Abstract
BACKGROUND/OBJECTIVE:
Reevaluation of the appropriateness of acetylcholinesterase inhibitors (AChEIs) is recommended in older adults with severe dementia, given the lack of strong evidence to support their continued effectiveness and risk for medication-induced adverse events. We sought to evaluate the impact of deprescribing AChEIs on risk of all-cause events (hospitalizations, emergency department visits, and mortality) and serious falls or fractures in older nursing home (NH) residents with severe dementia.
DESIGN:
Analysis of 2015 to 2016 data from Medicare claims, Part D prescriptions, Minimum Data Set (MDS) version 3.0, Area Health Resource File, and Nursing Home Compare. Marginal structural models with inverse probability of treatment weights were used to evaluate the association of deprescribing AChEIs and all-cause negative events as well as serious falls or fractures.
SETTING:
US Medicare certified NHs.
PARTICIPANTS:
Nonskilled NH residents, aged 65 years and older, with severe dementia receiving AChEIs within the first 14 days of an MDS assessment in 2016 (n = 37 106).
RESULTS:
The sample was primarily white (78.7%), female (75.5%), and aged 80 years or older (77.4%). Deprescribing AChEIs was associated with an increased likelihood of all-cause negative events in unadjusted models (odds ratio [OR] = 1.17; 95% confidence interval [CI] = 1.11-1.23; P < .01), but not in fully adjusted models (adjusted OR [aOR] = 1.00; 95% CI = 0.94-1.06; P = .94). By contrast, deprescribing was associated with a reduced likelihood of serious falls or fractures in unadjusted models (OR = 0.59; 95% CI = 0.52-0.66; P < .001) and remained significant in adjusted models (aOR = 0.64; 95% CI = 0.56-0.73; P < .001).
CONCLUSION:
Deprescribing AChEIs was not associated with a significant increase in the likelihood for all-cause negative events and was associated with a reduced likelihood of falls and fractures in older NH residents with dementia. Our findings suggest that deprescribing AChEIs is a reasonable approach to reduce the risk of serious falls or fractures without increasing the risk for all-cause events. J Am Geriatr Soc 68:699-707, 2020.
Keywords: dementia, nursing home, cholinesterase inhibitors, deprescribing, pharmacoepidemiology
Deprescribing acetylcholinesterase inhibitors (AChEIs) may be a reasonable strategy to reduce medication burden and the potential for adverse events in patients with severe dementia, given the lack of strong evidence for their long-term effectiveness in this population.1 Although one of the main barriers to deprescribing AChEIs is the potential worsening of behavioral symptoms,2–4 studies suggest that, in patients with severe dementia, any worsening of these symptoms that may be observed is likely not clinically significant.5–7 Equally important clinical end points in the decision to discontinue AChEIs may be the occurrence of all-cause events (ie, hospitalizations, emergency department [ED] visits, and mortality) that result from deprescribing vs the occurrence of adverse events that may be induced by AChEIs (ie, medication-related adverse events).
Although randomized trials report a consistent and positive effect of AChEIs on cognitive function compared to placebo, nearly all have been conducted in individuals with mild or moderate dementia.1 The limited number of studies that have included patients with severe dementia report findings that had minor clinical significance or were inconclusive, putting into question the cognitive benefits of these agents in patients with more advanced disease.1 There is some evidence from clinical trials8,9 and observational studies10,11 to suggest that use of AChEIs may be associated with decreased mortality risk; however, it is uncertain by what mechanism this is achieved. It is also uncertain to what degree these benefits may apply to patients with severe dementia. For example, a recently published retrospective observational study12 found that community-dwelling patients receiving AChEIs had significantly reduced mortality rates compared to patients not receiving treatment. However, when analyses were stratified by baseline mortality risk, this benefit was not seen for patients with a high risk for mortality, a designation that would likely apply to individuals with severe dementia.
Despite the uncertain impact of discontinuing AChEIs on all-cause events, one potential benefit is a reduction in the occurrence of serious medication-related adverse events that may result in hospitalization,13 specifically syncope.14 AChEI induced syncopal events or related symptoms may lead to avoidable hospitalizations for cardiac procedures, as well as an increased risk for falls and fractures, as demonstrated in a large population based study by Gill et al.15 However, no studies to date have examined whether discontinuing AChEIs actually results in reduced rates of serious fall or fracture.
The objective of this study was to evaluate the impact of deprescribing AChEIs on the occurrence of all-cause negative events (ie, hospitalizations, ED visits, and mortality), as well as hospitalizations or ED visits for serious falls and fractures in nursing home (NH) residents with severe dementia. The findings presented will address a critical knowledge gap with regard to the potential risks and benefits that may be associated with deprescribing AChEIs in this population, in which deprescribing is most likely to occur.
METHODS
Design and Data Sources
This study was a retrospective, longitudinal analysis of Medicare Part A and B claims, Master Beneficiary Summary File (MBSF), Part D prescription drug event data, the Minimum Data Set (MDS) version 3.0, the Area Health Resource File (AHRF), and Nursing Home Compare (NHC) for 2015 to 2016. The University of Pittsburgh Institutional Review Board deemed this study exempt.
