Abstract
Advances in our understanding of thyroid lesions, especially those entities with an indolent behavior, has led clinicians to question the most appropriate surgical management of such thyroid nodules. Several studies have shown that the non-invasive encapsulated follicular variant of papillary thyroid carcinomas (NI-EFVPC) exhibits poor histopathologic diagnostic reproducibility and have been over-treated as conventional thyroid cancer. In 2015, an international thyroid working group re-evaluated NI-EFVPC and its diagnostic criteria. The new terminology of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP) was accordingly introduced to replace NI-EFVPC. The literature has emphasized that NIFTPs are biologically similar to follicular adenomas lacking lymph node metastases and/or recurrence. While the definition of NIFTP is based on specific morphological parameters, recent studies have questioned whether the criterion allowing less than 1% of true papillae should be revised to a total absence of papillae. The motivation for this revision is the rare finding, in some studies, of lymph nodes with metastatic NIFTP. This review evaluates the existing published series of NIFTP cases, clinical consequences of NIFTP, and emerging changes in the diagnostic criteria for NIFTP. The introduction of NIFTP has resulted in significant impact on the clinical management of thyroid nodules. Recent revisions in the morphological criteria for NIFTP emphasize the need to adhere to very stringent histomorphologic criteria when making a diagnosis of NIFTP. The adoption of NIFTP terminology instead of NI-EFVPC is associated with conservative lobectomy without radioactive iodine treatment in the majority of cases.
Keywords: Papillary thyroid carcinoma, Follicular variant of PTC, NIFTP, Thyroid cytology, Molecular testing, Personalized medicine
Introduction
In recent decades, the incidence of thyroid carcinoma, including small nodules, has nearly tripled even though no change in mortality has been reported [1–3]. In fact, increased screening employing radiologic modalities and the use of fine needle aspiration (FNA) in the evaluation of thyroid nodules have resulted in the detection of subcentimeter malignant nodules. This approach has led to the conclusion that most thyroid tumors are likely indolent and being overtreated. Among them was the follicular variant of papillary thyroid carcinoma (FVPTC) [4–11]. FVPTC comprises 9 to 22% of papillary thyroid carcinomas (PTCs), including neoplasms with encapsulated or infiltrative features, which differ prognostically and in their respective molecular profiles [7–12]. The infiltrative FVPTCs (I-FVPTC) are associated with a higher risk of recurrence, frequent lymph node metastases, and mutations in BRAFV600E, whereas one-third of encapsulated FVPTC (E-FVPTC) especially those lacking any invasive characteristics (NI-EFVPTC) do not show lymph node metastases, BRAFV600E mutations and do not recur locally or at distant sites [5]. Nevertheless, NI-EFVPTC continued to be diagnosed as “carcinoma” resulting in psychosocial stigmata, over-treatment such as total thyroidectomy, and radioactive iodine (RAI) [4, 6–14].
The Endocrine Pathology Society working group (ESP-WG) reviewed a large series (n = 268) of NI-EFVPTCs and concluded that the absence of invasion was associated with indolent biologic behavior, similar to follicular adenomas, even when patients were treated conservatively with thyroid lobectomy or without RAI [10]. Thus, the ESP-WG proposed a new diagnostic term, the “noninvasive follicular thyroid neoplasm with papillary like nuclear features” (NIFTP), which underscores the key features of this indolent thyroid neoplasm. The reclassification of NI-EFVPTC as NIFTP had important implications for the cytological interpretation of thyroid nodules as it significantly impacts the risk of malignancy (ROM) for the diagnostic categories of The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) [12–18].
This review highlights the clinicopathological specifics of NIFTP and discusses the recently proposed revisions of diagnostic criteria for NIFTP [19] as well as the impact on patient management.
Methods
A literature search was performed to identify studies published in English that analyzed the rate of NIFTP with surgical follow-up since its introduction in April 2016 [10]. The following resources were employed for systematic data collection: the electronic databases from PubMed, Scopus, and Web of Science. Searches used the keywords [Thyroid] AND [Carcinoma, neoplasm, cancer or nodule] AND [NIFTP] AND [follicular variant of PTC]. Evaluation of various journal websites and references of previous review articles were also employed.
Results
Of 118 retrieved references, 42 were retained and their full text was assessed. The majority of the studies included retrospective series. Included studies were published by authors from the USA, Canada, Europe, and Asia. The majority of these studies reported cases of histologically proven NIFTP. Extracted data included: (i) number of thyroid carcinoma cases; (ii) number of PTC, FVPTC, I-FVPTC, and NIFTP cases with follow-up; and (iii) number of NIFTP cases with ≤ 1% papillae. An additional search for the recent revision of NIFTP morphological criteria was conducted using PubMed.
