Abstract
Cancer cells are characterized by dysregulation in signal transduction and metabolic pathways leading to increased glucose uptake, altered mitochondrial function, and the evasion of antigrowth signals. Fasting and fasting-mimicking diets (FMDs) provide a particularly promising intervention to promote differential effects in normal and malignant cells. These effects are caused in part by the reduction in IGF-1, insulin, and glucose and the increase in IGFBP1 and ketone bodies, which generate conditions that force cancer cells to rely more on metabolites and factors that are limited in the blood, thus resulting in cell death. Here we discuss the cellular and animal experiments demonstrating the differential effects of fasting on normal and cancer cells and the mechanisms responsible for these effects.
Introduction
Cancer, the second leading cause of mortality globally, accounted for 8.8 million deaths in 2015. According to WHO data, lung, prostate, colorectal, stomach, and liver cancer are the most common types in men, while breast, colorectal, lung, cervical, and stomach cancer are the most common malignancies among women. Cancer is a multifactorial disease characterized by the accumulation of multiple DNA mutations in specific genes called oncogenes and tumor suppressor genes [1]. Primary functions of activated oncogenes and inactivated tumor suppressors are promoting and sustaining cancer proliferation and evading growth suppression and cell death [2–4]. These mutations are also responsible for cellular metabolic reprogramming, which involves altered bioenergetics, enhanced biosynthesis, and interference with mitochondrial function resulting in cancer cell survival, growth, and metastasis [5].
According to a number of studies, between 30% and 50% of cancer deaths could be prevented by modifying or avoiding key risk factors, such as reducing alcohol consumption, avoiding tobacco products, maintaining a healthy body weight, exercising regularly, and addressing infection-related risk factors [6–8]. Several epidemiological studies have demonstrated that diet plays an important role in the initiation, promotion, and progression of many common cancers in Western countries [9,10]. Excessive adiposity due to overconsumption of unhealthy foods and associated with a sedentary lifestyle increases the risk of developing cancer. The chronic metabolic imbalance generated by excessive consumption of food is associated with increased oxidative stress, insulin resistance, inflammation, and changes in hormone and growth factor concentrations that play key roles in the pathogenesis of many cancers [11,12]. Thus, cancer risk could be reduced by consuming a diet with a high intake of plant foods (e.g., vegetables, whole grains, beans, fruits) and limited consumption of animal fat, meat, and dairy products [8,13]. Various human studies suggest a strong positive correlation between the consumption of this type of diet and risk reduction for the development of colon, lung, oral, esophageal, and stomach cancer [6,9,14,15].
However, because dietary interventions can clearly affect cellular protection, aging, and cancer incidence, they can also play an important role in cancer treatment. To date, the main treatment options for cancer include surgery when possible and chemotherapy and/or radiotherapy, plus a long list of target-specific drugs such as tyrosine kinase inhibitors, immunotherapy, hormone therapy, and others. Treatment planning is usually guided by tumor type and stage and available resources. Although these novel target-specific drugs may eventually largely replace chemotherapy and radiotherapy, traditional treatments are unlikely to be phased out for decades. In addition, the high cost of many of the novel cancer therapies such as immunotherapy will limit their availability to a large portion of the world population, making chemotherapy a viable treatment option for many years to come. Most chemotherapeutic drugs target rapidly dividing cancer cells but can also damage normal cells (e.g., bone marrow, gastrointestinal tract, heart, hair follicle) generating various side effects including myelosuppression, fatigue, vomiting, diarrhea, and even, in some cases, death. This greatly limits the use of chemotherapy and treatmentremains suboptimal [16]. Thus, dietary approaches have the potential to both promote the protection of normal cells against chemotherapy, radiotherapy, and other treatments and enhance their efficacy by generating a hostile environment for cancer cells.
In this review we discuss how wide-acting nutritional intervention such as fasting (see Glossary) and FMDs can be effective in promoting the protection of mice and possibly humans against chemotherapy treatment and enhancing cancer cell death by exploiting their dysregulated metabolism and hallmarks such as insensitivity to antigrowth signals.
Altered Metabolism: One of the Emerging Hallmarks of Tumorigenesis
Cancer is characterized by dysregulated metabolism leading to high glucose consumption due to upregulation of glycolysis (the Warburg effect) and downregulation of oxidative phosphorylation [17]. This altered metabolism is often compensating for genetic and epigenetic alterations forcing tumor cells to adopt alternative strategies to generate energy as well as amino acids, fatty acids (FAs), and other metabolites necessary for cancer cell survival and proliferation [18] and can lead to the production and accumulation of reactive oxygen species, contributing to the generation of additional mutations [19].
Cancer cells are characterized by the accumulation of mutations in oncogenes [e.g., the IGF-1 receptor (IGF-1R) or its downstream effectors such as the GTP proteins RAS/RAF and mitogen-activated protein kinase (MAPK), the tumor suppressor phosphatase and tensin homolog (PTEN), phosphatidylinositol 3-kinase (PI3K), the serine/threonine kinase AKT] that result in constitutive activation of proliferation pathways independently or partially independently of external growth factors [5,20,21].
