (%) | 9 (14) |
| Diarrhea, n (%) | 9 (14) |
| Hypoxia, n (%) | 9 (16) |
| Hypothermia, n (%) | 3 (5) |
| Rash, n (%) | 3 (5) |
| Hypotension, n (%) | 2 (3) |
| Apnea, n (%) | 2 (3) |
| Seizure, n (%) | 2 (3) |
| Asymptomatic, n (%) | 3 (5) |
| Laboratory and imaging studies | |
| WBC count, cells × 109/L, median (IQR) | 7.04 (4.80-8.94) (n = 44) |
| Neutrophil count, cells × 109/L median (IQR) | 1.20 (0.87-1.99) (n = 36) |
| Neutropenia, n/total (%) | 22/36 (56) |
| Lymphocyte count, cells × 109/L, median (IQR) | 2.92 (1.83-4.87) (n = 42) |
| Lymphopenia, n/total (%) | 7/45 (16) |
| Platelet count, cells/μL, median (IQR) | 348 500 (284 750-408 500)∗ (n = 24) |
| Thrombocytopenia, n/total (%) | 2/27 (7) |
| CRP, mg/L, median (IQR) | 2.1 (0.9-4.5)† (n = 27) |
| Procalcitonin, ng/mL, median (IQR) | 0.13 (0.10-0.22)† (n = 20) |
| AST, U/L, median (IQR) | 61.5 (46.25-66.5)‡ (n = 11) |
| ALT, U/L, median (IQR) | 26.5 (18-38.25) (n = 10) |
| Elevated cardiac biomarkers, n (%) | 3 (5) |
| Chest radiograph abnormal, n/n obtained (%) | 13/28 (46) |
| Chest CT abnormal, n/n obtained (%) | 9/9 (100) |
| Blood culture, n positive/n obtained (%) | 3/37 (8) |
| Urine culture, n positive/n obtained (%) | 3/28 (11) |
| CSF culture, n positive/n obtained (%) | 0/26 |
| Viral coinfection, n (%) | 5 (8) |
| Treatment and disposition | |
| Hospital admission, n (%) | 58 (92)§ |
| ICU admission, n/total (%) | 13/61 (21) |
| Supplemental oxygen via nasal cannula, n/total (%) | 12/55 (22) |
| CPAP, n/total (%) | 3/59 (5) |
| Mechanical ventilation, n/total (%) | 2/62 (3) |
| Vasopressor, n/total (%) | 1/59 (2) |
| Antibiotic therapy, n/total (%) | 25/39 (64) |
| COVID-19–specific treatment, n (%) | 8/56 (14) |
| Hospital length of stay, d, median (IQR) | 3 (2, 8) (n = 46) |
| Death, n (%) | 0/63 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPAP, continuous positive airway pressure; CRP, C-reactive protein; CSF, cerebrospinal fluid; CT, computed tomography; WBC, white blood cell.
Clinical and demographic characteristics of infants younger than 3 months of age with community-onset SARS-CoV-2 infection. Not all variables of interest were reported for all infants; denominators reported for individual variables as appropriate. Age was reported with variable units and degree of precision; summary statistics were therefore not performed. Neutropenia was defined as a neutrophil count <1500/μL. Lymphopenia was defined as a lymphocyte count <1500/μL. Thrombocytopenia was defined as a platelet count <150 000/μL. For WBC count, lymphocytes, neutrophils, and platelet count, if multiple values were reported, the lowest value was included.
Two infants were described as thrombocytopenic without reported platelet count.
Values reported in 1 study were excluded as they represented significant outliers, with concern for possible incorrect reporting of units. Study authors were contacted for clarification with no response.
One infant was reported to have significant aminotransferase elevation with an AST >500 U/L. Precise value was not provided and therefore not included in statistical analysis.
One infant did not require admission on initial presentation but was admitted after blood culture was positive, ultimately determined to be a contaminant. Infant not included in n of infants requiring hospitalization.