Data originated from a random sample of 1 million Medicare beneficiaries, aged 65 years and older, with continuous enrollment in Medicare Parts A, B, and D in 2015 and a dementia diagnosis prior to 2016 based on the Chronic Conditions Warehouse algorithm for identifying Alzheimer disease or related disorders with International Classification of Diseases (ICD) codes.16 The MDS, a comprehensive health assessment tool administered to residents of Centers for Medicare and Medicaid Services–certified NHs at admission and at least every 90 days thereafter, served as the primary source of variables. The Medicare MBSF and Part A/B claims were used to identify comorbidities, inpatient and outpatient healthcare utilization in the year prior, and death date. Medicare Part D data provided information on prescriptions dispensed in outpatient and long-term care settings, including drug name, National Drug Code, date filled, dose, strength, quantity, days’ supply, and prescriber characteristics. The NHC and AHRF provided facility characteristics.17,18
Sample
The final cohort consisted of nonskilled nursing stays for patients with severe dementia receiving AChEIs at index (Figure 1). Skilled NH stays require more advanced care, including intravenous medications and physical therapy, and are covered by Medicare Part A and, thus, medication data are not available. We used the MDS reason for assessment fields (A0310A, A0310B) to identify all MDS assessments for nonskilled NH stays,19,20 beginning in 2016. NH episodes (n = 335 487) were constructed by matching the first assessment in 2016 to the closest discharge form or assigning the end of the study period (December 31, 2016) as the end date. The first day in which AChEI supply was observed was assigned as the AChEI index date. We required that residents had continuous enrollment in Medicare Parts A, B, and D for the duration of all episodes and the year prior (n = 14 388; 4.3% excluded). Episodes in which the resident had any Medicare skilled nursing facility claims overlapping the index date to episode end date were excluded (n = 52 390; 16.3% excluded). Episodes in which residents had severe dementia were identified using cognitive assessments in the MDS (n = 149 727; 55.6%). Specifically, the Brief Interview for Mental Status (BIMS)21 was used, or if unable to complete the BIMS, the Cognitive Performance Scale (CPS) was used.22 We used a BIMS score of 7 or less or a CPS score of 4 or greater to identify severe dementia, which have demonstrated acceptable sensitivity and specificity for severe cognitive impairment when compared to the modified Mini-Mental State Examination.23 We then limited to residents receiving AChEIs at index by searching Part D records for generic drug names (donepezil, rivastigmine, and galantamine). Residents were considered treated if there was a prescription for an AChEI with an estimated days’ supply overlapping one of the initial 14 days of the episode (n = 43 996; 29.4%). We excluded episodes with 30 days or less of follow-up to allow time to observe potential discontinuation (n = 4158; 9.5% excluded). Finally, if residents had more than one episode meeting criteria, only the first was included (n = 2729; 6.9% excluded).
Figure 1.
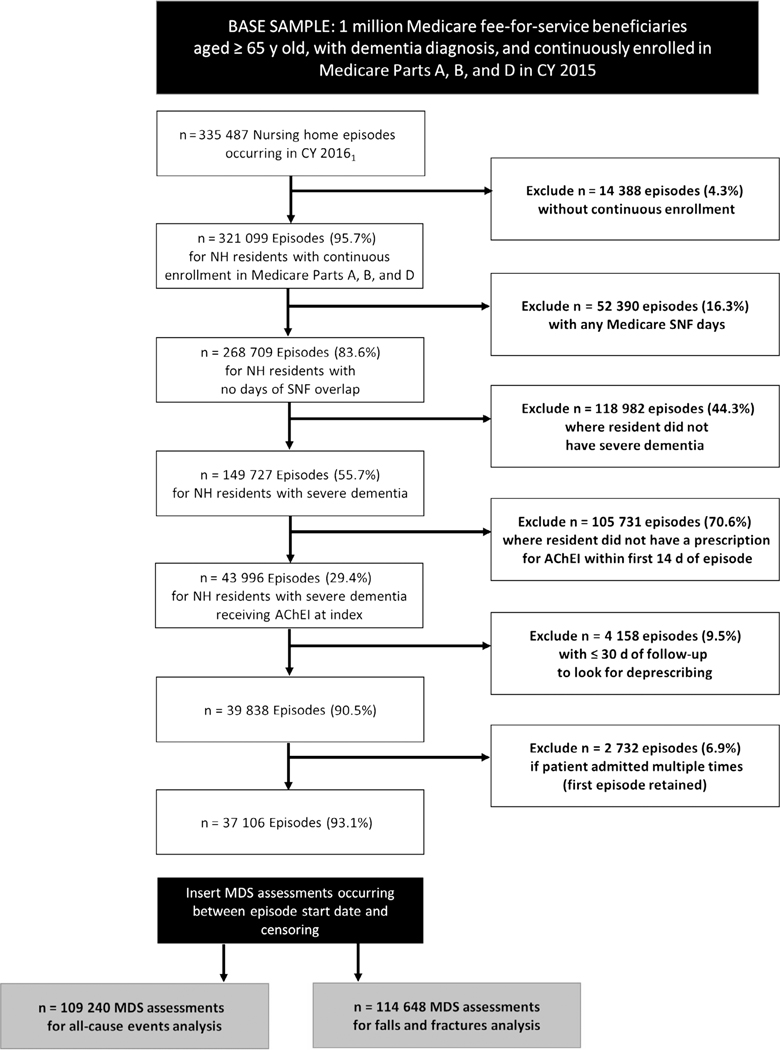
Sample construction. AChEI indicates acetylcholinesterase inhibitor; CY, Calendar Year; MDS, Minimum Data Set; NH, nursing home; SNF, skilled nursing facility.
