Abstract
Background:
The distress thermometer and problem list (DT&PL) is a recommended screening measure but the utility of the physical problem list (PPL) has not been evaluated in patients with metastatic lung cancer who typically have high rates of both physical and psychological symptoms. We hypothesized that the PPL will provide an accurate representation of lung cancer symptoms and be associated with concomitant distress, anxiety, depression, and worsened survival.
Methods:
Stage IV lung cancer patients (n = 116) reported physical symptoms from 22 PPL variables and completed the DT&PL for distress, general anxiety disorder-7 for anxiety, and Patient Health Questionnaire 9 for depression. Inferential analyses were controlled for demographic and clinical characteristics.
Results:
The average number of physical problems was 4.7 (SD = 3.8) while the median was 3.0. Fatigue, sleep, pain, and breathing problems were most common. Physical symptom burden was associated with nonmarried/partnered status (P = .003) and depression (P < .001) on multivariate analysis accounting for 43% of physical symptom burden variance. Greater number of physical symptoms and lower BMI were associated with worsened survival. Individual physical symptoms were most often associated with depression.
Conclusion:
The PPL of the DT&PL appears to have clinical utility given its associations with the most common lung cancer symptoms, depression, and worsened survival. In addition to its potential role in clinics worldwide already using the DT&PL, physical symptom burden on the DT&PL should trigger a concomitant psychological assessment.
Keywords: cancer, depression, distress screening, distress thermometer and problem list, lung cancer, oncology, physical problem list, physical symptom burden, survival analysis
1 |. INTRODUCTION
Physical symptom burden is highly prevalent in patients with lung cancer and remains an unmet treatment need.1–3 At the same time, psychological symptoms such as depression are also commonly seen in this setting.4,5 While physical symptom burden and depression tend to co-occur, this has not been evaluated specifically in the context of lung cancer using the physical problem list (PPL) of the distress thermometer and problem list (DT&PL).
The DT&PL is recommended by the National Comprehensive Cancer Network (NCCN) for the evaluation of psychological distress symptoms and problems in other domains of life such as practical, familial, emotional, spiritual, and physical. The problem list (PL) of the DT&PL contains 39 separate items to which patients endorse the presence or absence of particular symptoms over the past week, allowing for the identification of patient reported problems across these five domains. The later domain, the PPL contains 22 distinct physical symptom items that can be used as a triaging tool to assign patients to appropriate clinical treatments.
The DT&PL is the most broadly accepted and implemented measure of distress internationally given its convenience and proven acceptability in busy oncology clinics.6 It has been translated into many languages and validated in many settings.7 Therefore, it is being used to not only screen for distress, but also triage patients to appropriate resources based on associated problems. The PL, and specifically the PPL, is meant to be a convenient measure to identify pertinent issues that accompany distress on the DT&PL. The PPL provides readily accessible information about physical symptomatology and can be tracked over time relatively easily. Few studies have tried to determine which physical problems are most salient and related to other cancer factors,8,9 but evidence exists that patient characteristics and psychological symptoms may influence certain physical problems in the setting of cancer.10
Understanding the association between physical and psychological symptoms on the DT&PL in the setting of lung cancer can potentially help many patients with lung cancers who suffer with some of the highest rates of both physical and psychological symptom burden.11,12 It is clinically useful to know the predictive value of these measures and how they relate to other symptoms, especially using the DT&PL as a single tool. It may be an efficient means of capturing distressing physical problems among lung cancer patients across their disease trajectory and is translatable across many oncology clinic settings internationally.7 The prevalence of individual physical symptoms (PPL variables) that are contained in the DT&PL has not been adequately described in the setting of lung cancer. Physical symptoms captured on the DT&PL have not been explored for their associations with psychological symptoms, such as depression, or with patient characteristics or cancer treatments. Treatments recently implemented into standard practice, such as immunotherapy/targeted therapies, may significantly alter this association since these agents are effective at ameliorating symptoms but may lengthen the disease course.
A mounting number of physical symptoms or their intensity is generally associated with shorter life expectancy in oncology. Although elevated distress is not associated with worsened survival,13 physical symptom captured on the PPL of the DT&PL may be associated with worse survival consistent with this known association. Understanding survival associations of patient endorsed physical symptoms would support using the PPL of the DT&PL as an indicator of physical symptom burden.
Specific time points are recommended for distress screening; however, distressing physical symptoms may be present at any time. Physical symptom burden may vary based on patient (demographics), disease (type of lung cancer, length of time with lung cancer), treatment (type of treatment), and associated clinical characteristics (presence of distress, anxiety, or depression).1,14 Co-occurring symptoms (eg, depression or anxiety) and physical symptoms need to be addressed concomitantly in order to adequately address physical symptom burden. It is most helpful to obtain this information from one measure that is already widely in use. Understanding the clinical psychological correlates of physical symptom burden is critical for the effective palliation of lung cancer symptoms.