Discussion
Pathology
For the past four decades, nuclear features of thyroid follicular cells have been considered the most reliable morphological criteria to diagnose PTC [20–26]. In fact, these nuclear features in thyroid lesions trump the architectural features such as the presence or absence of a tumor capsule, invasive characteristics, or papillary architecture. The FVPTC was described in the mid-1970s as a tumor with a predominant follicular growth pattern and concomitant nuclear features of PTC. Following this, two subtypes of FVPTC were recognized: infiltrative (I-FVPTC) and encapsulated (E-FVPTC) forms; the latter can be invasive or non-invasive [24–29]. While I-FVPTC frequently metastasizes to cervical lymph nodes similar to classical PTC, E-FVPTC behaves in a more indolent fashion especially when there is no capsular or vascular invasion (NI-EFVPTC). The variable morphology and clinical course of these subtypes of PTC caused controversies in the diagnosis and management of E-FVPTC, especially for NI-EFVPTC. The confusion was accentuated by a lack of uniform criteria to diagnose E-FVPTC even among endocrine pathology experts. Several publications, including some with lengthy clinical follow-up, emphasized that NI-EFVPTC behaved in a benign manner and that the majority of these cases were overtreated. This argument was further strengthened by the knowledge provided by molecular profiling which showed that I-FVPTC and E-FVPTC (including both invasive and non-invasive entities) have a unique set of gene mutations and fusions. In order to resolve this controversy, the ESP-WG recommended that NI-EFVPTC be re-defined as a neoplasm rather than carcinoma and consequently be termed NIFTP [10]. This was intended to lessen the clinical and psychological implication related to the over-diagnosis of this lesion as “cancer,” as well as reducing comorbidity associated with overtreatment.
Clinical Presentation
NIFTP presents similarly to most thyroid nodules that are detected on physical examination or by imaging. Ultrasound findings are typically those of a well-circumscribed oval/ round nodule that is hypervascular with a hypoechoic rim, compared to I-FVPTC which have more irregular or lobulated margins and are mostly hypervascular [Fig. 1a; [30]. The ultrasound of I-FVPTC also shows at least one suspicious feature for malignancy including marked hypoechogenicity, microcalcifications, blurred margins, “taller than wide” nodule, and a mostly avascular color Doppler pattern [30].
Fig. 1.
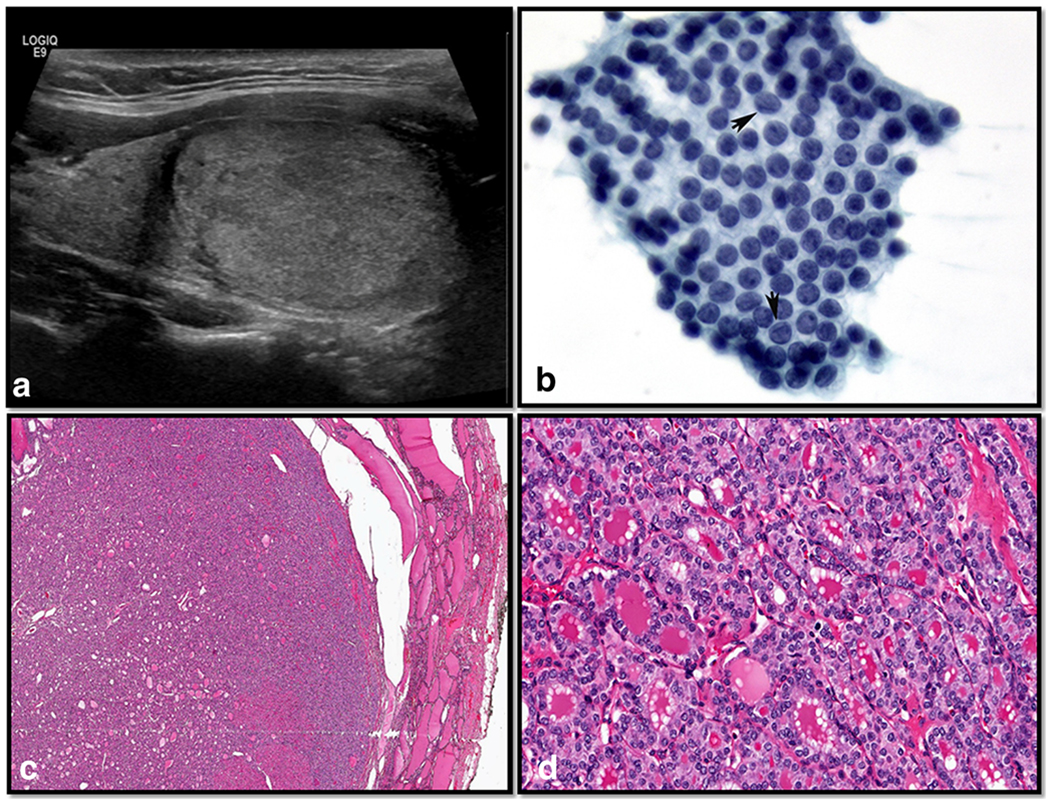
Ultrasonographic, cytomorphologic, and histopathologic features of a case of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). a The ultrasound examination showed a solid isoechoic nodule; cytological smear stained with Papanicolaou stain shows a monolayer sheets of follicular cells containing elongated nuclei with nuclear chromatin pallor, intranuclear grooves (arrows), and eccentrically placed nucleoli. b The case was diagnosed as suspicious for malignancy based on the morphological features (conventional Pap smear, × 400). c The histopathologic evaluation demonstrated a well-demarcated follicular-patterned nodule without invasive characteristics (H&E × 200), and d cells lining the follicles show nuclear features diagnostic of papillary thyroid carcinoma (H&E × 400)
Since the introduction of NIFTP, several studies have provided insight into the impact of this new terminology on the interpretation of thyroid fine needle aspiration (FNA) [9, 12–18, 31–35]. If NIFTP was classified as a non-malignant lesion, the risk of malignancy (ROM) in each diagnostic category of TBSRTC would be reduced, particularly for nodules classified as indeterminate [9, 12–18]. Several studies compared the cytological features of NIFTP to classical PTC and/or FVPTC [9, 12–18, 31–35]. These studies confirmed that the majority ofNIFTP cases belong to TBSRTC categories III, IV, and V (Fig. 1b), and that three cytomorphological features (namely microfollicular pattern, lack of papillary structures, and intranuclear pseudoinclusions) are helpful to predict a possible NIFTP diagnosis on a subsequent surgical pathology specimen (Fig. 1b). Unfortunately, these cytomorphological features cannot reliably distinguish NIFTP from FVPTC, and at present, the majority of NIFTP cases are frequently diagnosed as indeterminate or suspicious for malignancy [Fig. 1b; [36–40].