A longitudinal data set was created in which each resident could have multiple MDS assessments from episode start until deprescribing or censoring. The final cohort included 37 106 residents with 109 240 assessments for all-cause events and 114 648 assessments for falls and fractures.
Dependent Variables
The dependent variables in this analysis were all-cause negative events and medication-related events of serious falls or fractures. All-cause negative events included ED visits, hospitalizations, and death for any reason. Hospitalizations and ED visits were identified by searching for Medicare claims occurring since AChEI index date until the earliest of the following: NH discharge date, death date, or end of study (December 31, 2016). Death dates were extracted from the Medicare MBSF. Serious falls and fractures were identified in a similar fashion, but using claims for hospitalizations or ED visits associated with specific ICD-9 or ICD-10 codes.24
Independent Variables
The primary independent variable in this analysis was whether AChEIs were deprescribed. Deprescribing was defined as discontinuation, signaled by a subsequent gap in therapy of at least 30 days based on prescription fill dates and last day of supply, with the 31st gap day in the period serving as the discontinuation date. The discontinuation date was defined as such to avoid the potential for immortal time bias, in which a period of immortal time, usually required for the observation of a specific event, may be incorrectly attributed to the exposed group.25,26 Deprescribing was treated as a time-varying exposure in our data set and was coded as positive if the 31st gap day in medication supply occurred on or after the assessment start date and before either the assessment stop date or the event date.
Covariates were extracted from the MDS, Medicare Part D Prescription Drug Event records, Medicare Part A and B claims, NHC, and the AHRF. We previously identified that a number of clinical factors corresponding to limited prognosis or deteriorating clinical status were associated with increased likelihood for deprescribing, while system-level factors may act as barriers to deprescribing.27 The full list of covariates included: demographics (age, sex, race/ethnicity, and marital status), clinical assessment factors (MDS assessment form type, resident ability to be understood, poor appetite, urinary incontinence, swallowing disorder, parenteral nutrition or tube feeds, mechanically altered diet, recent weight loss, shortness of breath, dehydration, cancer, end-stage renal disease, heart failure, activities of daily living, limited prognosis or hospice utilization, antidepressant use, antipsychotic use, benzodiazepine use, strong anticholinergic use, AChEI type, memantine use, total number of medications, Charlson Comorbidity Index, all-cause and cause-specific hospitalizations in 90 days prior to index date, and location prior to NH residence), environment of care (NH geographic region, facility size, and rurality), and provider specialty (AChEI prescriber specialty: primary care, geriatrics, or other).
Demographic, environment of care, and provider specialty variables were treated as time invariant and were measured at the time of the index MDS assessment, while clinical assessment factors were created as time-varying and were measured at the time of each MDS assessment.
Statistical Analyses
Analyses were performed using SAS, version 9.4 (SAS Institute Inc) and STATA 14 (StataCorp). Missing observations (<5% total) were addressed with single imputation using chained equations, including all covariates in the imputation.28
We used marginal structural models with inverse probability of treatment weights (IPTW) to address the potential for time dependent confounding.29–34 IPTWs were used to model each subject’s propensity for being deprescribed, considering the subject’s history of covariates.30,35 We also addressed the potential for loss to follow-up by using inverse probability of censoring weights (IPCW), calculated as the probability of remaining uncensored at the time of each MDS assessment. The final stabilized inverse propensity weight for each assessment was the product of IPTW and IPCW. Additional information on the derivation of IPTWs and IPCWs and the implementation of marginal structural models can be found in supplementary materials.
Each record ended with the event of interest, censoring due to death or discharge, or the day before the next MDS assessment start date (if no event or censoring). Sample characteristics were calculated on both the patient level and the assessment level (ie, at the time of each MDS assessment). We evaluated the balance of covariates across deprescribing status after weighting using standardized differences in each sample.36
Analyses were conducted using an intent-to-treat approach for deprescribing, where residents remained in this category until censoring or end of follow-up. The primary analysis of the association of deprescribing and each outcome used a pooled logistic regression model for discrete events, analogous to a Cox proportional hazards model, and was weighted by the product of IPTWs and IPCWs. We used robust SEs to account for the correlation between observations from the same individual.
We conducted several sensitivity analyses. We first evaluated the influence of extreme weights in our sample. In the second sensitivity analysis, we reconducted our analyses in a per-protocol fashion, where residents who were deprescribed but then later filled a new AChEI prescription (ie, “restarters”) were censored in the period following the new AChEI prescription. Finally, we examined the effect of deprescribing AChEIs on both outcomes in subgroups stratified by whether residents were receiving memantine at baseline.