This study will evaluate self-reported physical symptom burden among lung cancer patients receiving care at an outpatient dedicated lung cancer clinic using the PPL of the DT&PL. It will assess whether physical symptoms identified through the DT&PL accurately reflect the most commonly endorsed physical symptoms in lung cancer patients. It will additionally examine whether physical symptom burdens vary systematically by patient demographic, medical characteristics (eg, lung cancer type), and psychological state (eg, distress, anxiety, or depression). We hypothesized that (1) physical symptom burden (number, as opposed to degree of symptoms) using the PPL of the will be representative of the most commonly endorsed physical symptoms in lung cancer, and (2) the number of physical symptoms will be associated with psychological symptoms (ie, distress, anxiety, depression, and worsened survival). Therefore, physical symptom burden captured on the PPL of the DT&PL may alert clinicians to the possibility and likelihood of additional psychological symptoms and survival implications.
2 |. METHODS AND MATERIALS
The Memorial Sloan Kettering Cancer Center Institutional Review Board (IRB) approved this study MED18-165, “Survey of Routine Markers of Inflammation and Psychological Variables in Patients with Metastatic Lung Cancer” on 15 August 2018. Surveys and lab values were collected from participants from May 2017 to November 2017 as part of standard of care practice.
2.1 |. Participants
Inclusion criteria consisted of a confirmed histologic diagnosis of stage IV lung cancer including non-small cell lung cancer (NSCLC), such as squamous cell carcinoma and nonsquamous cell carcinoma (eg, adenocarcinoma and other), and small cell lung cancer (SCLC) who were undergoing active treatment, spoke English, and had a performance status of Eastern Cooperative Group (ECOG) less than or equal to 2.15 Patients who were not undergoing treatment for their metastatic lung cancer or with other concomitant cancers were excluded. Patients had to be on active treatment for at least 1 month and had to be more than 1 month from receiving the diagnosis of lung cancer to be included.
2.2 |. Procedure
Patients were approached by their treating oncologist or nurse practitioner and asked to fill out a voluntary survey to gather information about their symptoms. Patients filled out the questionnaire containing standardized survey questions either prior to the appointment or during chemotherapy or other treatment infusions. Patients were asked to raise any concerns with clinic staff and to notify a staff member if they felt significantly depressed or had suicidal thoughts. Survey results were reviewed with patients during the same visit. Referrals to available psychological services were provided in the survey.
2.3 |. Measures
2.3.1 |. Patient demographic and medical characteristics
Patient demographic information including age, race/ethnicity, and marital status were gathered from the electronic medical record. Medical information gathered from the medical record included disease type, treatment type, length of time with diagnosis, antidepressant use, and BMI.
2.3.2 |. Physical problems
Patients endorsed whether a physical symptom had been a problem for them over the past week using the PPL on the DT&PL. The PPL contains 22 separate items to which patients endorse the presence of particular symptoms allowing identification of patient reported problems. The DT&PL has been used widely by cancer institutions to meet the Commission on Cancer distress-screening mandate for accreditation in 2015.16,17
2.3.3 |. Distress
Distress thermometer (DT) scores range from 0 (Not at all distressed) to 10 (Extremely distressed) and a cutoff of ≥4 has been accepted by the NCCN to indicate clinically meaningful distress.18 Distress scores were evaluated for their associations with individual PPL variables and with the total number of endorsed PPL variables by patient. The DT&PL (including the DT and PL) has been validated among international cancer populations.7
2.3.4 |. Anxiety
The general anxiety disorder-7 (GAD-7) is a seven-item brief measure used to identify probable cases of generalized anxiety disorder that has been used extensively and validated in the cancer context.19 Patients rate their frequency of symptoms within the last 2 weeks on a 4-point scale. Scores can range from 0 to 21 with higher values indicating greater anxiety symptoms.
2.3.5 |. Depression
The Patient Health Questionnaire 9 (PHQ-9) is a nine-item measure of depression that is self-administered and used to find probable cases of depression.20 It has been validated in cancer settings and has designated cutoff points for grading depression severity.21
In addition, depression was measured by the Hospital Anxiety and Depression Scale (HADS) and provided comparative sensitivity analysis that was used for evaluating depression without questions that refer to physical symptoms. The HADS is a 14-item symptom rating scale that was developed to identify clinically significant cases of anxiety and depressive disorders among medically ill patients without the use of physical symptoms.22 Only the seven-item depression subscale (HADS-D) was used, which has been validated in the lung cancer setting.22,23
2.4 |. Statistical analysis
2.4.1 |. Primary analyses
Demographic and clinical characteristics were compared with number of physical symptoms on the DT&PL using correlational analysis with Pearson correlation coefficients for quasi-normally distributed independent variables and Spearman correlational coefficients for non-parametric analysis. Number of physical symptoms endorsed (dependent variable) was transformed using the square root function to account for a Skewness and Kurtosis. Skewness transformed from 1.206 (SD = 23) to −0.193 and kurtosis transformed from 1.358 (SD = 45) to 0.127. Independent samples t tests and analysis of variance (ANOVA) were used to test for group differences between categorical independent variables. A multivariate linear regression model was created for number of physical symptoms using variables that were statistically significant in univariate analyses and included covariates that were identified a priori based on known associations. To assess the predictive accuracy of number of physical symptoms in identifying clinically significant depressive symptoms, receiver operating characteristic (ROC) curve analysis was used, with the area under the curve (AUC) statistic used to quantify sensitivity and specific across the numerical range of physical symptoms. PHQ-9 score of 10 was used to define clinically significant depression.