Activation of PI3K–AKT leads to enhanced glucose uptake by promoting expression of the glucose transporter GLUT1 and glycolysis. Downstream of PI3K–AKT, the mammalian target of rapamycin (mTOR) plays an important role in mitochondrial metabolism [21,22]. mTOR enhances protein synthesis and promotes mitochondrial biogenesis and lipogenesis. mTOR is also a major downstream target of the AMP-dependent kinase (AMPK) pathway [22]. The tumor suppressor liver kinase B1 (LKB1) and AMPK control cell growth in response to environmental nutrient changes and downregulate the mTOR pathway [23,24]. Mutations in these pathways can reduce the incidence and progression of various tumors [25].
Another important oncogene altered in cancer isthe transcription factor c-Myc [26]. It supports anabolic growth, enhances glycolysis and FA synthesis, and promotes mitochondrial gene expression and mitochondrial biogenesis [26]. Oncogenes like KRAS, which is frequently mutated in various cancers, coordinate the physiological functions of the PI3K and c-Myc pathways to promote tumorigenicity [5,27].
By contrast, insensitivity to growth inhibitory signals is due to loss-of-function mutations in tumor suppressor genes (e.g., Rb, p53, p21, PTEN) that enable cancer cells to disregard antiproliferative signals [5]. The loss of p53 not only has an impact on DNA repair, cell cycle arrest, and apoptosis, but promotes tumorigenesis by increasing glycolytic flux to promote anabolism and redox balance [5,27].
The dependence of cancer cells on glycolysis can be further accentuated by hypoxic conditions that are common to many tumor cells. The hypoxia response system upregulates glucose transporters and multiple enzymes of the glycolytic pathway [28]. Hypoxia can independently increase levels of hypoxia-inducible transcription factor 1α (HIF-1α) and HIF-2α, which in turn upregulate glycolysis [25,29,30]. HIFs are also responsible for an increase in the pool of cancer stem cells by inducing the expression of genes such as OCT4, SOX2, and NANOG or activation of the Notch signaling pathway and of self-renewal and differentiation [31,32]. Furthermore, HIF-1α increases telomerase activity in hypoxic cancer cells, maintaining the immortal lifespan of the tumor mass [32].
More recently, sirtuin proteins have also been found to finely regulate energy metabolism by facilitating the tricarboxylic acid cycle (TCA), oxidative phosphorylation, and FA metabolism [33,34]. Sirtuins are NAD-dependent deacetylase enzymes that play key roles in the regulation of metabolism, inflammation, and DNA repair [34]. They have a protective effect against DNA damage and oxidative stress and against the accumulation of mutations and genomic instability. Loss of sirtuins in cancer cells results in the accumulation of mutations and genomic instability allowing cell division to proceed without proper DNA repair [34,35].
Mutation in c-Myc, mTOR, and other oncogenes are also responsible for alterations in amino acid synthesis and metabolism [4,36]. The amino acid pathways are activated in subsets of cancer and drive the production of specific amino acids and their utilization as intermediate metabolites for the production of important biomolecules such as nucleotides, lipids,and glutathione [18]. Cancer cells show a high demand for non-essential amino acids such as glutamine and serine. Glutamine is the main source of nitrogen used by cancer cells for the biosynthesis of new molecules [18]. Alterations in serine synthesis pathway (SSP) enzymes reflect the requirement for serine by cancer cells to maintain nucleotide synthesis [37].
To meet their catabolic and anabolic needs, cancer cells also increase their intake and synthesis of FAs [4]. FAs essential for cellular proliferation, lipid membrane synthesis, energy production, and cellular signaling [18,38]. In addition to glucose, amino acids and FAs can supply substrates tothe TCA cycle to sustain mitochondrial ATP production in cancer cells and promote growth [39].
Thus, cancer cells have a variety of mutations and alterations that render them potentially sensitive to the extensive changes in growth factors and nutrients caused by extreme dietary conditions such as fasting or certain ketogenic diets (KDs).
The Differential Effects of Fasting and FMDs on Normal and Cancer Cells
Comparative studies indicate that different organisms (yeast, bacteria, worms, flies, mice) are capable of adapting and surviving under different forms and durations of food deprivation [40,41]. Since during periods of food scarcitythe organisms can be exposed to a widerange of insults (UV radiation, heat, cold, and other stressors), adaptation to starvation requires that they enter into a multistress-resistance mode and invest their energy in protective systems to minimize damage and protect their genetic material so it can be unaltered when passed on to their progeny [42,43].
Experiments in yeast have shown that when this unicellular organism is switched from glucose or ethanol medium to water, it becomes more resistant to multiple types of stress and lives longer [44–47]. In response to starvation, yeast cells downregulate two main pathways, the Ras–PKA pathway (glucose response) and the Tor–S6K (Sch9) pathway (amino acid response), and inhibit the serine/threonine kinase Rim 15 and the downstream stress resistance transcription factors Msn2/4 and Gis1, which are required for the protective effects, in part by increasing the expression of stress resistance genes including SOD2 and catalase [45]. Notably, when starved yeast cells enter a hypometabolic mode that allows them to minimize the use of reserve carbon sources and can also accumulate high levels of the ketone body-like acetic acid, analogously to the accumulation of ketone bodies in mammals [45,46]. Thus, under challenging conditions such as starvation, energy expenditure is reduced and diverted from growth to maintenance, thereby enhancing protection and survival [14,48].