RESULTS
Sample Characteristics
Time-invariant sample characteristics, including demographics, environment of care, and provider specialty, are presented at baseline in Table 1. Time-varying clinical characteristics are presented at baseline on the resident level and during follow-up at the assessment level in Table 2. At the index date, the sample was primarily white (78.7%), aged 80 years or older (77.4%), and female (75.5%). Most residents in the sample had already been residing in the NH at their index date (87.7%) as opposed to newly admitted (12.3%). Weighted sample characteristics are presented in Supplementary Tables S1 and S2. All sample characteristics showed adequate balance after applying IPTWs, with the exception of MDS assessment type, hospitalizations, memantine use, total number of medications, geographic region, and rurality.
Table 1.
Baseline Time Invariant Sample Characteristics (N = 37 106 Residents)
| Characteristics | No. (%) of Residents |
|---|---|
| Demographics | |
| Age, y | |
| 65–69 | 901 (2.4) |
| 70–79 | 7496 (20.2) |
| 80–89 | 17 922 (48.3) |
| ≥90 | 10 787 (29.1) |
| Sex | |
| Male | 9092 (24.5) |
| Female | 28 014 (75.5) |
| Race/ethnicity | |
| White | 29 210 (78.7) |
| Black | 4405 (11.9) |
| Hispanic | 2049 (5.5) |
| Other | 1442 (3.9) |
| Current marital status | |
| Married | 8111 (22.1) |
| Not Married | 28 918 (77.9) |
| Environment of Care | |
| Geographic region | |
| Midwest | 10 131 (27.3) |
| Northeast | 6631 (17.9) |
| South | 17 279 (46.6) |
| West | 3065 (8.3) |
| Certified beds | |
| <50 | 1904 (5.1) |
| 50–99 | 10 613 (28.6) |
| 100–199 | 20 335 (54.8) |
| ≥200 | 4254 (11.5) |
| Rural/urban continuum | |
| Urban | 25 715 (69.3) |
| Rural | 9925 (26.7) |
| Highly rural | 1466 (4.0) |
| Provider Specialty | |
| Prescriber specialty | |
| Geriatrics | 3167 (8.5) |
| Primary care | 3963 (10.7) |
| Other | 29 976 (80.8) |
Table 2.
Time-Varying Clinical Characteristics
| Variable | Full Sample At Index Date (N = 37 106 Residents) | All-Cause Events Analysis, Assessment Level (N = 109 240 Assessments) | Falls/Fractures Analysis, Assessment Level (N = 114 647 Assessments) |
|---|---|---|---|
| Entered from | |||
| Community | 7575 (20.4) | 22 018 (20.2) | 22 850 (19.9) |
| Hospital | 25 275 (68.1) | 74 676 (68.4) | 78 630 (68.6) |
| NH or other LTC facility | 4256 (11.5) | 12 546 (11.5) | 13 168 (11.5) |
| MDS assessment type | |||
| Admission | 4576 (12.3) | 4576 (4.2) | 4576 (4.0) |
| Quarterly | 24 434 (65.8) | 78 051 (71.4) | 81 972 (71.5) |
| Annual | 6001 (16.2) | 19 598 (17.9) | 20 575 (17.9) |
| Significant change in status | 2095 (5.6) | 7015 (6.4) | 7525 (6.6) |
| Charlson Comorbidity Index | |||
| 0–1 | 6054 (16.3) | 17 095 (15.6) | 17 639 (15.4) |
| 2–3 | 9162 (24.7) | 28 203 (25.8) | 29 371 (25.6) |
| 4–5 | 8702 (23.5) | 26 667 (24.4) | 27 994 (24.4) |
| ≥6 | 13 188 (35.5) | 37 275 (34.1) | 39 644 (34.6) |
| Makes self understood | |||
| Understood | 14 721 (39.7) | 41 022 (37.6) | 42 987 (37.5) |
| Usually understood | 9961 (26.8) | 29 437 (26.9) | 30 859 (26.9) |
| Sometimes understood | 8084 (21.8) | 24 000 (22.0) | 25 148 (21.9) |
| Rarely/never understood | 4340 (11.7) | 14 781 (13.5) | 15 654 (13.7) |
| PHQ 9 score, mean (SD) | 2.22 (3.40) | 2.16 (3.39) | 2.16 (3.40) |
| Aggressive behavior scale score, mean (SD) | 0.56 (1.34) | 0.53 (1.29) | 0.53 (1.29) |
| Activities of daily living score | |||
| 1–7 | 2849 (7.7) | 7968 (7.3) | 8359 (7.3) |
| 8–14 | 6033 (16.3) | 16 502 (15.1) | 17 268 (15.1) |
| 15–21 | 19 426 (52.4) | 56 793 (52.0) | 59 437 (51.8) |
| 22–28 | 8798 (23.7) | 27 977 (25.6) | 29 584 (25.8) |
| Urinary incontinence | |||