Recently, some authors have reported NIFTP cases that were associated with loco-regional micrometastases, as well as BRAFV600E mutation and/or BRAF-like mutation (RET/PTC rearrangement) or high-risk TERT promoter mutations [19, 24–29]. Hence, substituting the criterion of “less than 1% papillary structures” with “no well-formed papillary structures” has been proposed, along with pathologic examination of the entire nodule, especially in the presence of florid nuclear features of PTC (nuclear score 3) by a subset of the original consensus group. A comparison between the initial diagnostic criteria and current revised criteria is displayed in Table 1.
Table 1.
Comparison of NIFTP diagnostic criteria before and after proposed revisions
| Original diagnostic criteria10 | Revised diagnostic criteria19 | |
|---|---|---|
| Encapsulation or clear demarcation | ||
| X | X | |
| Follicular growth pattern | X | X |
| < 1% papillary structures | X | |
| No well-formed papillae | X | X |
| Nuclear features of PTC | X | X |
| No psammoma bodies | X | X |
| Nuclear features | X | X |
| Score 2–3 | X | X |
| Nuclear elongation/grooves/chromatin clearing | X | X |
| < 30% solid/trabecular/insular growth pattern | X | X |
| No vascular and capsular invasion | X | X |
| No tumor necrosis | X | X |
| No high mitotic activity | X | X |
| Lack of BRAFV600E mutation with IHC or molecular testing | X | X |
| Lack of BRAF-like mutations or other high-risk mutations (TERT and TP53) | X | X |
PTC, papillary thyroid carcinoma; IHC, immunohistochemistry; X, inclusion criteria
Partially adapted from a table by Rossi ED and Faquin WC in Cancer Cytopathology 2018 [41]
Assessment and Diagnosis
As reported by Nikiforov et al. in the seminal paper describing NIFTP, the histological diagnosis of NIFTP is rendered by strictly adhering to inclusion and exclusion morphological criteria [10]. The key parameters include nuclear cytology of PTC (i.e., nuclear membrane irregularities, ground glass appearance of nuclei, and large nuclear size) in the setting of a non-invasive follicular-patterned tumor (Fig. 1c, d). The presence of an exclusive follicular growth pattern and lack of any invasive characteristics is of paramount importance in the diagnosis of NIFTP. Complete histological evaluation of the entire tumor capsule and careful examination to exclude any papillary architecture tops the list of inclusion criteria. While the original paper by Nikiforov et al. advocated the presence of < 1% papillary structures, a recent study by Cho et al. demonstrated that even the presence of 1% papillae can be associated with lymph node metastases [27]. Therefore, in a follow-up review by a subset of the NIFTP working group, a recommendation was made that no papillary structures should be allowed in thyroid nodules diagnosed as NIFTP [19]. Concerning papillary structures and their definition, the detection of well-formed papillary structures thus cannot be present to make a definitive diagnosis of NIFTP. A papillary structure is a thin, delicate fibrovascular core covered by neoplastic thyroid follicular epithelial cells that show the nuclear features of papillary carcinoma. Abortive papillae do not contain a fibrovascular core and are just stacked or piled up nuclei. Follicles within a fibrovascular core space are also not true papillary structures. A papillary structure should also have at least three cells on each side surrounding a fibrovascular core. While this may be arbitrary, only 1 or 2 cells on either side are more abortive than true papillae. While papillary structures can be sought at a × 20 magnification, careful high-power examination is also required.