Among 62 infants with specified test site for SARS-CoV-2 PCR testing, 60 (97%) had at least 1 positive respiratory specimen (Table II). Two infants tested positive only from an anal swab.48 All infected infants who had nasopharyngeal testing (n = 48) had a positive test, as did 12 (83%) infants who had an oropharyngeal sample tested. PCR testing of cerebrospinal fluid, blood, and urine was infrequently performed and was positive in 1 of 6 (17%), 1 of 3 (33%), and 1 of 2 (50%) samples, respectively. Eight (80%) of 10 infants tested had a positive PCR result from stool or an anal swab, with persistent viral shedding in stool reported in several infants. Han et al24 reported serial quantitative PCR testing in an infant and her mother who also was hospitalized with COVID-19. The infant had positive PCR testing from nasopharyngeal, oropharyngeal, saliva, blood, urine, and stool samples, with gradual decline in viral load over a 3-week hospital admission. Her mother had detectable virus in nasopharyngeal/oropharyngeal, sputum, and stool samples; testing of blood and urine samples was negative.
Fever was the most common symptom reported among infants (n = 46, 73%), followed by respiratory symptoms (rhinitis, cough, or respiratory distress) (n = 40, 66%) (Table III). Reported gastrointestinal symptoms included diarrhea (n = 9) and emesis (n = 9). Two infants (3%) presented with seizures. Three infants (5%) were asymptomatic, including 1 infant with cystic fibrosis who had been tested due to known exposure to a family member with COVID-19.28
Laboratory tests were performed and reported in up to two-thirds of cases. Reported hematologic abnormalities included neutropenia (n = 22/39, 56%), lymphopenia (n = 7/45, 16%), and thrombocytopenia (n = 2/27, 7%). Median C-reactive protein was 2.1 mg/L (IQR 0.9-4.5; range, below limit of detection to 172); 5 (19%) infants had an elevated C-reactive protein ≥10 mg/L. Median procalcitonin was 0.13 ng/mL (IQR 0.10-0.22; range, 0.01-9.3); 4 (20%) infants had an elevated procalcitonin (≥0.5 ng/mL). Elevated aminotransferases were documented in 9 of 11 infants tested (82%), although only 1 infant was reported to have substantial elevation of hepatic enzymes with aspartate aminotransferase >500 units/L. Two of the infants with abnormal aminotransferase values also had elevated cardiac biomarkers.18 , 35 An additional infant with suspected myocarditis had elevated inflammatory markers and markers of cardiac dysfunction in the absence of either respiratory or hepatic involvement.22 Chest imaging was performed in 34 infants. Of 28 infants for whom chest radiography was performed, 13 (46%) had abnormal findings. Among infants who had chest computed tomography performed (n = 9), all had noted abnormal findings.
Forty-three infants (68%) were evaluated for bacterial infection. Of 37 reported blood cultures, 3 (8%) were positive, although 2 cultures positive for Staphylococcus epidermidis and Streptococcus salivarius were thought to be due to contamination.29 , 36 One critically ill infant with septic shock and an admission blood culture positive for S epidermidis was treated.16 Among 28 infants with a reported urine culture, 3 (11%) were found to have a urinary tract infection. Urinary tract infection pathogens included Escherichia coli (n = 2) and Klebsiella oxytoca (n = 1).23 38 None of the 26 reported cerebrospinal fluid cultures was positive. Testing for viral coinfection was reported in 30 infants, of whom 5 (17%) had positive testing; identified pathogens included RSV (n = 2), rhinovirus/enterovirus (n = 2), and seasonal coronavirus (n = 1).17 , 19 , 31 , 41 , 49 One neonate was diagnosed with human metapneumovirus coinfection after initial SARS-CoV-2 hospital admission; this neonate presented 5 days after initial discharge with poor feeding and respiratory distress and was readmitted.29
Fifty-eight (92%) infants were hospitalized with 13 (21%) requiring ICU admission. Fourteen (24%) infants required respiratory support, but only 3 (5%) required continuous positive airway pressure and 2 (3%) required invasive mechanical ventilation. One neonate presenting with clinical sepsis required mechanical ventilation and vasopressor support, with course complicated by pneumothorax and thoracostomy tube placement.17 The neonate recovered quickly and was discharged home without respiratory support 9 days after admission. Cook et al16 reported a neonate with a history of extreme prematurity at 27 weeks gestation who had been discharged from the neonatal ICU 10 days before hospitalization with respiratory failure and SARS-CoV-2 infection. The neonate was successfully extubated but remained hospitalized at the time of publication. Most infants received supportive care only; COVID-specific pharmacotherapies employed included hydroxychloroquine/azithromycin (n = 2), inhaled interferon (n = 5), and remdesivir (n = 1). Two neonates were treated with intravenous immunoglobulin, one in the setting of suspected myocarditis and the other with RSV coinfection and severe pneumonia requiring continuous positive airway pressure.22 , 31
Among admitted infants, length of hospital stay ranged from 1 to 30 days. Almost all infants with known disposition were discharged home; 1 infant was transferred to another hospital and 2 remained admitted at time of publication. Among infants discharged after hospital admission, 2 required readmission, 1 with newly diagnosed human metapneumovirus infection and the other due to persistent fever.29 , 38 Feld et al36 reported an infant who initially did not require hospital admission but was subsequently admitted due to a positive blood culture, determined to be a contaminant. There were no reported deaths among infants with community-onset SARS-CoV-2 infection.