| Continent | 3954 (10.7) | 10 220 (9.4) | 10 720 (9.4) |
| Occasionally incontinent | 5439 (14.7) | 14 420 (13.2) | 15 114 (13.2) |
| Frequently incontinent | 12 281 (33.1) | 35 465 (32.5) | 37 093 (32.4) |
| Always incontinent | 14 644 (39.5) | 47 338 (43.3) | 49 734 (43.4) |
| Indwelling catheter | 788 (2.1) | 1797 (1.6) | 1987 (1.7) |
| Cancer | 1555 (4.2) | 4462 (4.1) | 4677 (4.1) |
| Heart failure | 5809 (15.7) | 16 697 (15.3) | 17 584 (15.3) |
| End-stage renal disease | 3419 (9.2) | 9960 (9.1) | 10 537 (9.2) |
| Short of breath | 2469 (6.7) | 6922 (6.3) | 7353 (6.4) |
| Poor appetite | 4946 (13.3) | 14 490 (13.3) | 15 276 (13.3) |
| Weight loss | 2389 (6.4) | 6952 (6.4) | 7416 (6.5) |
| Swallowing difficulty | 1247 (3.4) | 3790 (3.5) | 3999 (3.5) |
| Mechanically altered diet | 20 311 (54.7) | 56 341 (51.6) | 59 107 (51.6) |
| IV/parenteral nutrition or feeding tube | 1262 (3.4) | 2961 (2.7) | 3463 (3.0) |
| Hospice or limited prognosis | 1875 (5.1) | 6777 (6.2) | 7278 (6.3) |
| Hospitalizations/ED visits (90 d prior to index) | |||
| None | 27 980 (75.4) | ||
| Cause specific (fall, fracture, or syncope) | 3150 (8.5) | ||
| Other cause | 5976 (16.1) | ||
| AChEI at index date | |||
| Donepezil | 28 877 (77.8) | 84 455 (77.3) | 88 587 (77.3) |
| Donepezil/memantine | 832 (2.2) | 2485 (2.3) | 2564 (2.2) |
| Galantamine | 483 (1.3) | 1927 (1.8) | 2044 (1.8) |
| Rivastigmine (oral) | 5403 (14.6) | 15 825 (14.5) | 16 653 (14.5) |
| Rivastigmine (transdermal) | 1511 (4.1) | 4548 (4.2) | 4800 (4.2) |
| Memantine use | 15 199 (41.0) | 44 342 (40.6) | 46 535 (40.6) |
| Benzodiazepine and/or Z drug | 5442 (14.7) | 14 846 (13.6) | 15 706 (13.7) |
| Antipsychotic use | 9003 (24.3) | 23 999 (22.0) | 25 226 (22.0) |
| Antidepressant use | 21 071 (56.8) | 60 832 (55.7) | 63 791 (55.6) |
| Highly anticholinergic drugs (AGS Beers Criteria®) | 5519 (14.9) | 14 776 (13.5) | 15 524 (13.5) |
| Total no. of medications, mean (SD) | 5.9 (3.1) | 5.7 (3.1) | 5.7 (3.1) |
Note: Data are given as number (percentage), unless otherwise indicated.
Abbreviations: AChEI, acetylcholinesterase inhibitor; ED, emergency department; IV, intravenous; LTC, long-term care; MDS, Minimum Data Set; NH, nursing home; PHQ, Patient Health Questionnaire.
Primary Analyses
All-cause negative events occurred in 31.6% of residents, and hospitalizations for falls or fractures occurred in 9.2% of residents, over a median follow-up time of 226 days (interquartile range [IQR] = 92–312 days) and 259 days (IQR = 110–318 days), respectively. This equated to event rates of 0.56 all-cause negative events and 0.15 falls or fractures per person-year. ED visits were the most common all-cause event, occurring in 16.1% of residents, followed by hospitalizations (8.4%) and death (7.1%). All-cause events occurred at a higher rate in residents who had AChEIs deprescribed vs those who did not (0.62 vs 0.55 events per person-year). Falls or fractures had a lower event rate in residents who had AChEIs deprescribed vs those who did not (0.09 vs 0.16 events per person-year).
Model results are presented in Figure 2. In unadjusted analyses, deprescribing AChEIs was associated with an increased likelihood for all-cause negative events and a reduced likelihood for falls or fractures. In adjusted analyses, deprescribing AChEIs was no longer significantly associated with all-cause negative events, but remained significantly associated with a reduced likelihood of falls or fractures.
Figure 2.
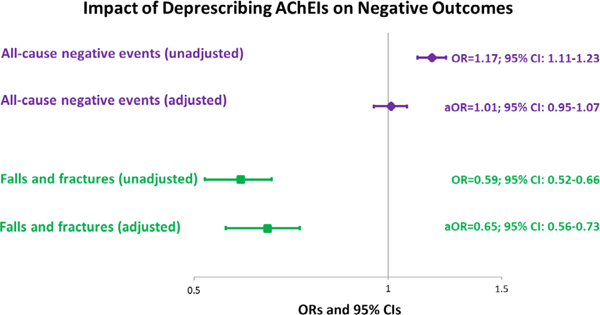
Association of deprescribing acetylcholinesterase inhibitors (AChEIs) with all-cause negative events and falls or fractures. aOR indicates adjusted OR; CI, confidence interval; OR, odds ratio.