Concerning the nuclear features of PTC, Nikiforov et al. proposed the presence of six features to be grouped into three categories including: (1) size and shape (nuclear enlargement/overlapping/crowding); (2) nuclear membrane irregularities (irregular contours), intranuclear grooves, and pseudo-inclusions; and (3) chromatin characteristics (clearing with margination/glassy nuclei). These nuclear features can be focal or diffuse with some gradation permitted in different areas of the lesion [10]. These features are to be analyzed employing a 3-point scoring scheme in which each class of nuclear features receives a score of 0 or 1, yielding a range from 0 to 3. This scoring scheme demonstrated high sensitivity (98.6%), specificity (90.1%), and diagnostic accuracy (94.3%). The more recently revised criteria emphasize that NIFTP usually exhibits a score of 2 (moderately expressed nuclear features of PTC) and that in cases of NIFTP with a nuclear score of 3, the entire tumor should be evaluated in order to exclude the presence of papillary structures [19]. The working group still maintained the following exclusion criteria: (1) presence of psammoma bodies, (2) tumor necrosis or high mitotic activity, and (3) presence of any lymphovascular invasion and/or lymph node metastases.
Albeit limited, so far, several specific genetic alterations have been associated with NIFTP (Table 2). In many NIFTP cases, a RAS mutation is the most typical genetic alteration detected, similar to those found in follicular adenomas and follicular carcinomas. Of note, Howitt et al. [8] found RAS mutations in 46% of E-FVPTC while Rivera et al. [42] found that 0% of E-FVPTC and 26% of I-FVPTC have a BRAF mutation. Zhao et al., comparing NIFTP with I-FVPTC, reported one case of NIFTP with a BRAFV600E mutation whereas 36% of I-FVPTC were BRAF mutated [18]. BRAFV600E is a prototypic mutation found in PTC and is not expected to be seen in encapsulated follicular-patterned neoplasms, including NIFTP. The majority of studies discussing the genetic alterations in NIFTP demonstrated that BRAFV600E was wild type. Nonetheless, 5% of NIFTP harbor a BRAFK601E mutation having a different signaling and gene expression signature, which is closer to that of RAS mutation. However, single cases of mutated BRAFV600E have been described suggesting that the discrepancy might be due to inappropriate application of the diagnostic criteria for NIFTP. It remains to be determined if BRAFV600E represents an exclusion criterion. Other point mutations and gene fusions have also been linked with NIFTP. EIF1AX mutations are found in 5 to 10% of NIFTP and frequently coexist with RAS mutations. Also, mutations affecting the PTEN gene can be found in 5% of NIFTP. Young patients with NIFTP also show DICER1 mutations in 5% of cases. A higher percentage of NIFTP (20 to 30%) may show either PAX8/PPARG fusion or THADA fusion. According to the recent revision of NIFTP, the application of molecular testing can be used as a secondary criterion (Table 2 and Fig. 2).
Table 2.
Overview of the most frequent molecular changes seen in NIFTP compared to FA, PTC, E-FVPTC, and I-FVPTC
| Testing method | FA | NIFTP | cPTC | E-FVPTC | I-FVPTC |
|---|---|---|---|---|---|
| Immunohistochemistry | |||||
| HBME-1 | − | + | ++ | +/++ | ++ |
| GALECTIN-3 | − | + | ++ | +/++ | ++ |
| CD56 | +/++ | − | − | − | − |
| Molecular testing | |||||
| BRAFV600E | − | +* | ++ | + | + |
| BRAFK601E | + | + | + | ++ | + |
| NRAS | ++ | ++ | + | ++ | ++ |
| HRAS | ++ | ++ | + | ++ | + |
| KRAS | ++ | + | + | ++ | + |
| PTEN | ++ | − | + | − | − |
| TERT | − | +* | + | + | + |
| RET/PTC | − | +* | ++ | + | + |
| PAX8/PPARγ | + | ++ | ++ | ++ | ++ |
| ALK fusion | − | − | + | − | − |
| BRAF fusion | − | − | + | − | − |
| ETV6/NTRK3 | − | − | ++ | − | − |
| NTRK1/3 fusion | − | − | + | − | − |
| GEC/GSC | Mostly benign | Mostly suspicious | Frequently suspicious | Frequently suspicious | Frequently suspicious |
FA, follicular adenoma; NIFTP, non-invasive follicular thyroid neoplasm with papillary-like nuclei; cPTC, classical variant of PTC; E-FVPTC, encapsulated follicular variant of PTC; I-FVPTC, infiltrative follicular variant of PTC; GEC/GSC, gene expression and gene sequencing classifier;
Sometimes present
Commonly present
Absent
The detection of these genetic alterations should trigger an exhaustive search for invasive features and papillary formations
Fig. 2.