In the same manner, in response to starvation mammalian cells enter either a non-dividing or a low-dividing state and invest energy resources in cellular protection against various insults. Low levels of IGF-1 reduce intracellular mitogenic signaling pathways, downregulating two of the major pathways downstream of IGF-1R – those regulated by Ras and AKT – which can induce cell cycle arrest by activation of p53 and p21 [42,49,50] and of protective transcription factors including FOXO and Egr1, which regulate SODs, and other stress resistance genes [51,52].
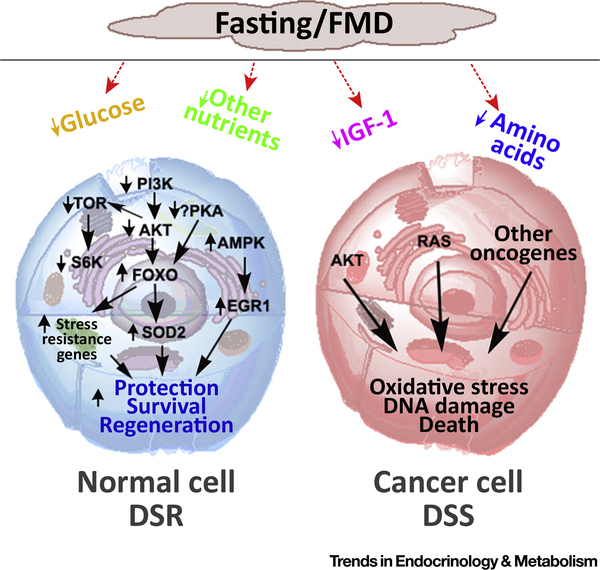
However, in both yeast and mammals the constitutive activation of Ras or of other oncoproteins can block entry into this protective mode, thus providing a method by which fasting induces protection in normal cells but not in oncogene-driven cancer cells, an effect that we termed differential stress resistance (DSR) [42] (Figure 1). The reason for this is that Ras, Tor, PKA, and other proteins found in a hyperactive state in many cancer cells negatively regulate stress resistance, as described above.
Figure 1. Schematic Representation of Differential Stress Resistance (DSR) and Differential Stress Sensitization (DSS) Mechanisms in Response to Fasting and Fasting-Mimicking Diets (FMDs).
In normal cells, proteins/enzymes downstream of glucose, IGF-1, and other growth factor pathways, including TOR, PKA, and AKT, are downregulated in response to fasting and FMDs. This down regulation arrests or reduces growth and promotes the activation of stress resistance genes resulting in protection against chemotherapy (DSR) and other drugs, survival, and regeneration. By contrast, cancer cells are sensitized by fasting/FMDs because of the constitutive activity of oncoproteins, which negatively regulate stress resistance and promote the generation of reactive oxygen species and cell death (DSS). The picture was drawn by using Photoshop CS6 and ChemDrawn Professional 17.
In in vivo studies, 48–72 h of fasting protects mice from lethal doses of doxorubicin, a drug that usually induces cardiotoxicity [43], and etoposide, a chemotherapeutic drug that damages DNA with a broad toxicity profile ranging from myelosuppression to liver and neurological damage [42]. This resistance to chemotherapy is due in part to the reduction in blood glucose and IGF-1 and, in cardiomyocytes, to the activation of transcription factor Egr1, an orthologof the yeast Msn2/4 transcription factors [52]. Recently, short-term starvation (STS) was also shown to promote hematopoietic stem cell (HSC) protection and self-renewal and reverse chemotherapy-induced DNA damage and immunosuppression [53].
In addition to protecting normal cells from chemotherapy toxicity, fasting conditions can be as effective as chemotherapy in killing cancer cells and, more importantly, render chemotherapy and other therapies much more effective against a wide variety of tumor types; an effect termed differential stress sensitization (DSS). Although the mechanisms for the broad toxic effect of fasting on many cancer cell types remain poorly understood, they probably involve the inability of cancer cells to adapt to complex environments. Cancer cells evolve in an environment in which most nutrients are in excess, thus making it possible for them toincrease functions such as glycolysis or protein biosynthesis [4,5]. Multiple studies in a variety of cancer mouse models support this hypothesis [54–57]. Cycles of fasting delay the progression of melanoma, glioma, and breast cancer and increase the effectiveness of cyclophosphamide (CP) and doxorubicin (DXR) against a variety of tumors. In 4T1 breast cancer cells, the effect of fasting is mediated by an increase of AKT and S6 kinases phosphorylation, increased oxidative stress, caspase-3 cleavage, DNA damage, and apoptosis [54].