2.4.2 |. Secondary analyses
A sensitivity analysis was performed in order to understand the influence of somatic items on the PHQ-9 and endorsing physical symptom burden on the PPL. Two alternate regression models were creating that substituted depression (PHQ-9) with either 1) the PHQ-9 without somatic items (questions 3, 4, 5 & 8) or 2) the HADS-D. A survival analysis was conducted on 14 December 2019 as part of this retrospective analysis and found that 79 patients (out of 116 patients) had died using hospital electronic medical records in addition to the Social Security Death Index. A Cox regression analysis was performed to evaluate covariate associations with survival using censored data. A median split was performed andparticipants were divided into high and low physical symptom burden. These two groups were used to conduct a Kaplan-Meier survival analysis. Statistical procedures were performed using the SPSS version 24 software (SPSS, Chicago, Illinois) and were two-tailed with a 5% significance level.
3 |. RESULTS
3.1 |. Primary analyses
3.1.1 |. Cohort characteristics
Out of 150 potential participants who were asked to fill out survey questionnaires, 116 returned survey information (77.3% response rate). Sample characteristics are presented in Table 1. The average age was 65.5 years old and the majority of the sample was female (65.5%), Non-Hispanic White (86.2%), married (70.7%), and living with lung cancer for 15.4 months on average. Most patients had adenocarcinoma NSCLC (75.0%) and were receiving chemotherapy (40.5%), followed by immunotherapy (29.3%), and targeted biologic therapy (20.7%). Antidepressant medication use was reported among 15.5% of the cohort. The average score for distress was 4.01 (3.1) (DT&PL) with 40.2% meeting screening criteria (DT&PL ≥4), anxiety was 3.91 (SD = 4.9) (GAD-7) with 13.4% meeting screening criteria (GAD-7 ≥ 10), and depression was 6.3 (SD = 5.0) (PHQ-9) with 27.6% meeting screening criteria (PHQ-9 ≥ 10).
TABLE 1.
Study sample characteristics and association with number of endorsed physical symptoms on the physical problem list of the distress thermometer and problem list
| Total (n = 116) M (SD) |
Number of endorsed physical symptoms (PPL) |
||||||
|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
||||||
| r | P | Regression coefficient | B | t value | P | ||
| PPL (DT&PL) (0-22) | 4.7 (3.8) | - | - | - | - | - | - |
| Age (years) | 65.9 (9.3) | .11 | .23 | 0.01 (0.01) | .14 | 1.831 | .07 |
| Body mass index | 25.9 (5.0) | −.01 | .88 | −0.02 (0.02) | −.09 | −1.129 | .26 |
| Time with disease (months) | 15.4 (17.3) | .04 | .67 | ||||
| Distress (DT&PL) (0-10) | 4.01 (3.1) | .42 | <.001*** | 0.03 (0.04) | .09 | .820 | .41 |
| Anxiety (GAD-7) (0-21) | 3.91 (4.4) | .37 | <.001*** | 0.01 (0.02) | .05 | .494 | .62 |
| Depression score (PHQ-9) (0-30) | 6.30 (5.0) | .60 | <.001*** | 0.09 (0.02) | .49 | 5.002 | <.001*** |
| n (%) | F | P | |||||
| Disease type | 1.084 | .40 | |||||
| Adenocarcinoma | 87 (75.0%) | ||||||
| Squamous cell carcinoma | 6 (5.2%) | ||||||
| Small cell lung cancer | 18 (15.5%) | ||||||
| Unspecified | 5 (4.3%) | ||||||
| Treatment type | .141 | .87 | |||||
| Chemotherapy | 47 (40.5%) | ||||||
| Immunotherapy | 34 (29.3%) | ||||||
| Targeted therapy | 24 (20.7%) | ||||||
| Missing | 11 (9.5%) | ||||||
| Line of treatment | 2.453 | .09 | |||||
| First | 56 (53.3%) | ||||||
| Second | 34 (32.4%) | ||||||
| Third or beyond | 15 (14.3%) | ||||||
| Missing | 5 (4.5%) | ||||||
| Sex | t | P | |||||
| Female | 76 (65.5%) | .948 | .35 | −0.01 (0.17) | −.00 | −.054 | .96 |
| Male | 40 (32.5%) | ||||||
| Race/Ethnicity | |||||||
| Nonwhite | 16 (13.8.0%) | .25 | .79 | ||||
| White | 100 (86.2%) | ||||||
| Married | |||||||
| Yes | 82 (70.7%) | 3.959 | <001*** | −0.52 (0.17) | −.25 | −3.022 | .003** |
| No | 33 (29.3%) | ||||||
| Antidepressant | |||||||
| Yes | 18 (15.5%) | −1.022 | .31 | ||||
| No | 98 (84.5%) | ||||||
| F: 11.494 Adjusted R2: 43, P < .001 | |||||||
Abbreviations: DT&P, Distress Thermometer and Problem List; GAD-7, Generalized Anxiety Disorder 7 item; PHQ-9, Patient Health Questionnaire-9.