Discussion
Although the spectrum of clinical disease of community-onset SARS-CoV-2 infection among young infants ranges from asymptomatic to critical illness, most identified infants appear to have mild-to-moderate illness and to recover quickly with supportive treatment alone. We were unable to draw conclusions regarding prevalence of infant disease, given the preponderance of case reports among publications to date. Larger pediatric studies have reported epidemiologic data of interest; however, aggregate reporting of data in broader age groups (ie, children <12 months of age or children <5 years) prohibits commentary on prevalence among young infants. There was a notable male predominance among reported infants, mirroring observations in other groups.50 , 51 It is unclear whether male sex could predispose infants to infection or to more significant clinical symptomatology, increasing likelihood of seeking care and of SARS-CoV-2 testing.
Clinical presentation in infants largely was nonspecific, with predominance of fever and respiratory symptoms among ill infants. Laboratory abnormalities such as neutropenia, leukopenia, and elevated inflammatory markers seen in these cases also can be observed in a number of common illnesses in this age group. In infants diagnosed with coinfection, such as urinary tract infection or other concomitant respiratory viral disease, it is unclear whether presentation and observed laboratory anomalies were primarily reflective of SARS-CoV-2 infection.
Unusual but noteworthy presentations reported among infants included cardiac and neurologic manifestations. Elevated cardiac biomarkers were reported in only 3 infants; however, these studies were not obtained routinely in infants presenting with community-onset disease.18 , 22 , 35 Cardiac manifestations have been reported in other age groups, including myocarditis in older children during SARS-CoV-2 infection.52 , 53 Among children with multisystem inflammatory syndrome in children, cardiac manifestations including elevated cardiac biomarkers, diminished cardiac function, arrhythmias, and coronary artery aneurysms can occur.6
Clinical seizures were reported only in 2 infants, only one of whom had an abnormal electroencephalogram.15 , 19 Bhatta et al54 reported new-onset seizures as the only manifestation of SARS-CoV-2 infection in a school-aged child. Neurologic manifestations of SARS-CoV-2 infection also have been reported in older age groups, including in more than one-third of patients in a large case series of adults with COVID-19 admitted to 3 hospitals in Wuhan, China.55
There are important limitations of this systematic review, including the quality of the available evidence (with a preponderance of case reports), the potential impact of publication bias, and variable reporting of outcomes of interest among included studies. In addition, we cannot exclude perinatal COVID-19 transmission among young neonates who presented from the community and who had symptomatic mothers; however, only 2 of the infants included in this systematic review came to medical attention in the first week of life.
Given the variability of manifestations described in reports, clinicians should have a high index of suspicion for SARS-CoV-2 infection in young infants presenting from the community with systemic symptoms, even in the absence of fever. Evaluation for serious bacterial illnesses should continue based on community guidelines, especially among febrile neonates. Diagnosis of SARS-CoV-2 infection does not preclude coinfection with other respiratory pathogens. We suggest that a thorough evaluation include PCR testing by a respiratory viral panel in children with respiratory symptoms. There are no studies to date reporting efficacy of COVID-specific therapies in this age group; however, critical illness in this age group is rare and infants appear to recover well with supportive care.