In mesothelioma and lung carcinomas, fasting activates the ATM–Chk2–p53 stress response signaling pathway, which increases sensitization to cisplatin (CDDP), whereas in normal cells serum starvation activates AMPK, which stabilizes p53 and p21 resulting in proliferation arrest and protection of normal cells against CDDP toxicity [56]. The combination of fasting and CDDP treatment dramatically increases the sensitivity of human cancer cells to CDDP, with complete remission in approximately 60% of animals bearing mesothelioma xenografts and in 40% of animals with lung carcinoma xenografts [56]. Fasting cycles are also able to enhance gemcitabine’s effect in a pancreatic cancer xenograft mouse model by induction of human equilibrative nucleoside transporter 1 (hENT1), the transporter of gemcitabine across the cell membrane, and decreased ribonucleotide reductase M1 (RRM1), a key enzyme involved in the homeostasis of nucleotide pools affecting cell proliferation, migration, and metastasis [55]. Furthermore, fasting cycles increased the effect of the tyrosine kinase inhibitor sorafenib in hepatocellular cancer cells by inhibition of cell growth and glucose uptake [57]. In another mouse study, fasting alone reversed the progression of both B cell and T cell acute lymphoblastic leukemia (B-ALL and T-ALL) but did not affect acute myeloid leukemia (AML). Modulation of the leptin receptor (LEPR) by fasting was central to these effects [58].
Analogously to starved mice, IGF-1 genetic models such as LID transgenic mice characterized by a conditional liver IGF-1 gene deletion and reduction of circulating IGF-1 (70–80%) display increased resistanc et ohig hdose so fchemotherap ydrug ssuc ha sCP,DXR,an d5-fluorouracil (5-FU) but also sensitization of melanoma cells to chemotherapy leading to cancer-free survival [43]. Growth hormone receptor deficiency in humans is also associated with a major reduction in IGF-1 and insulin, as well as with a very low cancer, and diabetes incidence [59].
Together with the results discussed above, these data indicate that fasting promotes protection in multiple cell types and sensitization of a range of cancer cell types in part by reducing IGF-1 levels and signaling and in part by reducing glucose and its signaling.
Therefore, the reduction in circulating IGF-1 already obtained after 48–60 h of fasting can retard tumor growth, with effects similar to those caused by chemotherapy, but fasting used in combination with chemotherapeutic drugs is able to enhance toxicity, often promoting cancer-free survival [1,54]. Thus, cycles of short-term fasting or FMDs have the potential to be effective in both cancer prevention and treatment by promoting the death of cancer but not normal cells.
Fasting, Immunity, and Cancer
Fasting may also affect the killing of cancer cells by activating the immune system and/or allowing immune cells to recognize malignant cells. Bimonthly FMD cycles started at middle age rejuvenate the immune system, reduce cancer incidence, and extend longevity in mice [60]. This may be explained in part by the ability of the FMDs to enhance immune cell-dependent attack on cancer cells. In a murine model of cancer, a FMD in combination with chemotherapy stimulated T cell-dependent cytotoxicity against breast and melanoma cancer cells. The FMD promoted the infiltration of CD3+/CD8+ TILs in the tumor bed and was associated with delayed cancer progression [61–63]. In breast tumors this effect was partially mediated by downregulation of the stress-responsive enzyme heme oxygenase-1 (HO-1). Downregulation of HO-1 in the tumor by the FMD was necessary for the decrease of Tregs and for the immune-dependent attack of cancer cells [62,64].
Treatment with caloric restriction (CR) mimetics such as hydroxycitrate, an inhibitor of ATP citrate lyase, and spermidine improved the inhibition of tumor growth by chemotherapy. These effects of fasting and CR mimetics could be mediated in part by autophagy, the induction of which was shown to improve immunosurveillance by depletion of tumor-infiltrating Tregs in an in vivo mutant KRAS-induced lung cancer mouse model [65].
It is becoming increasingly apparent that microbiota – host-associated microbial communities – can also influence the development of cancer [66]. The microbiota plays an important role in modulating various host physiological process such as cellular metabolism and immune function that are highly dysregulated during carcinogenesis [67,68]. Perturbations to the microbiota promote the development of numerous diseases, including inflammatory bowel disease (IBD) and colorectal cancer (CRC). Previous studies revealed that fasting and feeding rhythms significantly alter the gut microbiota [69,70] and reduce the probability of pathogenic invasion in mice [71], but whether starvation induces changes in the composition or function of the microbiome to enhance the killing of cancer cells is unknown [66].
Fasting, Autophagy, and Cancer
Autophagy is a lysosomal degradation process that serves as a vehicle for clearing dysfunctional organelles and for maintaining genomic stability by clearing excised genomic fragments. It regulates the responses of eukaryotic cells to cellular stress such as hypoxia, genomic instability, endoplasmic reticulum stress, nutrient stress, and fasting [72]. Various signaling pathways have been implicated in the upregulation or downregulation of autophagy, including PI3K–mTOR and AMPK and various tumor suppressor (p53, PTEN, TSC1/TSC2) and tumor-associated (p21, AKT) genes [72]. For this reason autophagy appears to have a dual role in carcinogenesis: on the one hand it promotes tumor growth, while on the other hand it induces tumor suppression. Autophagy is often inhibited in early-stage cancer, allowing tumor development, but in late-stage cancers autophagy and accompanying chemotherapy resistance is high [73]. Because starvation is one of the most efficient ways to promote autophagy in most cells [74], and in cancer mouse models it improves immunosurveillance and cancer treatment [65,75], it will be important to study further the role of autophagy in the effects of fasting on cancer cells.