P < .05;
P < .01;
P < .001.
3.1.2 |. Associations with number of endorsed physical symptoms
The average number of physical symptoms reported was 4.7 (SD = 3.8) with a range from 0 to 18. The most commonly reported physical symptoms were fatigue (65%), sleep (37%), pain (32%), breathing (31%), itchy dry skin (30%), constipation (28%), tingling hands/feet (28%), nose dry/congested (28%), memory/concentration (23%), nausea (23%), getting around (23%), and eating (21%) (Table 2). The median number of physical symptoms was 3.0.
TABLE 2.
Most commonly endorsed physical problem list symptoms and their association with psychological variables (distress, anxiety, and depression) and survival
| N | % | Survival HR | 95% CI | Distress (DT&PL) | Anxiety (GAD-7) | Depression (PHQ-9) | |
|---|---|---|---|---|---|---|---|
| Fatigue | 75 | 65 | 1.497 | 0.27-0.94* | NS | NS | t = 5.48, P < .001*** |
| Sleep | 43 | 37 | 1.131 | 0.50-1.52 | t = 3.13, P = .002** | t = 3.61, P = .001** | t = 4.79, P < .001*** |
| Pain | 37 | 32 | 1.438 | 0.33-0.97* | t = 3.63, P < .001*** | t = 3.30, P = .002** | t = 3.84, P < .001*** |
| Breathing | 36 | 31 | 1.415 | 0.34-1.02 | NS | NS | t = 2.79, P = .006** |
| Skin dry/itchy | 35 | 30 | 1.085 | 0.51-1.63 | t = 3.05, P = .003** | t = 2.04, P = .05* | t = 2.75, P = .009** |
| Constipation | 33 | 28 | 1.377 | 0.36-1.08 | t = 2.78, P = .008** | t = 2.19, P = .02* | t = 3.04, P = .004** |
| Tingling hands/feet | 32 | 28 | 1.313 | 0.39-1.22 | NS | NS | t = 2.11, P = .04* |
| Nose dry/congested | 32 | 28 | 1.282 | 0.41-1.26 | NS | NS | t = 2.56, P = .02* |
| Memory/concentration | 27 | 23 | 1.078 | 0.50-1.70 | t = 2.39, P = .02* | t = 3.36, P = .002** | t = 4.58, P < .001*** |
| Nausea | 27 | 23 | 1.305 | 0.37-1.32 | t = 2.53, P =.01* | NS | t = 2.34, P = .02* |
| Getting around | 27 | 23 | 1.407 | 0.34-1.04 | t = 2.50, P = .01* | NS | t = 3.10, P = .004** |
| Eating | 24 | 21 | 1.469 | 0.30-.95* | t = 3.58, P = .002 | NS | t = 4.61, P < .001*** |
Abbreviations: DT&P, Distress Thermometer and Problem List; GAD-7, Generalized Anxiety Disorder 7 item; PHQ-9, Patient Health Questionnaire-9.
P < .05;
P < .01;
P < .001.
Overall, 90.5% of patients reported at least one PPL physical symptom. An increasing number of physical symptoms reported was associated with unmarried/nonpartnered status (P < .001). The average number of physical complaints was 6.8 (SD = 4.3) for unmarried/partnered patients and 3.8 (SD = 3.1) for married/partnered patients. Physical symptom burden (number of physical complaints) was associated with elevated distress (P < .001), anxiety (P < .001), and depression (P < .001) (Table 1). Number of physical symptoms endorsed was not significantly correlated with other treatment or demographic variables such as treatment type (immunotherapy, targeted therapies), disease type, line of treatment, sex, or race.
Multivariate regression analysis revealed that depression (P < .001) and nonmarried/partnered status (P = .003) were associated with a higher number of physical symptoms and accounted for a 43% variance in the number of physical symptom complaints (adjusted R2 = .43) (Table 1).
Classification analyses, using ROC analysis was used to identify the optimal cut point for identifying a clinically significant number of physical symptoms with depression. A PHQ-9 score of 10 to indicate depression was used to determine the optimal cut point for maximizing sensitivity and specificity. This analysis suggested that four or more physical symptoms on the PPL of the DT&PL provided the optimal discrimination between those with and without depression, with sensitivity of 75% and specificity of 70%. The AUC associated with this model was 0.818 (P < .001).