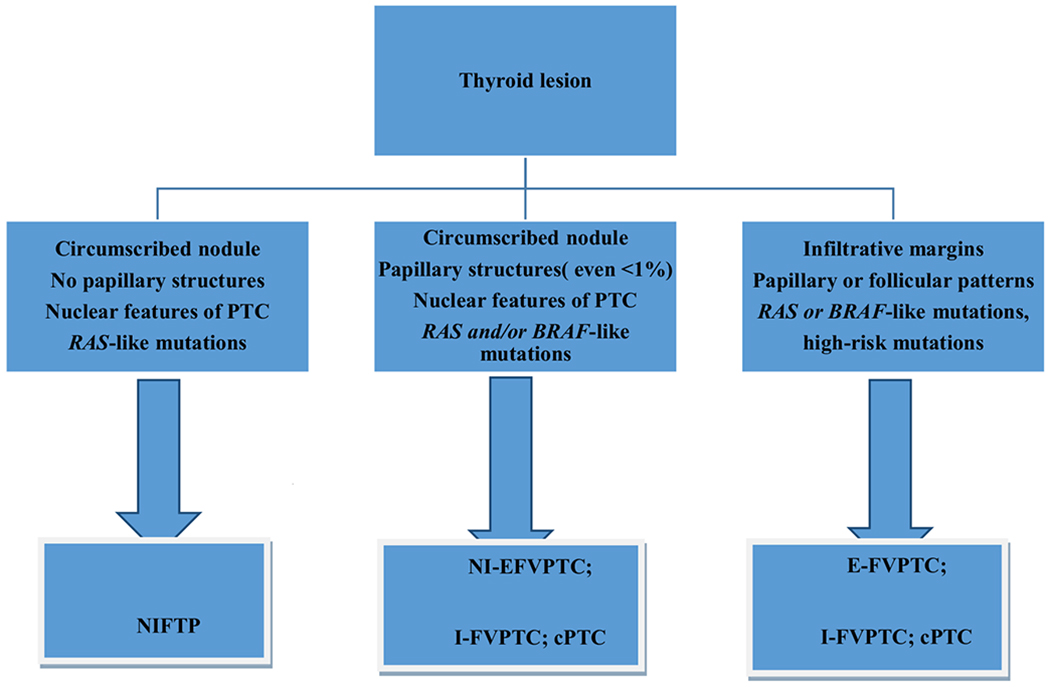
Diagnostic algorithm approach for NIFTP using revised morphological criteria. Legend: cPTC, classic variant of papillary thyroid carcinoma; NI-FVPTC, noninvasive-encapsulated follicular variant of PTC; E-FVPTC, encapsulated follicular variant of PTC; I-FVPTC, infiltrative follicular variant of PTC
Treatment
Few studies have evaluated the treatment and clinical outcome of NIFTP. The majority of these publications emphasized the indolent outcome in E-FVPTC [41, 43–49]. Ghossein et al. reported in their series that all 78 patients with E-FVPTC were alive and free of recurrence after 11 years compared with 6% of individuals with I-FVPTC [9]. The pivotal paper by Nikiforov et al. confirmed that none of the 109 patients diagnosed with NIFTP had any disease recurrence at follow-up after 10–26 years, while 12% with I-FVPTC experienced recurrences [10]. The introduction of NIFTP accordingly supported conservative management (i.e., lobectomy alone) as well as avoiding any subsequent radioactive iodine therapy [41, 50–52]. The American Thyroid Association (ATA) strongly suggests that well-differentiated thyroid cancers are followed-up by means of serial serum thyroglobulin measurements, serum thyroid stimulating hormone (in patients receiving thyroid hormone therapy), and neck ultrasound [20]. This approach has been adopted for NIFTP [50–54]55]. With NIFTP now associated with the lowest point in the “risk stratification” of thyroid neoplasms, there are consequently fewer emotional and financial consequences to patients with this diagnosis.
Prognosis
As underlined in the original study and endorsed by different publications and Endocrine Societies, NIFTP is defined as a tumor with indolent behavior and an excellent prognosis [19]. Since its introduction, authors such as Cho et al. have reported a few cases presenting with recurrences and/or distant metastases [27]. In those cases, strict adherence to the diagnostic criteria may not have been used. To date, there remain several issues that have yet to be answered such as how to manage subcentimeter lesions, lesions larger than 4 cm, and lesions with an oncocytic component that fulfill diagnostic criteria of NIFTP [25]. Of note, E-FVPTC with oncocytic features seems to at least share the same favorable clinical course as their non-oncocytic counterparts.
Conclusions
The introduction of NIFTP was an essential step to address a subset of low-risk thyroid neoplasms that are overtreated. Acceptance of NIFTP as a non-malignant lesion will spare patients from the psychological impact being diagnosed with a “malignancy” as well as additional surgical and clinical treatment and follow-up. The emerging literature indicates that the adoption of strict inclusion and exclusion criteria is essential to correctly diagnose NIFTP. This has led to a revision of the morphological criteria and is a testimony to the fact that embracing NIFTP as a new entity is only the first step toward a more evidence-based approach to managing thyroid neoplasms (Fig. 2). Despite the fact that NIFTP is currently based on a histological diagnosis, we await reliable criteria that might lead to a diagnosis of NIFTP on cytological specimens [53].
Footnotes
Conflict of Interest The authors declare that they have no conflict of interests.