Fasting and FMDs in Cancer Treatment in Humans
Because most patients have difficulties in tolerating water-only fasting for multiple days in combination with their chemotherapy sessions, a FMD that enables the patient to eat while achieving effects similar to fasting was developed [60]. Day 1 of the FMD supplies ~4600kJ (11% protein, 46% fat, 43% carbohydrate) whereas days 2–5 provide ~3000kJ per day (9% protein, 44% fat, 47% carbohydrate); thus, fat and complex carbohydrates are the major source of calories in the FMD. This low-calorie, low-protein, high-complex-carbohydrate, and high-fat diet mimics the effects of fasting on markers associated with stress resistance, including the reduction of the levels of glucose and IGF-1 and the increase in the levels of ketone bodies and IGFBP-1 [60,76]. The discovery that cycles of a FMD can increase the efficacy of chemotherapy in cancer cells while at the same time protecting mice from its toxicity has stimulated several clinical trials [18], which are currently evaluating its effect in combination with chemotherapy as a therapeutic strategy in humans (e.g., ClinicalTrials.gov NCT01802346, NCT02126449, NCT02710721, and NCT01954836). In one study, some cancer patients have fasted voluntarily during their chemotherapy and have reported fewer side effects [77,78]. From 2008 to 2009, ten unrelated patients diagnosed with a variety of cancers (breast, prostate, lung, etc.) tried fasting with their chemotherapy treatments; in all patients the fasting waswell tolerated and was associated with a self-reported reduction in multiple chemotherapy-induced side effects such as fatigue, weakness, nausea, and others [77]. The safety of fasting before chemotherapy was also demonstrated in a different clinical trial at the Norris Comprehensive Cancer Center (USC) and the Los Angeles County/USC Medical Center where 18 subjects were enrolled from 2009 to 2012 to test fasting before platinum chemotherapy administration. A potential reduction of side effects and a reduction of leukocyte DNA damage were observed in the 72-h- but not the 24-h-fasted group receiving chemotherapy. At Leiden University Medical Center, 13 women with early-stage HER2-negative breast cancer were included and randomized into a clinical trial (NCT01304251) to test the safety of 48 h of fasting before chemotherapy. This pilot study confirms that short-term fasting is well tolerated and safe and may have beneficial effects on hematological toxicity and possibly on DNA damage in healthy cells (lymphocytes and myeloid cells) [79]. There are now several ongoing clinical trials testing the role of the FMD in reducing chemotherapy side effects and enhancing the killing of cancer cells in breast and prostate cancer patients. In these trialsthe FMD is started 3 days before and continues for 1 day after chemotherapy. Although randomized trials with much larger numbers of patients are needed to determine the effects of FMDs on protection against side effects and sensitization of cancer cells, the results of these pilot clinical trials give initial support for the potential efficacy of FMDs in cancer therapy.
Alternative Dietary Interventions for Cancer Treatment
Other dietary interventions (e.g., ketogenic and protein-restricted diets) that promote some of the metabolic responses caused by fasting have also been tested in cancer treatments [80,81]. KDs, which are rich in fats and poor in simple and complex carbohydrates, increase blood ketones and can decrease blood glucose, thus leading to high rates of fatty acid oxidation and an increase of acetyl coenzyme A (acetyl-CoA) production. When the amount of acetyl-CoA exceeds the capacity of the TCA cycle to utilize it, there is an increase in the production of the ketone bodies β-hydroxybutyrate (βHB) and acetoacetate (ACA), which can be used as an energy source in normal cells. By contrast, the alterations found in cancer cells reduce their ability to easily adapt to this metabolic change [82–84]. As a consequence, the ketotic state exacerbates metabolic oxidative stress in cancer cells, and it selectively enhances radio/ chemotherapy responses when combined with standard therapy [84,85]. The effects of ketogenic diets in animal cancer models are varied [86–88]. Various trials have tested the feasibility and tolerability of different KDs in combination with chemotherapy and radiotherapy in patients with neuronal cancer, but studies to demonstrate their efficacy are required [85,89–91]. Further research is needed to determine how the effects of chronic ketogenicdiets compare with those of periodic fasting/FMD on glucose, ketone bodies, IGF-1, and IGFBP1 and how these changes affect cancer progression and stress resistance.
Another dietary intervention is based on protein-restricted diets that could inhibit tumor growth by reducing the amino acid supply to tumor cells and consequently affect protein synthesis, mTOR activation, and other metabolic processes [92,93]. Preclinical experiments have shown that protein restriction inhibits in vivo tumor growth of melanoma but not breast cancer [92]or glioma [94]. Reduction in dietary protein intake is highly effective in inhibiting tumor growth in human xenograft prostate and breast cancer mouse models, an effect associated with a reduction in serum PSA and IGF-1 levels and downregulation of mTOR activity in tumor cells [93]. Because prolonged protein deprivation can stimulate tumor-induced muscle degradation and sarcopenia, dietary restriction of single amino acids could represent a potential alternative. For example, methionine-restricted diets have been tested in populations of patients with advanced cancers and have shown a good tolerability profile [95,96]. Notably, normal cells in the tumor microenvironment, including fibroblasts, endothelial cells, and immune cells, can supply tumor cells with amino acids derived from autophagic degradation of their proteins, thus potentially limiting the impact of this type of specific dietary restriction.