Sensitivity Analyses
Excluding observations with IPTWs outside of the 1st and 99th percentiles and top-coding values at the 1st and 99th percentiles resulted in no substantive changes to our findings (Supplementary Tables S3 and S4). Applying a per-protocol approach resulted in less than 2% of MDS assessments being excluded from analyses due to potential medication restarts, but our findings remained essentially unchanged (all-cause events: adjusted odds ratio [aOR] = 1.03 [95% confidence interval [CI] = 0.97-1.10] [P = .34]; falls or fractures: aOR = 0.66 [95% CI = 0.57-0.75] [P < .001]).
Analyses stratified by memantine use at baseline are presented in Supplementary Table S5. Results remained consistent regardless of baseline memantine use. Deprescribing AChEIs was not significantly associated with all-cause events (memantine at baseline: aOR = 1.04 [95% CI = 0.95-1.15] [P = .40]; no memantine at baseline: aOR = 0.99 [95% CI = 0.92-1.07] [P = .89]), and remained associated with a reduced likelihood of falls or fractures (memantine at baseline: aOR = 0.70 [95% CI = 0.57-0.87] [P < .001]; no memantine at baseline: aOR = 0.60 [95% CI = 0.51-0.70] [P < .001]).
DISCUSSION
In a large national sample of NH residents with severe dementia, we found that deprescribing AChEIs was not associated with the occurrence of all-cause negative events and was associated with a reduced likelihood of hospitalization due to falls or fractures. The findings from this analysis address a major gap in the literature related to the tolerability and safety of deprescribing AChEIs in NH residents and suggest that the discontinuation is reasonable in individuals with severe dementia given that it does not increase the likelihood of negative events.
To our knowledge, no large observational studies have examined the impact of discontinuing AChEIs on the occurrence of negative events. Smaller studies that have examined the impact of discontinuing AChEIs on all-cause negative events present conflicting results. Two observational studies37,38 reported higher mortality rates among patients who experienced gaps in AChEI therapy. By contrast, a recent meta-analysis of five randomized controlled trials reported no significant differences in dropout rates due to deaths or adverse events, such as falls and gastrointestinal symptoms, between individuals who discontinued AChEIs and those who continued therapy.39 Only one of the above studies included any patients with severe dementia,40 despite the fact that these patients may be most likely to discontinue these agents due to lack of perceived benefit relative to risks.21,22,41,42
The strengths of the present study overcome the limitations of prior investigations and enhance the clinical significance of our findings, while contributing new information to the literature on deprescribing. We used a large, nationally representative sample of NH residents and focused on those with severe dementia, who are arguably the most clinically relevant population for deprescribing AChEIs.43 In addition to all-cause negative events, we also evaluated the effect of deprescribing AChEIs on medication-related adverse events (ie, falls and fractures) in an effort to address the potential benefits of deprescribing, as well as the potential risks. To our knowledge, no prior studies have examined the effect of deprescribing on falls and fractures.
In unadjusted analyses, deprescribing AChEIs was associated with an increased likelihood for all-cause negative events. Interestingly, this association was no longer significant in adjusted models, suggesting that there is a substantial degree of confounding by indication, where those who are most likely to be deprescribed are those also at high risk for hospitalizations, ED visits, and/or death. This is not surprising given that severe dementia itself is considered to be a life limiting condition,44 and in our prior work, we identified that patients with clinical characteristics that signal a decline in health status were more likely to discontinue AChEIs.27,45,46 Thus, the observed increase in all-cause negative events in unadjusted analyses was likely attributable to a higher baseline mortality risk due to severe dementia rather than the effects of AChEI discontinuation.
Deprescribing AChEIs may confer some benefits in NH residents with severe dementia, as evidenced by the reduction in the likelihood of hospitalizations for serious falls or fractures that was observed in both adjusted and unadjusted analyses. Previous investigations have identified bradycardia and syncope as significant adverse effects of AChEIs that can precipitate the occurrence of falls and fractures.14,15 This is also supported by our prior work, in which we identified that discontinuing AChEIs was more likely in NH residents with a hospitalization for bradycardia, fall, or fracture in the prior 90 days,27 potentially in response to one of these negative events. We do acknowledge that while our outcome definition for falls and fractures was sensitive, it was not specific to events due to bradycardia or syncope and we did not have measures of blood pressure at the time of event available in claims to adjudicate these events. As a result, it is possible that some of this effect is not directly attributable to a reduction in adverse events achieved through deprescribing AChEIs.
There are several limitations that should be acknowledged. Although it is unlikely that a gap in days supply of greater than 30 days would occur unintentionally in a NH population, we were not able to confirm intentional vs unintentional discontinuation based on refill records. We also acknowledge that the rate of falls or fractures in our study may have been underestimated due to goals of care or advance care planning that may prevent some residents from being transferred to an acute setting. We also may have missed early adverse events by imposing a minimum length of stay requirement of 30 days as well other less serious adverse events not captured by claims. We only included the first qualifying episode for residents who had multiple stays during follow-up. Thus, there may have been differences in terms of overall health status and, thus, vulnerability to adverse events between their first and subsequent stays. However, the proportion of residents with multiple stays was small, so it is unlikely that this would have influenced our results substantially. Finally, although we used marginal structural models to address the potential for time-varying confounding, we cannot rule out the possibility of residual confounding or the potential unmeasured confounders.