3.1.3 |. Psychological associations of individual physical symptoms
The most common physical symptoms endorsed by at least 20% of patients (12 of 22 symptoms) based on study methodology were analyzed for their associations with psychological symptoms. Of the 12 evaluated variables, depression was associated with the most individual symptoms (11 of 12), followed by distress (8 of 12), and anxiety (5 of 12) (Table 2).
3.2 |. Secondary analyses
3.2.1 |. Sensitivity analysis using alternate measures of depression: 1) PHQ-9 without somatic items and 2) HADS-D
These alternate models found that the same covariates (unmarried/partnered status and depression) predicted number of physical symptoms endorsed on the PPL (Table 3) and both were statistically significant (P < .001). Model 1 used the PHQ-9 without somatic items and predicted 37% of variance while Model 2 used the HADS-D and predicted 48% of variance.
TABLE 3.
Alternate models of physical symptom number endorsed on the Distress Thermometer and Problem List using independent variables of depression that do not include somatic items of depression such as 1) the Patient Health Questionnaire-9 (PHQ-9) without somatic question items #3-5 & 8, and 2) the Hospital Anxiety and Depression Scale-Depression
| Model 1 Controls |
Number of endorsed physical symptoms | |||
|---|---|---|---|---|
| Regression coefficient | B | t | P | |
| Age | 0.02 (0.01) | .18 | 2.143 | .04 |
| Sex | 0.17 (0.18) | .08 | .877 | .38 |
| BMI | −0.02 (0.02) | −.13 | −1.475 | .14 |
| Married | −0.68 (0.19) | −.32 | −3.664 | <.001 |
| DT | 0.03 (0.04) | .11 | .788 | .43 |
| Anxiety (GAD-7) | 0.02 (0.03) | .08 | .650 | .52 |
| Independent Variable: Depression (PHQ-9) without somatic symptoms | 0.13 (0.04) | .40 | 3.445 | .001 |
| F: 8.541, Adjusted R2: .37, P < .001 | ||||
| Model 2 Controls |
Number of endorsed physical symptoms | |||
| Regression coefficient | B | t | P | |
| Age | 0.01 (0.01) | .13 | 1.788 | .08 |
| Sex | −0.04(0.17) | −.01 | −.199 | .84 |
| BMI | −0.02 (0.01) | −.11 | −1.513 | .13 |
| Married | −0.54 (0.17) | −.26 | −3.299 | .001 |
| DT | 0.02 (0.04) | .05 | .458 | .65 |
| Anxiety (GAD-7) | 0.01 (0.02) | .06 | .582 | .56 |
| Independent Variable: HADS-D | 0.14 (0.02) | .55 | 5.930 | <.001 |
| F: 14.075, Adjusted R2: .48, P < .001 | ||||
3.2.2 |. Survival analyses
When controlling for demographic covariates (age, sex, BMI, race, and married/partnered status), two variables were associated with shortened survival in univariate analysis (a)lower BMI, and (b) increased number of PPL physical problems and remained significant in multivariate analysis. Cox multivariate analysis demonstrated that risk of death increased with number of physical symptoms, HR: 1.077 (95% CI: 1.02-1.14, P = .01) but decreased with greater BMI, HR: 944 (95%, CI: 0.89-0.99). Two of the four most commonly endorsed physical symptoms were associated with worse survival: fatigue, HR: 1.497 (95% CI: 1.06-3.72, P = .03) and pain, HR: 1.438 (95% CI: 1.03-3.07, P = .04). In addition, problems with eating was associated with worse survival, HR: 1.469 (95% CI: 0.30-0.95, P = .03) (Table 2). Overall, three of the 12 physical symptoms (fatigue, pain, eating) were specifically associated with worse overall survival while several physical symptoms trended towards statistical significance (eg, breathing P = .06).
A median split divided patients with high and low symptom burden. Since most patients endorsed at least one symptom but only a minority endorsed many physical symptom problems, the median was 3.0, which was lower than the average number of physical symptoms endorsed (M = 4.7 SD = 3.8). Survival analysis for high vs low physical symptom burden (PPL≥3 vs <3) revealed a difference in survival where those patients with high symptom burden (PP≥3) survived 472 days vs low physical symptom burden who survived 578 days (log rank Mantel-Cox 4.214) (P = .04) (Figure 1).
FIGURE 1.