Concluding Remarks and Future Perspectives
Preclinical and clinical studies have demonstrated the important role of dysregulated metabolism in tumor initiation and progression. As discussed in this review, some of the most common alterations in cancer cells are mutations leading to the activation of signal transduction proteins and alterations of metabolic pathways. As a consequence, tumor cells require an abnormally high level of glucose to produce the glycolytic ATP and other metabolites needed to proliferate and survive. These alterations render cancer cells particularly vulnerable to the glucose, metabolite, and growth factor deficiencies and other changes generated by fasting conditions. These DSR and DSS responses provide the foundation for the ability of fasting and FMDsto promote cancer-free survival in animal models, particularly in combination with chemotherapy as well as more novel therapies (see Outstanding Questions). These differential effects in normal and cancer cells appear to be partly dependent on the deficiency of glucose and IGF-1 butare likely to involve many additional factors and metabolites. Since this approach is based onthe combination of a dietary therapy with standard-of-care drugs already approved by the FDA,it could become rapidly available once large randomized studies proving its efficacy are terminated.
Outstanding Questions.
How will fasting and FMDs affect novel cancer therapies including hormone therapy and immunotherapy?
Can fasting and/or low levels of glucose and IGF-I protect patients against chemotherapy?
Can the differential protection/sensitization mechanism of fasting and FMDs in combination with chemotherapy and other therapies lead to cancer-free survival in metastatic cancer patients?
Highlights.
Dysregulated metabolism is one of the emerging hallmarks of cancer cells.
Differential stress resistance (DSR) and differential stress sensitization (DSS) responses are the mechanisms caused by fasting and fasting-mimicking diet (FMDs) to promote protection of normal cells and induce cancer cell death.
Fasting-dependent reduction in glucose and IGF-1 mediates part of the DSR and DSS effects.
Fasting and FMDs have the potential for applications in both cancer prevention and treatment.
Glossary
- Differential stress resistance (DSR)
mechanism used on fasting and FMDs to protect normal cells but not cancer cells from chemotherapy and other stressors.
- Differential stress sensitization (DSS)
mechanism used on fasting and FMDs to preferentially kill cancer cells and other damaged cells in combination with chemotherapy or other cytotoxic agents.
- Fasting
in humans achieved by ingesting no or minimal amounts of food and caloric beverages for periods that typically range from 12 h to 3 weeks.
- Fasting-mimicking diet (FMD)
a plant-based diet designed to achieve fasting-like effects on the serum levels of IGF-I, IGFBP1, glucose, and ketone bodies while providing both macro- and micronutrients to minimize the burden of fasting and adverse effects.
- Short-term starvation (STS)
obtained by ingestion of only water for 24–72 h.
- Starvation
a chronic nutritional insufficiency that is commonly used as a substitute for the word fasting, particularly in lower eukaryotes, but is also used to define extreme forms of fasting.
References
- 1.Hanahan D and Weinberg RA (2000) The hallmarks of cancer. Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D and Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 3.Ward PS and Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21, 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlova NN and Thompson CB (2016) The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeBerardinis RJ and Chandel NS (2016) Fundamentalsof cancer metabolism. Sci. Adv. 2, e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kushi LH et al. (2012) American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 62, 30–67 [DOI] [PubMed] [Google Scholar]
- 7.Kohler LN et al. (2016) Adherence to diet and physical activity cancer prevention guidelines and cancer outcomes: a systematic review. Cancer Epidemiol. Biomarkers Prev. 25, 1018–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Cancer Research Foundation and American Institute for Cancer Research (2007) Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective, WCRF/AICR [Google Scholar]
- 9.Kushi LH et al. (2006) American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 56, 254–281 quiz 313–314 [DOI] [PubMed] [Google Scholar]
- 10.Renehan AG et al. (2010) Incidentcancer burdenattributable to excess body mass index in 30 European countries. Int. J. Cancer 126, 692–702 [DOI] [PubMed] [Google Scholar]
- 11.Calle EE and Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591 [DOI] [PubMed] [Google Scholar]
- 12.Renehan AG et al. (2010) Interpreting the epidemiological evidence linking obesity and cancer: a framework for population-attributable risk estimations in Europe. Eur. J. Cancer 46, 2581–2592 [DOI] [PubMed] [Google Scholar]
- 13.Mayne ST et al. (2016) Diet, nutrition, and cancer: past, present and future. Nat. Rev. Clin. Oncol. 13, 504–515 [DOI] [PubMed] [Google Scholar]
- 14.Longo VD and Fontana L (2010) Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol. Sci. 31, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Go VL et al. (2004)Dietand cancerprevention: evidence-based medicine to genomic medicine. J. Nutr. 134 (12 Suppl), 3513S–3516S [DOI] [PubMed] [Google Scholar]
- 16.Chabner BA and Roberts TG (2005) Timeline: chemotherapy and the war on cancer. Nat. Rev. Cancer 5, 65–72 [DOI] [PubMed] [Google Scholar]
- 17.Warburg O (1956) On the origin of cancer cells. Science 123, 309–314 [DOI] [PubMed] [Google Scholar]
- 18.Hirschey MD et al. (2015) Dysregulated metabolism contributes to oncogenesis. Semin. Cancer Biol. 35 (Suppl), S129–S150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vander Heiden MG et al. (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robey RB and Hay N (2009) Is Akt the “Warburg kinase”? Akt-energy metabolism interactions and oncogenesis. Semin. Cancer Biol. 19, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S and Yu D (2010) PI3king apart PTEN’s role in cancer. Clin. Cancer Res. 16, 4325–4330 [DOI] [PubMed] [Google Scholar]