References
- 1.Carling T, Udelsman R. Thyroid tumors In: DeVita VT Jr, Lawrence TS, Rosenberg SA. Cancer: Principles and practice of oncology. 9th ed Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, p. 1457–1472. [Google Scholar]
- 2.Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM (2010) Analysis of the rising incidence of thyroid cancer using the surveillance, epidemiology and end results national cancer data registry. Surgery 148:1147–1153. [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG (2006) Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 295:2164–2167. [DOI] [PubMed] [Google Scholar]
- 4.Vivero M, Kraft S, Barletta JA (2013) Risk stratification of follicular variant of papillary thyroid carcinoma. Thyroid 23 (3): 273–279 [DOI] [PubMed] [Google Scholar]
- 5.Welch HG, Doherthy GM (2018) Saving thyroid-overtreatment of small papillary cancers. N Engl J Med 379 (4): 310–312 [DOI] [PubMed] [Google Scholar]
- 6.Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, Chan JK, DeLellis RA, Harach HR, Kakudo K, LiVolsi VA, Rosai J, Sebo TJ, Sobrinho-Simoes M, Wenig BM, Lae ME (2004) Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol 28 (10):1336–1340 [DOI] [PubMed] [Google Scholar]
- 7.Baloch Z, LiVolsi VA, Henricks WH, Sebak BA (2002) Encapsulated follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 118 (4):603–605 [PubMed] [Google Scholar]
- 8.Howitt BE, Chang S, Eslinger M, Paschke R, Drage MG, Krane JF, Barletta JA (2015) Fine-needle aspiration diagnoses of noninvasive follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 144 (6): 850–857 [DOI] [PubMed] [Google Scholar]
- 9.Ganly I, Wang L, Tuttle MR, Katabi N, Ceballos GA, Harach HR, Ghossein R.(2015) Invasion rather than nuclear features correlates with outcome in encapsulated follicular tumors: further evidence for the reclassification of the encapsulated papillary thyroid carcinoma follicular variant. Hum Pathol 46 (5): 657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LDR, Barletta JA, Wenig BM, al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, el-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nosé V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle RM, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA (2016). Nomenclature revision for encapsulated follicular variant fo papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2 (8): 1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson LD (2016) Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: a namechange to noninvasive follicular thyroid neoplasm with papillary like nuclear features would help to prevent overtreatment. Mod Pathol 29 (7): 698–707 [DOI] [PubMed] [Google Scholar]
- 12.Bizzarro T, Martini M, Capodimonti SStraccia P, Lombardi CP, Pontecorvi A, Larocca LM, Rossi ED (2016) The morphologic analysis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) on liquid based cytology: Some insights of their identification in our institutional experience. Cancer Cytopathol 124 (10):699–710 [DOI] [PubMed] [Google Scholar]
- 13.Faquin W, Wong L, Afrogheh A, Ali SZ, Bishop JA, Bongiovanni M, Pusztaszeri MP, VandenBussche CJ, Gourmaud J, Vaickus LJ, Baloch ZW (2016) Impact of reclassifying non invasive FVPC on the risk of malignancy in the Bethesda system for reporting thyroid Cytopathology. Cancer Cytopathol 124 (3):181–187 [DOI] [PubMed] [Google Scholar]
- 14.Strickland KC, Howitt BE, Marquesee E, Alexander EK, Cibas ES, Krane JF, Barletta JA (2015) The impact of noninvasive follicular variant of papillary thyroid carcinoma on rates of malignancy from fine needle aspiration diagnostic categories. Thyroid 25 (9): 987–992 [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim A, La Fortune KA, Wu H (2016) Fine needle aspiration cytology of noninvasive follicular thyroid neoplasm with papillary like nuclear features (NIFT), single institutional study with comparison to invasive follicular variant of papillary thyroid carcinoma. Mod Pathol SS 29; 86–125 [Google Scholar]
- 16.Maletta F, Massa F, Torregorssa L, Duregon E, Casadei GP, Basolo F, Tallini G, Volante M, Nikiforov YE, Papotti M (2016) Cytological features of “non-invasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum Pathol 54:134–142 [DOI] [PubMed] [Google Scholar]
- 17.Pusztaszeri M, Triponez F, Meyer P,Sadowski SM (2017) Non Invasive follicular thyrid neoplasm with papillary like nuclear features (NIFTP): report of an institutional experience with 86 cases. J Basic Clin Med 6 (1): 29–35 [Google Scholar]
- 18.Zhao L, Dias-Santagata D, Sadow PM, Faquin WC (2017) Cytological, molecular, and clinical features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features versus invasive forms of follicular variant of papillary thyroid carcinoma. Cancer Cytopathol 125 (5): 323–331 [DOI] [PubMed] [Google Scholar]