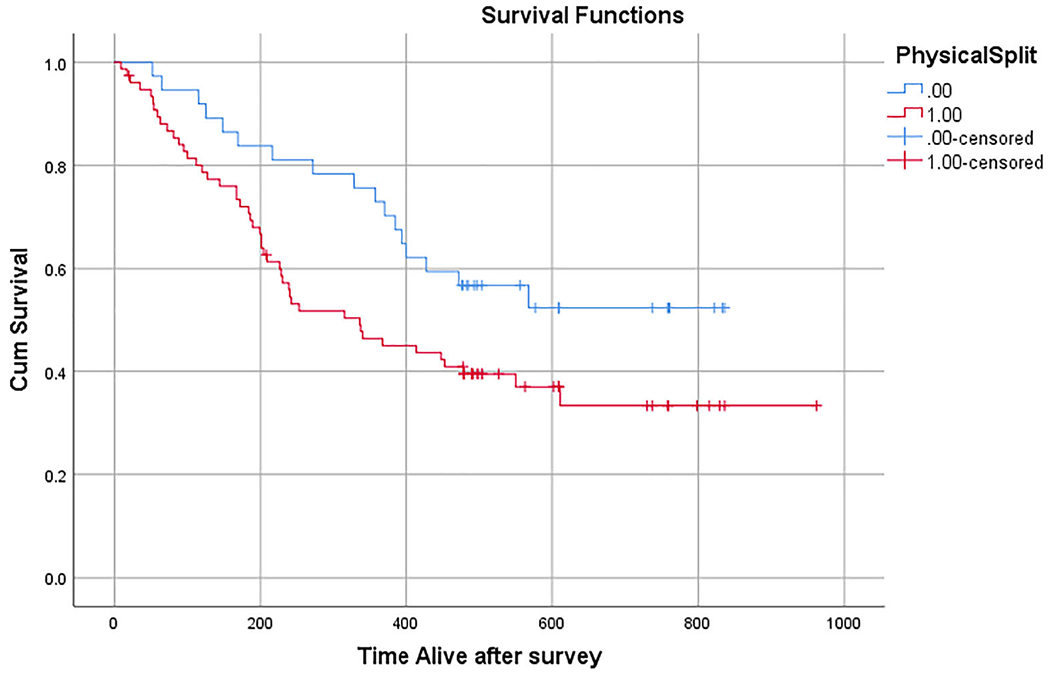
Kaplan–Meier curve survival analysis of patients with high physical symptom burden (≥3 endorsed physical symptoms) vs low physical symptom burden (<3 endorsed physical symptoms)
4 |. DISCUSSION
This study evaluated the utility of using the PPL contained within the DT&PL in identifying patients with lung cancer who were also experiencing physical symptoms. The DT&PL is a screening measure that is used worldwide to screen for cancer-related distress. The most commonly endorsed physical symptoms (ie, fatigue, sleep, pain, and breathing) reflect the most common physical symptoms of metastatic lung cancer in general.2,24 While there is long-standing recognition of the overlap between physical and psychological symptoms, especially in the context of medical illness such as advanced cancer,25 our model revealed that depression and nonmarried/partnered status accounted for 43% of physical symptom burden variance. Eleven out of 12 physical symptoms endorsed by the PPL were associated with the occurrence of depression in a nonrandom fashion. In other words, physical symptoms measured by the PPL were highly correlated with depression and to a lesser extent, distress, and anxiety. Additionally, we demonstrated the survival implications of worsened physical symptom burden as captured on the PPL by total and individual physical symptoms. Thus, the PPL performed well as a unique self-report measure providing significant clinical information in this population.
The sensitivity analysis using another measure of depression and the PHQ-9 without somatic items revealed the same associations. For the PHQ-9 model without somatic items, goodness of fit was reduced because somatic items are generally endorsed in depression but, PHQ-9 items are also highly correlated and therefore nonsomatic items were also associated with depression, as anticipated. In other words, the association between depression and endorsing physical problems was due in part to the somatic items on the PHQ-9. But, depression without somatic items is still strongly associated with endorsing physical symptoms as evidenced by the representation of the same associations and a similar goodness of fit statistic using the HADS-D, which does not contain physical symptoms.
This study demonstrated the tight association between depression and physical symptom burden captured by the PPL that accompanies the DT&PL (a well-known tool that is already widely in use) in the context of the growing implications of patient-reported outcomes (PROs) data. It is clinically meaningful to understand the likelihood of associated symptoms when doing universal screening using a straightforward, easily adoptable measure like the DT&PL. This study reveals that number of physical symptoms endorsed on the DT&PL may indicate greater psychological symptom burden (eg, depression) that is not necessarily caught on the stand alone DT itself. Interestingly, the correlation between number of physical symptoms endorsed and depression or anxiety was stronger than its correlation with distress on the DT&PL, which was seen specifically in multivariate analysis. Other studies have found that distress level measured on the DT&PL does not accurately detect mood disorders thus highlighting a potential weakness of the DT by itself.26 In effect, that makes looking at physical symptom burden more important than only looking at just distress level in screening for psychological symptoms.
There is an increasing attention to PROs in clinical cancer settings, which has been associated with improved overall survival in the setting of diverse cancers.27 In the study by Basch et al., physical symptoms were not just reported but were also addressed appropriately, which likely explains the survival benefit.27 In contrast, the addition of distress screening above and beyond standard of care has not been shown to improve survival.28 Therefore, identifying large physical symptom burden on the DT&PL may help institutions that use the DT&PL to identify patients who are suffering from physical symptom burden and may be at risk of worsened survival as well as depression. Of note, physical symptoms identified by the PPL of the DT&PL were also associated with worsened survival in this patient sample. In fact, the presence of a limited number of physical symptoms (≥3 physical symptoms) was associated with worse survival.