- 22.Zoncu R et al. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shackelford DB and Shaw RJ (2009) The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nat. Rev. Cancer 9, 563–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shackelford DB et al. (2009) mTOR and HIF-1α-mediated tumor metabolism in an LKB1 mouse model of Peutz–Jeghers syndrome. Proc. Natl. Acad. Sci. U. S. A. 106, 11137–11142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroemer G and Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13, 472–482 [DOI] [PubMed] [Google Scholar]
- 26.Dang CV et al. (2009) MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin. Cancer Res. 15, 6479–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones RG and Thompson CB (2009) Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 23, 537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carnero A and Lleonart M (2016) The hypoxic microenvironment: a determinant of cancer stem cell evolution. Bioessays 38 (Suppl. 1), S65–S74 [DOI] [PubMed] [Google Scholar]
- 29.Semenza GL (2010) Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29, 625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeBerardinis RJ et al. (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 [DOI] [PubMed] [Google Scholar]
- 31.Tafani M et al. (2016) The interplay of reactive oxygen species, hypoxia, inflammation, and sirtuins in cancer initiation and progression. Oxid. Med. Cell. Longev. 2016, 3907147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keith B and Simon MC (2007) Hypoxia-inducible factors, stem cells, and cancer. Cell 129, 465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalkiadaki A and Guarente L (2015) The multifaceted functions of sirtuins in cancer. Nat. Rev. Cancer 15, 608–624 [DOI] [PubMed] [Google Scholar]
- 34.Saunders LR and Verdin E(2007) Sirtuins: critical regulatorsat the crossroads between cancer and aging. Oncogene 26, 5489–5504 [DOI] [PubMed] [Google Scholar]
- 35.Roth M and Chen WY (2014) Sorting out functions of sirtuins in cancer. Oncogene 33, 1609–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hensley CT et al. (2013) Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Invest. 123, 3678–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M and Vousden KH (2016) Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16, 650–662 [DOI] [PubMed] [Google Scholar]
- 38.Raffaghello L and Longo V (2017) Metabolic alterations at the crossroad of aging and oncogenesis. Int. Rev. Cell Mol. Biol. 332, 1–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullen AR et al. (2011) Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longo VD (2003) The Ras and Sch9 pathways regulate stress resistance and longevity. Exp. Gerontol. 38, 807–811 [DOI] [PubMed] [Google Scholar]
- 41.Parrella E and Longo VD (2010) Insulin/IGF-I and related signaling pathways regulate aging in nondividing cells: from yeast to the mammalian brain. ScientificWorldJournal 10, 161–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raffaghello L et al. (2008) Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. U. S. A. 105, 8215–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee C et al. (2010) Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 70, 1564–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madia F et al. (2008) Longevity mutation in SCH9 prevents recombination errors and premature genomic instability in a Werner/Bloom model system. J. Cell Biol. 180, 67–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei M et al. (2008) Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/ PKA, Tor, and Sch9. PLoS Genet. 4, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Longo VD et al. (1997) Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J. Cell Biol. 137, 1581–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu J et al. (2014) Tor–Sch9 deficiency activates catabolism of the ketone body-like acetic acid to promote trehalose accumulation and longevity. Aging Cell 13, 457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Longo VD (1999) Mutations in signal transduction proteins increase stress resistance and longevity in yeast, nematodes, fruit flies, and mammalian neuronal cells. Neurobiol. Aging 20, 479–486 [DOI] [PubMed] [Google Scholar]
- 49.Keyomarsi K and Pardee AB (2003) Selective protection of normal proliferating cells against the toxic effects of chemotherapeutic agents. Prog. Cell Cycle Res. 5, 527–532 [PubMed] [Google Scholar]
- 50.Blagosklonny MV and Pardee AB (2001) Exploiting cancer cell cycling for selective protection of normal cells. Cancer Res. 61, 4301–4305 [PubMed] [Google Scholar]
- 51.Longo VD et al. (2008) Turning anti-ageing genes against cancer. Nat. Rev. Mol. Cell Biol. 9, 903–910 [DOI] [PubMed] [Google Scholar]
- 52.Di Biase S et al. (2017) Correction: Fasting regulates EGR1 and protects from glucose- and dexamethasone-dependent sensitization to chemotherapy. PLoS Biol. 15, e1002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng CW et al. (2014) Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 14, 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee C et al. (2012) Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 4, 124ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Aronzo M et al. (2015) Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget 6, 18545–18557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi Y et al. (2012) Starvation-induced activation of ATM/Chk2/ p53 signaling sensitizes cancer cells to cisplatin. BMC Cancer 12, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lo Re O et al. (2018) Fasting inhibits hepatic stellate cells activation and potentiates anti-cancer activity of sorafenib in hepatocellular cancer cells. J. Cell Physiol. 233, 1202–1212 [DOI] [PubMed] [Google Scholar]