- 19.Nikiforov Y, Baloch ZW, Hodak SP, Giordano TJ, Lloyd RV, Seethala RR, Wenig BM (2018) Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillary like nuclear features. Jama Oncol 14. doi: 10.1001/jamaoncol.2018.1446 4, 1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P, Doherty GM, Mandel SJ, Morris JC, Nassar A, Pacini F, Schlumberger M, Schuff K, Sherman SI, Somerset H, Sosa JA, Steward DL, Wartofsky L, Williams MD (2017) American thyroid association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary like nuclear features. Thyroid 27 (4): 481–483 [DOI] [PubMed] [Google Scholar]
- 21.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L (2016) 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26 (1): 1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baloch ZW, Seethala RR, Faquin WC, Papotti MG, Basolo F, Fadda G, Randolph GW, Hodak SP, Nikiforov YE, Mandel SJ (2016) Noninvasive follicular thyroid neoplasm with papillary like nuclear features (NIFTP): a changing paradigm in thyroid surgical pathology and implications for thyroid cytopathology. Cancer Cytopathol 124 (6):616–620 [DOI] [PubMed] [Google Scholar]
- 23.LiVolsi VA, Baloch ZA (2017) Coming to terms with diagnosis noninvasive follicular thyroid neoplasm with papillary like nuclear features (NIFTP): practice changer in endocrine pathology. J Basic Clin Med 6 (1):8–13 [Google Scholar]
- 24.Rosario PW Mourao GF, Nunes MB, Nunes MS, Calsolari MR (2016) Noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Endocr Relat Cancer 23 (12): 893–897 [DOI] [PubMed] [Google Scholar]
- 25.Xu B, Tallini G, Scognamiglio T, Roman BR, Tuttle RM, Ghossein RA (2017) Outcome of large noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid 27 (4): 512–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parente DN, Kluijghout WP, Bongers PJ, Verzijl R, Devon KM, Rotstein LE, Goldstein DP, Asa SL, Mete O, Pasternak JD (2018) Clinical safety of renaming encapsulated follicular variant of papillary thyroid carcinoma. Is NIFTP truly benign? World J Surg 42 (2):321–326 [DOI] [PubMed] [Google Scholar]
- 27.Cho U, Mete O, Kim MH, Bae JS, Jung CK (2017) Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: The impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol 30 (6): 810–825 [DOI] [PubMed] [Google Scholar]
- 28.Alves VAF, Kakudo K, LiVolsi V, Lloyd RV, Nikiforov YE, Nose V, Papotti M, Thompson LDR (2018) Noninvasive Follicular Thyroid Neoplasm With Papillary-Like Nuclear Features (NIFTP): Achieving Better Agreement By Refining Diagnostic Criteria. Clinics (Sao Paulo) 73:e576. doi: 10.6061/clinics/2018/e576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakudo K, El-Naggar AK, Hodak SP, Khanafshar E, Nikiforov YE, Nosé V, Thompson LDR (2018) Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in thyroid tumor classification. Pathol Int 68 (6): 327–333. [DOI] [PubMed] [Google Scholar]
- 30.Yang GCH, Fried KO, Scognamiglio T (2017) Sonographic and Cytological Differences of NIFTP from infiltrative or invasive encapsulated follicular variant of papillary thryoid carcinoma: A review of 179 cases. Diagn Cytopathol 45 (6): 533–541 [DOI] [PubMed] [Google Scholar]
- 31.Zhou H, Baloch ZW, Nayar R, Bizzarro T, Fadda G, Adhikari-Guragain D, Hatem J, Larocca LM, Samolczyk J, Slade J, Rossi ED (2018) Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): Implications for the risk of malignancy (ROM) in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). Cancer 126 (1): 20–26 [DOI] [PubMed] [Google Scholar]
- 32.Layfield LJ, Baloch ZW, Esebua M, Kannuswamy R, Schmidt RL (2017) Impact of the Reclassification of the Non-Invasive Follicular Variant of Papillary Carcinoma as Benign on the Malignancy Risk of the Bethesda System for Reporting Thyroid Cytopathology: A Meta-Analysis Study Acta Cytol 61 ( 3): 187–193. [DOI] [PubMed] [Google Scholar]
- 33.Shahi M, Yousaf H, Amin K, Li F (2017) Impact of New Nomenclature “Non-Invasive Follicular Neoplasm with Papillary Like Nuclear Features” on the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). An Institutional Experience. Mod Pathol 30:116A. [Google Scholar]
- 34.Nishino M (2016) Molecular cytopathology for thyroid nodules: A review of methodology and test performance. Cancer Cytopathol 124 (1): 14–27 [DOI] [PubMed] [Google Scholar]
- 35.Song SJ, LiVolsi VA, Montone K, Baloch Z (2017) Pre-operative features of non-invasive follicular thyroid neoplasms with papillary-like nuclear features: An analysis of their cytological, Gene Expression Classifier and sonographic findings. Cytopathol l28 (6):488–494. [DOI] [PubMed] [Google Scholar]