Footnotes
Supported by the National Institutes of Health (KL2TR003099 [to A.S.], K24AI141580 [to A.M.], and K23HD100594 [to J.J.]). The authors declare no conflicts of interest.
Appendix
Table I.
Electronic search strategy
| Databases | Search terms |
|---|---|
| PubMed | (Coronavirus[tw] OR COVID[tw] OR “SARS-CoV-2”[tw]) AND (Neonat∗[tw] OR infant[tw]) AND (2019/11/01:2020/06/15[dp]) |
| Embase | ‘Coronavirus infection’ OR ‘Severe acute respiratory syndrome coronavirus 2’ AND Infant OR Newborn |
Electronic search strategy for PubMed and Embase. The electronic search identified 415 records via PubMed and 378 records via Embase.
References
- 1.World Health Organization WHO Coronavirus disease (COVID-19) Dashboard 2020. https://covid19.who.int Accessed August 10, 2020.
- 2.Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Q., Xing Y., Shi L., Li W., Gao Y., Pan S. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;146 doi: 10.1542/peds.2020-0961. [DOI] [PubMed] [Google Scholar]
- 5.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Segal J.B. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 6.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan X., Chen D., Xia Y., Wu X., Li T., Ou X. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Joanna Briggs Institute Critical appraisal checklist for case reports. http://joannabriggs.org/research/critical-appraisal-tools.html Accessed July 12, 2020.
- 10.The Joanna Briggs Institute. Critical appraisal checklist for case series 2017. Available from: http://joannabriggs.org/research/critical-appraisal-tools.html. Accessed July 12, 2020.
- 11.The Joanna Briggs Institute. Critical appraisal checklist for cohort studies 2017. Available from: http://joannabriggs.org/research/critical-appraisal-tools.html. Accessed July 12, 2020.
- 12.Calderaro A., Arcangeletti M.C., De Conto F., Buttrini M., Montagna P., Montecchini S. SARS-CoV-2 infection diagnosed only by cell culture isolation before the local outbreak in an Italian seven-week-old suckling baby. Int J Infect Dis. 2020;96:387–389. doi: 10.1016/j.ijid.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canarutto D., Priolo A., Russo G., Pitea M., Vigone M.C., Barera G. COVID-19 infection in a paucisymptomatic infant: raising the index of suspicion in epidemic settings. Pediatr Pulmonol. 2020;55:e4–e5. doi: 10.1002/ppul.24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvalho W.B., Gibelli M.A.C., Krebs V.L.J., Calil V., Nicolau C.M., Johnston C. Neonatal SARS-CoV-2 infection. Clinics (Sao Paulo) 2020;75:e1996. doi: 10.6061/clinics/2020/e1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chacón-Aguilar R., Osorio-Cámara J.M., Sanjurjo-Jimenez I., González-González C., López-Carnero J., Pérez-Moneo-Agapito B. COVID-19: Fever syndrome and neurological symptoms in a neonate. An Pediatr (Engl Ed) 2020;92:373–374. doi: 10.1016/j.anpede.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook J., Harman K., Zoica B., Verma A., D'Silva P., Gupta A. Horizontal transmission of severe acute respiratory syndrome coronavirus 2 to a premature infant: multiple organ injury and association with markers of inflammation. Lancet Child Adolesc Health. 2020;4:548–551. doi: 10.1016/S2352-4642(20)30166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coronado Munoz A., Nawaratne U., McMann D., Ellsworth M., Meliones J., Boukas K. Late-onset neonatal sepsis in a patient with COVID-19. N Engl J Med. 2020;382:e49. doi: 10.1056/NEJMc2010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui Y., Tian M., Huang D., Wang X., Huang Y., Fan L. A 55-day-old female Infant infected with 2019 novel coronavirus disease: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020;221:1775–1781. doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dugue R., Cay-Martínez K.C., Thakur K.T., Garcia J.A., Chauhan L.V., Williams S.H. Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94:1100–1102. doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumpa V., Kamity R., Vinci A.N., Noyola E., Noor A. Neonatal coronavirus 2019 (COVID-19) infection: a case report and review of literature. Cureus. 2020;12:e8165. doi: 10.7759/cureus.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Färber K., Stäbler P., Getzinger T., Uhlig T. Suspected sepsis in a 10-week-old infant and SARS-CoV-2 detection in cerebrospinal fluid and pharynx. Monatsschr Kinderheilkd. 2020:1–4. doi: 10.1007/s00112-020-00942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giacomet V., Manfredini V.A., Meraviglia G., Peri C.F., Sala A., Longoni E. Acute inflammation and elevated cardiac markers in a two-month-old infant with severe acute respiratory syndrome coronavirus 2 infection presenting with cardiac symptoms. Pediatr Infect Dis J. 2020;39:e149–e151. doi: 10.1097/INF.0000000000002750. [DOI] [PubMed] [Google Scholar]
- 23.González Brabin A., Iglesias-Bouzas M.I., Nieto-Moro M., Martínez de Azagra-Garde A., García-Salido A. Neonatal apnea as initial manifestation of SARS-CoV-2 infection. An Pediatr (Barc) 2020;93:215–216. doi: 10.1016/j.anpedi.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han M.S., Seong M.W., Heo E.Y., Park J.H., Kim N., Shin S. Sequential analysis of viral load in a neonate and her mother infected with SARS-CoV-2. Clin Infect Dis. 2020:ciaa447. doi: 10.1093/cid/ciaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamali Aghdam M., Jafari N., Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond) 2020;52:427–429. doi: 10.1080/23744235.2020.1747634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kan M.J., Grant L.M.C., Muña M.A., Greenhow T.L. Fever without a source in a young infant due to SARS-CoV-2. J Pediatric Infect Dis Soc. 2020:piaa044. doi: 10.1093/jpids/piaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patek P., Corcoran J., Adams L., Khandhar P. SARS-CoV-2 infection in a 2-week-old male with neutropenia. Clin Pediatr (Phila) 2020;59:918–920. doi: 10.1177/0009922820920014. [DOI] [PubMed] [Google Scholar]
- 28.Poli P., Timpano S., Goffredo M., Padoan R., Badolato R. Asymptomatic case of COVID-19 in an infant with cystic fibrosis. J Cyst Fibros. 2020;19:e18. doi: 10.1016/j.jcf.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Precit M.R., Yee R., Anand V., Mongkolrattanothai K., Pandey U., Dien Bard J. A case report of neonatal acute respiratory failure due to severe acute respiratory syndrome coronavirus-2. J Pediatric Infect Dis Soc. 2020;9:390–392. doi: 10.1093/jpids/piaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins E., Ilahi Z., Roth P. Febrile infant: COVID-19 in addition to the usual suspects. Pediatr Infect Dis J. 2020;39:e81–e82. doi: 10.1097/INF.0000000000002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi B., Xia Z., Xiao S., Huang C., Zhou X., Xu H. Severe pneumonia due to SARS-CoV-2 and respiratory syncytial virus infection: a case report. Clin Pediatr (Phila) 2020;59:823–826. doi: 10.1177/0009922820920016. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Wang D., Chen G.C., Tao X.W., Zeng L.K. SARS-CoV-2 infection with gastrointestinal symptoms as the first manifestation in a neonate. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:211–214. doi: 10.7499/j.issn.1008-8830.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng L.K., Tao X.W., Yuan W.H., Wang J., Liu X., Liu Z.S. First case of neonate with COVID-19 in China. Zhonghua Er Ke Za Zhi. 2020;58:279–280. doi: 10.3760/cma.j.cn112140-20200212-00081. [DOI] [PubMed] [Google Scholar]