Future studies should continue to address the impact of deprescribing AChEIs on other medication-specific adverse events, such as gastrointestinal effects, weight loss, and urinary symptoms, which may significantly affect resident quality of life.
CONCLUSIONS
This study found that deprescribing AChEIs was not associated with a significant increase in the likelihood of all-cause negative events, but was associated with a decrease in the likelihood of hospitalizations due to serious falls and fractures in older NH residents with severe dementia. Our findings suggest that deprescribing AChEIs may be an effective strategy to reduce the occurrence of serious medication-related adverse events without substantial risk.
Supplementary Material
Editor’s Note.
Our Journal and many others have published papers like this one that call into question the benefit/risk ratio of acetylcholinesterase inhibitors (AChEIs), especially as cognitive impairment progresses. The initial decision about whether to start an AChEI is a person-centered one – like many of the decisions we make in treating older patients. The best evidence for the effectiveness of these drugs is in delaying the need for institutionalization. But, that is a very complicated outcome because it is dependent on so many factors other than any slight stabilization or improvement in cognitive function these drugs might provide.
There is more convincing evidence emerging about the potential harms of these drugs. Many students and residents do not think about the potential cardiac effects of AChEIs through causing bradycardia that can result in hypotension, cerebral hypo-perfusion, near syncope, falls and injuries. While making hospital rounds I have seen several older patients who have had pacemakers inserted while on an AChEI. These drugs have in fact been shown to be associated with syncope, pacemaker placement, and injurious falls, and their initiation is associated with emergency department visits and hospitalizations, as demonstrated by a paper recently published in this Journal.
The current paper by Niznik and colleagues adds further evidence to these associations, by demonstrating that deprescribing of AChEIs is associated with fewer falls and fractures among people with dementia residing in nursing homes.
-Joseph G. Ouslander, MD
ACKNOWLEDGMENTS
Financial Disclosure: Funding was provided by The Patrick and Catherine Weldon Donaghue Medical Research Foundation and the University of Pittsburgh Older American’s Independence Center (P30 AG024827). Dr Niznik was funded by a T32 Award from the National Institute on Aging (T32AG021885) at the University of Pittsburgh at the time this research was conducted.
Sponsor’s Role: Funding sources had no role in the study design, data collection and analysis, manuscript preparation, or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Buckley JS, Salpeter SR. A risk benefit assessment of dementia medications: systematic review of the evidence. Drugs Aging. 2015;32(6):453–467. [DOI] [PubMed] [Google Scholar]
- 2.Shega JW, Ellner L, Lau DT, Maxwell TL. Cholinesterase inhibitor and N-methyl-D-aspartic acid receptor antagonist use in older adults with end-stage dementia: a survey of hospice medical directors. J Palliat Med. 2009;12(9): 779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray R, Prettyman R. When do we discontinue anti dementia drugs? views expressed by clinicians in a national survey within the United Kingdom. Int Psychogeriatr. 2013;25(9):1559–1560. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann N, Black SE, Li A, Lanctôt KL. Discontinuing cholinesterase inhibitors: results of a survey of Canadian dementia experts. Int Psychogeriatr. 2011;23(4):539–545. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Inoue Y, Mikami K, Gen K. The influence and changes in the dosages of concomitantly used psychotropic drugs associated with the discontinuation of donepezil in severe Alzheimer’s disease with behavioral and psychological symptoms on dementia: a preliminary open-label trial. Ther Adv Psychopharmacol. 2014;4(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns A, Bernabei R, Bullock R, et al. Safety and efficacy of galantamine (Reminyl) in severe Alzheimer’s disease (the SERAD study): a randomised, placebo-controlled, double-blind trial. Lancet Neurol. 2009;8(1):39–47. [DOI] [PubMed] [Google Scholar]
- 7.Simpson S, Beavis D, Leddy A, Ball S, Johnson I. Naturalistic audit of NICE criteria for the use of cholinesterase inhibitors. Psychiatr Bull. 2005;29(11): 410–412. [Google Scholar]
- 8.Tricco AC, Ashoor HM, Soobiah C, et al. Comparative effectiveness and safety of cognitive enhancers for treating Alzheimer’s disease: systematic review and network metaanalysis. J Am Geriatr Soc. 2018;66(1):170–178. [DOI] [PubMed] [Google Scholar]
- 9.Blanco-Silvente L, Castells X, Saez M, et al. Discontinuation, efficacy, and safety of cholinesterase inhibitors for Alzheimer’s disease: a meta-analysis and meta-regression of 43 randomized clinical trials enrolling 16 106 patients. Int J Neuropsychopharmacol. 2017;20(7):519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasper MC, Ott BR, Lapane KL. Is donepezil therapy associated with reduced mortality in nursing home residents with dementia? Am J Geriatr Pharmacother. 2005;3(1):1–7. [DOI] [PubMed] [Google Scholar]