- 36.Basolo F, macerola E, Ugolini C, Poller DN,Baloch Z (2017) The molecular landscape of non invasive follicular thyroid neoplasm with papillary like-nuclear features (NIFTP): a literature review. Adv Anat Pathol 24 (5): 252–258 [DOI] [PubMed] [Google Scholar]
- 37.Kim TH, Lee M, Kwon AY, Choe JH, Kim JH, Kim JS, Hahn SY, Shin JH, Chung MK, Son YI, Ki CS, Yim HS, Kim YL, Chung JH, Kim SW, Oh YL (2017)Molecular genotyping of the non-invasive encapsulated follicular variant of papillary thyroid carcinoma. Histopathology. 72 (4): 648–661 [DOI] [PubMed] [Google Scholar]
- 38.Jiang XS, Harrison GP, Datto MB (2016) Young investigator challenge: Molecular testing in noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Cancer Cytopathol 124 (12): 893–900 [DOI] [PubMed] [Google Scholar]
- 39.Krane JF, Alexander EK, Cibas ES, Barletta JA (2016) Coming to terms with NIFTP. A provisional approach for cytologists. Cancer Cytopathol 124 (10): 767–772 [DOI] [PubMed] [Google Scholar]
- 40.Bongiovanni M, Giovannella L, Romanelli, Trimboli P (2018) Cytological Diagnoses Associated with Noninvasive Follicular Thyroid Neoplasms with Papillary-Like Nuclear Features According to the Bethesda System for Reporting Thyroid Cytopathology: A Systematic Review and Meta-Analysis. Thyroid. 21. doi: 10.1089/thy.2018.0394. [Epub ahead of print, 29, 222, 228 [DOI] [PubMed] [Google Scholar]
- 41.Rosario PW, Cancela Penna G, Calsolari MR (2014) Noninvasive encapsulated follicular variant of papillary thyroid carcinoma: is lobectomy sufficient for tumours ≥1 cm? Clin Endocrinol 81 (4): 630–632 [DOI] [PubMed] [Google Scholar]
- 42.Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, Ghossein RA. (2010) Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated versus infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol 23 (9): 1191–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H, Izevbaye I, Chen F, Weinstein B (2012) Recent advances in follicular variant of papillary thyroid carcinoma. N A J Med Sci 5 (1): 212–16 [Google Scholar]
- 44.Chan J (2002) Strict criteria should be applied in the diagnosis of encapsulated follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 117 (1): 16–18 [DOI] [PubMed] [Google Scholar]
- 45.Liu J Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, Tuttle RM, Ghossein RA (2006) Follicular variant of papillary thyroid carcinoma: A clinicopathologic study of a problematic entity. Cancer 107 (6): 1255–1261 [DOI] [PubMed] [Google Scholar]
- 46.Renshaw AA, Gould EW (2002) Why there is the tendency to overdiagnose the follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 117 (1): 19–21 [DOI] [PubMed] [Google Scholar]
- 47.Piana S, Frasoldati A, Di Felice E, Gardini G, Tallini G, Rosai J (2010) Encapsulated well-differentiated follicular patterned thyroid carcinoma do not play a significant role in the fatality rate from thyroid carcinoma. Am J Surg Pathol 34 (6): 868–872 [DOI] [PubMed] [Google Scholar]
- 48.Xing M (2007) Braf mutation in papillary thyroid cancer:pathogenic role,molecular bases,and clinical implications. Endocr Rev 28 (7):742–762. [DOI] [PubMed] [Google Scholar]
- 49.Puxeddu E, Durante C, Avenia N, Filetti S, Russo D (2008) Clinical implications of BRAF mutation in thyroid carcinoma. Trends Endocrinol Metab 19 (4):138–145. [DOI] [PubMed] [Google Scholar]
- 50.Seethala RR, Baloch ZW, Barletta JA, Khanafshar E, Mete O, Sadow PM, LiVolsi VA, Nikiforov YE, Tallini G, Thompson LD (2018) Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a review for pathologists, Mod Pathol 31 (1): 3955. [DOI] [PubMed] [Google Scholar]
- 51.Agrawal N, Abbott CE, Liu C, Kang S, Tipton L, Patel K, Persky M, King L, Deng FM, Bannan M, Ogilvie JB, Heller K, Hodak SP (2017) Noninvasive follicular tumor with papillary-like nuclear features: not a tempest in a teapot. Endocr Pract 23 (4): 451–457 [DOI] [PubMed] [Google Scholar]
- 52.Ferris RL, Nikiforov Y, Terris D, Seethala RR, Ridge JA, Angelos P, Duh QY, Wong R, Sabra MM, Fagin JA, McIver B, Bernet VJ, Harrell RM, Busaidy N, Cibas ES, Faquin WC, Sadow P, Baloch Z, Shindo M, Orloff L, Davies L, Randolph GW (2018) AHNS Series: Do you know your guidelines? AHNS Endocrine Section Consensus Statement: State-of-the-art thyroid surgical recommendations in the era of noninvasive follicular thyroid neoplasm with papillary-like nuclear features Head Neck doi: 10.1002/hed.25141 40, 1881, 1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossi ED, Faquin WC (2018) NIFTP revised: Chronicle of a change foretold. Cancer Cytopathol 126: 897–901 [DOI] [PubMed] [Google Scholar]
- 54.Tallini G, Tuttle RM, Ghossein RA (2017) The history of the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab 102 (1): 15–22 [DOI] [PubMed] [Google Scholar]