- 34.Buonsenso D., Costa S., Sanguinetti M., Cattani P., Posteraro B., Marchetti S. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am J Perinatol. 2020;37:869–872. doi: 10.1055/s-0040-1710541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai X., Ma Y., Li S., Chen Y., Rong Z., Li W. Clinical characteristics of 5 COVID-19 cases with non-respiratory symptoms as the first manifestation in children. Front Pediatr. 2020;8:258. doi: 10.3389/fped.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feld L., Belfer J., Kabra R., Goenka P., Rai S., Moriarty S. A case series of the 2019 novel coronavirus (SARS-CoV-2) in 3 febrile infants in New York. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1056. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y., Wen H., Rong D., Zhou Z., Liu H. Clinical characteristics and radiological features of children infected with the 2019 novel coronavirus. Clin Radiol. 2020;75:520–525. doi: 10.1016/j.crad.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaren S.H., Dayan P.S., Fenster D.B., Ochs J.B., Vindas M.T., Bugaighis M.N. Novel coronavirus infection in febrile infants aged 60 days and younger. Pediatrics. 2020;146:e20201550. doi: 10.1542/peds.2020-1550. [DOI] [PubMed] [Google Scholar]
- 39.Meslin P., Guiomard C., Chouakria M., Porcher J., Duquesne F., Tiprez C. Coronavirus disease 2019 in newborns and very young infants: a series of six patients in France. Pediatr Infect Dis J. 2020;39:e145–e147. doi: 10.1097/INF.0000000000002743. [DOI] [PubMed] [Google Scholar]
- 40.Nathan N., Prevost B., Corvol H. Atypical presentation of COVID-19 in young infants. Lancet. 2020;395:1481. doi: 10.1016/S0140-6736(20)30980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng K.F., Bandi S., Bird P.W., Wei-Tze Tang J. COVID-19 in neonates and infants: progression and recovery. Pediatr Infect Dis J. 2020;39:e140–e142. doi: 10.1097/INF.0000000000002738. [DOI] [PubMed] [Google Scholar]
- 42.Paret M., Lighter J., Pellett Madan R., Raabe V.N., Shust G.F., Ratner A.J. SARS-CoV-2 infection (COVID-19) in febrile infants without respiratory distress. Clin Infect Dis. 2020:ciaa452. doi: 10.1093/cid/ciaa452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salvatori G., De Rose D.U., Concato C., Alario D., Olivini N., Dotta A. Managing COVID-19-positive maternal-infant dyads: an Italian experience. Breastfeed Med. 2020;15:347–348. doi: 10.1089/bfm.2020.0095. [DOI] [PubMed] [Google Scholar]
- 44.Venturini E., Palmas G., Montagnani C., Chiappini E., Citera F., Astorino V. Severe neutropenia in infants with severe acute respiratory syndrome caused by the novel coronavirus 2019 infection. J Pediatr. 2020;222:259–261. doi: 10.1016/j.jpeds.2020.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei M., Yuan J., Liu Y., Fu T., Yu X., Zhang Z.J. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323:1313–1314. doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White A., Mukherjee P., Stremming J., Sherlock L.G., Reynolds R.M., Smith D. Neonates hospitalized with community-acquired SARS-CoV-2 in a Colorado neonatal intensive care unit. Neonatology. 2020:1–5. doi: 10.1159/000508962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z.J., Yu X.J., Fu T., Liu Y., Jiang Y., Yang B.X. Novel coronavirus infection in newborn babies aged <28 days in China. Eur Respir J. 2020;55 doi: 10.1183/13993003.00697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H., Liu F., Li J., Zhang T., Wang D., Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. 2020;80:e7–e13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L.Q., Huang T., Wang Y.Q., Wang Z.P., Liang Y., Huang T.B. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lara D., Young T., Del Toro K., Chan V., Ianiro C., Hunt K. Acute fulminant myocarditis in a pediatric patient with COVID-19 onfection. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1509. [DOI] [PubMed] [Google Scholar]
- 53.Gnecchi M., Moretti F., Bassi E.M., Leonardi S., Totaro R., Perotti L. Myocarditis in a 16-year-old boy positive for SARS-CoV-2. Lancet. 2020;395:e116. doi: 10.1016/S0140-6736(20)31307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhatta S., Sayed A., Ranabhat B., Bhatta R.K., Acharya Y. New-onset seizure as the only presentation in a child with COVID-19. Cureus. 2020;12:e8820. doi: 10.7759/cureus.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]