- 58.Lu Z et al. (2017) Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. Nat. Med. 23, 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guevara-Aguirre J et al. (2011) Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 3, 70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brandhorst S et al. (2015) A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 22, 86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andre F et al. (2013) Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin. Cancer Res. 19, 28–33 [DOI] [PubMed] [Google Scholar]
- 62.Di Biase S et al. (2016) Fasting-mimicking diet reduces HO-1to promote T cell-mediated tumor cytotoxicity. Cancer Cell 30, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Issa-Nummer Y et al. (2013) Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer – a substudy of the neoadjuvant GeparQuinto trial. PLoS One 8, e79775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Otterbein LE et al. (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 6, 422–428 [DOI] [PubMed] [Google Scholar]
- 65.Pietrocola F et al. (2016) Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zitvogel L et al. (2017) Anticancer effects of the microbiome and its products. Nat. Rev. Microbiol. 15, 465–478 [DOI] [PubMed] [Google Scholar]
- 67.Fulbright LE et al. (2017) The microbiome and the hallmarksof cancer. PLoS Pathog. 13, e1006480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zitvogel L et al. (2016) Microbiome and anticancer immunosurveillance. Cell 165, 276–287 [DOI] [PubMed] [Google Scholar]
- 69.Thaiss CA et al. (2014) Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529 [DOI] [PubMed] [Google Scholar]
- 70.Li G et al. (2017) Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 26, 672–685.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Becattini S et al. (2016) Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 22, 458–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sui X et al. (2013) Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell. Death. Dis. 4, e838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sehgal AR et al. (2015) You eat what you are: autophagy inhibition as a therapeutic strategy in leukemia. Leukemia 29, 517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mattson MP et al. (2017) Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 39, 46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun P et al. (2017) Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget 8, 74649–74660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Longo VD and Mattson MP (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab. 19, 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Safdie FM et al. (2009) Fasting and cancer treatmen ti nhumans: a case series report. Aging (Albany NY) 1, 988–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raffaghello L et al. (2010) Fasting and differential chemotherapy protection in patients. Cell Cycle 9, 4474–4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Groot S et al. (2015) The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer 15, 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vernieri C et al. (2016) Targeting cancer metabolism:dietary and pharmacologic interventions. Cancer Discov. 6, 1315–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bozzetti F and Zupec-Kania B (2016) Toward a cancer-specific diet. Clin. Nutr. 35, 1188–1195 [DOI] [PubMed] [Google Scholar]
- 82.Erickson N et al. (2017) Systematic review: isocaloric ketogenic dietary regimes for cancer patients. Med. Oncol. 34, 72. [DOI] [PubMed] [Google Scholar]
- 83.Morscher RJ et al. (2015) Inhibition of neuroblastoma tumor growth by ketogenic diet and/or calorie restriction in a CD1-nu mouse model. PLoS One 10, e0129802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Branco AF et al. (2016) Ketogenic diets: from cancer to mitochondrial diseases and beyond. Eur. J. Clin. Invest. 46, 285–298 [DOI] [PubMed] [Google Scholar]
- 85.Abdelwahab MG et al. (2012) The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One 7, e36197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Puchalska P and Crawford PA (2017) Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25, 262–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Feyter HM et al. (2016) A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth. Neuro Oncol. 18, 1079–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lv M et al. (2014) Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: a systematic review and meta-analysis. PLoS One 9, e115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aminzadeh-Gohari S et al. (2017) A ketogenic diet supplemented with medium-chain triglycerides enhancesthe anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget 8, 64728–64744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmidt M et al. (2011) Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr. Metab. (Lond.) 8, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Champ CE et al. (2014) Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J. Neuro-oncol. 117, 125–131 [DOI] [PubMed] [Google Scholar]
- 92.Levine ME et al. (2014) Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 19, 407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fontana L et al. (2013) Dietary protein restriction inhibits tumor growth in human xenograft models. Oncotarget 4, 2451–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brandhorst S et al. (2013) Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp. Gerontol. 48, 1120–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Durando X et al. (2008) Optimalmethionine-free diet duration for nitrourea treatment: a Phase I clinical trial. Nutr. Cancer 60, 23–30 [DOI] [PubMed] [Google Scholar]
- 96.Thivat E et al. (2009) Phase II trial of the association of a methionine-free diet with cystemustine therapy in melanoma and glioma. Anticancer Res. 29, 5235–5240 [PubMed] [Google Scholar]