Married/partnered status was protective for both total number of reported physical symptoms and individual physical problems. Marital status and social support certainly are beneficial for patients in several disease contexts.29 In the context of cancer, married/partnered status is associated with a generalized survival benefit, which may be reflected by less physical symptoms.30,31 The mechanism is unclear but may have to do with salutary effects of not being isolated and having help with various practical and disease related issues. Single status is a predictor of depressive symptoms in oncology settings.32 Perhaps minimal depressive symptoms in partnered/married patients lead to less physical complaints in this study population. At the very least, however, depression and physical symptoms should be carefully screened in single, unmarried patients.
While other studies have shown that depressed patients have higher physical symptom burdens, this study demonstrates that association using the PPL of the DT&PL: a validated standard scale that is generalizable and currently in use in many cancer treatment settings.33 Our group has studied the PPL of the DT&PL in other cohorts and found that lung cancer has higher physical symptom burden than breast or hematologic neoplasms but was also primarily associated with depression.34,35
This study underscores the importance of comanaging both physical and psychological symptoms and the inherent challenges therein. The psychological symptom with the most serious consequences is depression since cancer patients who are also depressed may have worsened overall survival.36 Their survival rates are restored to baseline if their depression is adequately addressed and treated.37 But, the identification of depression can be challenging, even when patients are exhibiting distress. For that reason, it can be helpful to use the PPL on the DT&PL to identify the psychologically high-risk individuals.
PRO initiatives should be comprehensive and identify underlying psychological issues that may be associated with physical and perhaps other symptom burdens. At the same time, well-integrated distress screening programs should also consider the concomitant use of physical symptom PRO data.38 Symptom-related research efforts should be pushed to address both simultaneously. The percentage of depressed patients was not accounted for in a recent randomized controlled trial using PRO data during routine cancer treatment that led to improved overall survival.39 The interactive effects of depression on addressing other PRO physical ailments should be understood.
4.1 |. Clinical implications
Physical symptom burden as captured on the PPL detects poorly controlled physical symptom burden among patients with lung cancer and may be useful in determining who may benefit from enhanced symptom management. Patients who noted a large degree of physical symptom burden should be evaluated closely for concomitant depression or other psychological issues and unmarried/partnered patients with depression or other psychological symptoms should be followed closely for symptomatic management of the physical symptoms.
4.2 |. Study limitations
This study has several limitations on its external validity. This study is limited most significantly by the yes/no dichotomous nature of the physical issues in the PPL because they do not provide information on symptom severity. For example, a patient could have only one symptom that is severe and limiting functioning while another patients has several physical symptoms with no limitation in functioning. The study is also cross sectional and observes a relatively low number of patients. Also, data were not collected on why patients declined to fill out surveys. The study was limited to stage IV lung cancer and symptom burden may vary in patients with localized lung cancer. More females than males were observed in this study but lung cancer is still more common in males. This cohort was also younger (65.9) than the average age of lung cancer and had a relatively extended time with disease (15.4 months). Antidepressant use was accounted for in the study (15.5%) but their indications (eg, pain, depression, and anxiety) and effect on physical and psychological symptoms is not clear. The study did not account for other medical comorbidities or certain key behaviors, like smoking that may have influenced or been related to physical or psychological symptoms or inflammation.
In summary, this study provides preliminary evidence for using the PPL of the DT&PL to identify physical symptom burden that appears to be indicative of increased depression risk in patients with lung cancer. The vast majority of patients reported that more than one physical symptom as problematic, and thus not adequately controlled or managed. This study highlights the close association between depression and physical symptom burden by both number and types of physical symptoms. There appears to be dose effect in terms of the associations between physical symptom burden and psychological symptoms (eg, depression) and overall survival. Future research should focus on the concomitant treatment of psychological states along with physical symptoms in order to quantify the benefit of a dual approach to physical and psychological symptom burden.
ACKNOWLEDGMENTS
D.R.J. was supported by National Institutes of Health with Grant No. T32 CA009461.
Footnotes
CONFLICT OF INTERESTS
The authors declare no potential conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007;37:16–21. [DOI] [PubMed] [Google Scholar]
- 2.Mendoza TR, Wang XS, Lu C, et al. Measuring the symptom burden of lung cancer: the validity and utility of the lung cancer module of the M. D. Anderson symptom inventory. Oncologist. 2011;16(2):217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walling AM, Weeks JC, Kahn KL, et al. Symptom prevalence in lung and colorectal cancer patients. J Pain Symptom Manage. 2015;49(2):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18(4):893–903. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan DR, Forsberg CW, Ganzini L, et al. Depression symptom trends and health domains among lung cancer patients in the CanCORS study. Lung Cancer. 2016;100:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell AJ. Pooled results from 38 analyses of the accuracy of distress thermometer and other ultra-short methods of detecting cancer-related mood disorders. J Clin Oncol. 2007;25(29):4670–4681. [DOI] [PubMed] [Google Scholar]
- 7.Donovan KA, Grassi L, McGinty HL, Jacobsen PB. Validation of the distress thermometer worldwide: state of the science. Psychooncology. 2014;23(3):241–250. [DOI] [PubMed] [Google Scholar]
- 8.VanHoose L, Black LL, Doty K, et al. An analysis of the distress thermometer problem list and distress in patients with cancer. Support Care Cancer. 2015;23(5):1225–1232. [DOI] [PubMed] [Google Scholar]
- 9.Clover KA, Oldmeadow C, Nelson L, Rogers K, Mitchell AJ, Carter G. Which items on the distress thermometer problem list are the most distressing? Support Care Cancer. 2016;24(11):4549–4557. [DOI] [PubMed] [Google Scholar]
- 10.Chopra D, De La Garza R. Depressive, anxiety, and distress symptoms among cancer patients who endorse appearance problems. Palliat Support Care. 2018;15:1–5. [DOI] [PubMed] [Google Scholar]
- 11.JC H NCCN Clinical Practice Guidelines in Oncology (NCCN GUidelines) Distress Management Version 2 2017; https://www.nccn.org/professionals/physiciangls/pdf/distress.pdf. Accessed August 31,2018.
- 12.Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2010;8 (4):487–494. [DOI] [PubMed] [Google Scholar]
- 13.de Mol M, den Oudsten BL, Aarts M, Aerts J. The distress thermometer as a predictor for survival in stage III lung cancer patients treated with chemotherapy. Oncotarget. 2017;8(22):36743–36749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwekkeboom KL, Tostrud L, Costanzo E, et al. The role of inflammation in the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. J Pain Symptom Manage 2018;55(5):1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 16.Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(19):2946–2954. [DOI] [PubMed] [Google Scholar]
- 17.Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32(17):1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland JC, Bultz BD, National comprehensive Cancer Network. The NCCN guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. 2007;5(1):3–7. [PubMed] [Google Scholar]
- 19.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thekkumpurath P, Walker J, Butcher I, et al. Screening for major depression in cancer outpatients: the diagnostic accuracy of the 9-item patient health questionnaire. Cancer. 2011;117(1):218–227. [DOI] [PubMed] [Google Scholar]
- 22.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. [DOI] [PubMed] [Google Scholar]
- 23.Schellekens MPJ, van den Hurk DGM, Prins JB, Molema J, van der Drift MA, Speckens AEM. The suitability of the Hospital Anxiety and Depression Scale, distress thermometer and other instruments to screen for psychiatric disorders in both lung cancer patients and their partners. J Affect Disord. 2016;203:176–183. [DOI] [PubMed] [Google Scholar]
- 24.LeBlanc TW, Nickolich M, Rushing CN, Samsa GP, Locke SC, Abernethy AP. What bothers lung cancer patients the most? A prospective, longitudinal electronic patient-reported outcomes study in advanced non-small cell lung cancer. Support Care Cancer. 2015;23 (12):3455–3463. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald P, Lo C, Li M, Gagliese L, Zimmermann C, Rodin G. The relationship between depression and physical symptom burden in advanced cancer. BMJ Support Palliat Care. 2015;5(4):381–388. [DOI] [PubMed] [Google Scholar]
- 26.Wagner LI, Pugh SL, Small W Jr, et al. Screening for depression in cancer patients receiving radiotherapy: feasibility and identification of effective tools in the NRG oncology RTOG 0841 trial. Cancer. 2017; 123(3):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell AJ, Vahabzadeh A, Magruder K. Screening for distress and depression in cancer settings: 10 lessons from 40 years of primary-care research. Psychooncology 2011;20(6):572–584. [DOI] [PubMed] [Google Scholar]
- 29.Bjornnes AK, Parry M, Lie I, Falk R, Leegaard M, Rustoen T. The association between hope, marital status, depression and persistent pain in men and women following cardiac surgery. BMC women’s Health. 2018;18(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121 (8):1273–1278. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Zheng Y, Zheng W, et al. Prevalence of depression and its related factors among Chinese women with breast cancer. Acta Oncol. 2009;48(8):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grotmol KS, Lie HC, Loge JH, et al. Patients with advanced cancer and depression report a significantly higher symptom burden than non-depressed patients. Palliat Support Care. 2018;10:1–7. [DOI] [PubMed] [Google Scholar]
- 34.McFarland DC, Shaffer KM, Tiersten A, Holland J. Prevalence of physical problems detected by the distress thermometer and problem list in patients with breast cancer. Psychooncology. 2018;27(5):1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McFarland DC, Shaffer KM, Polizzi H, et al. Prevalence of physical problems detected by the distress thermometer and problem list in patients with myeloproliferative disorders. J Natl Compr Canc Netw. 2017;15(12):1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22):5349–5361. [DOI] [PubMed] [Google Scholar]
- 37.Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29(4):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salmon P, Clark L, McGrath E, Fisher P. Screening for psychological distress in cancer: renewing the research agenda. Psychooncology. 2015;24(3):262–268. [DOI] [PubMed] [Google Scholar]
- 39.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]