- 11.Zhu CW, Livote EE, Scarmeas N, et al. Long-term associations between cholinesterase inhibitors and memantine use and health outcomes among patients with Alzheimer’s disease. Alzheimers Dement. 2013;9(6):733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilotto A, Polidori MC, Veronese N, et al. Association of antidementia drugs and mortality in community-dwelling frail older patients with dementia: the role of mortality risk assessment. J Am Med Dir Assoc. 2018;19(2):162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochon PA, Gruneir A, Gill SS, et al. Initial cholinesterase inhibitor therapy dose and serious events in older women and men. J Am Geriatr Soc. 2018; 66(9):1692–1699. [DOI] [PubMed] [Google Scholar]
- 14.Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev. 2012;9:CD009132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill SS, Anderson GM, Fischer HD, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009;169(9):867–873. [DOI] [PubMed] [Google Scholar]
- 16.Buccaneer Computer Systems & Service Inc. Chronic Condition Data Ware-house Medicare Administrative Data User Guide. Minneapolis, MN: Buccaneer Computer Systems & Service Inc; 2018; https://www.ccwdata.org/documents/10280/19002246/ccw-medicare-data-user-guide.pdf. Accessed June 18,2018. [Google Scholar]
- 17.Center for Medicare and Medicaid Services. Design for Nursing Home Compare Five Star Quality Rating System: Technical Users’s Guide. Baltimore, MD: Centers for Medicare and Medicaid Services; 2018; https://www.medicare.gov/NursingHomeCompare/Data/About.html. Accessed June 18, 2018. [Google Scholar]
- 18.US Department of Health and Human Services Health Resources and Services Administration, Bureau of Health Professions. Area Health Resource File (AHRF). Rockville, MD: Health Resources and Services Administration; 2018. [Google Scholar]
- 19.Intrator O, Hiris J, Berg K, Miller SC, Mor V. The residential history file: studying nursing home residents’ long-term care histories(*). Health Serv Res. 2011;46(1 pt 1): 120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei YJ, Simoni-Wastila L, Zuckerman IH, Brandt N, Lucas JA. Algorithm for identifying nursing home days using Medicare claims and minimum data set assessment data. Med Care. 2016;54(11):e73–e77. [DOI] [PubMed] [Google Scholar]
- 21.Umegaki H, Itoh A, Suzuki Y, Nabeshima T. Discontinuation of donepezil for the treatment of Alzheimer’s disease in geriatric practice. Int Psychogeriatr. 2008;20(4):800–806. [DOI] [PubMed] [Google Scholar]
- 22.Gardette V, Lapeyre-Mestre M, Piau A, et al. A 2-year prospective cohort study of antidementia drug non-persistency in mild-to-moderate Alzheimer’s disease in Europe: predictors of discontinuation and switch in the ICTUS study. CNS Drugs. 2014;28(2):157–170. [DOI] [PubMed] [Google Scholar]
- 23.Saliba D, Buchanan J, Edelen MO, et al. MDS 3.0: brief interview for mental status. J Am Med Dir Assoc. 2012;13(7):611–617. [DOI] [PubMed] [Google Scholar]
- 24.Hanlon JT, Zhao X, Naples JG, et al. Central nervous system medication burden and serious falls in older nursing home residents. J Am Geriatr Soc. 2017;65:1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. [DOI] [PubMed] [Google Scholar]
- 26.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492 499. [DOI] [PubMed] [Google Scholar]
- 27.Niznik JD, Zhao X, He M, et al. Factors associated with deprescribing acetylcholinesterase inhibitors in older nursing home residents with severe dementia. J Am Geriatr Soc. 2019;67:1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.StataCorp. Multiple Imputation Reference Manual. Stata Press; 2017: https://www.stata.com/bookstore/multiple-imputation-reference-manual/. Accessed April 18, 2017.
- 29.Dutcher SK, Rattinger GB, Langenberg P, et al. Effect of medications on physical function and cognition in nursing home residents with dementia. J Am Geriatr Soc. 2014;62(6):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 31.Mortimer KM, Neugebauer R, van der Laan M, Tager IB. An application of model-fitting procedures for marginal structural models. Am J Epidemiol. 2005;162(4):382–388. [DOI] [PubMed] [Google Scholar]
- 32.Hernan MA, Brumback BA, Robins JM. Estimating the causal effect of zidovudine on CD4 count with a marginal structural model for repeated measures. Stat Med. 2002;21(12):1689–1709. [DOI] [PubMed] [Google Scholar]
- 33.Thoemmes F, Ong AD. A primer on inverse probability of treatment weighting and marginal structural models. Emerg Adulthood. 2016;4(1): 40–59. [Google Scholar]
- 34.Fewell Z, Hernan MA, Wolfe F, et al. Controlling for time-dependent confounding using marginal structural models. Stata J. 2004;4(4):402–420. [Google Scholar]
- 35.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. [DOI] [PubMed] [Google Scholar]
- 36.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ku LE, Li CY, Sun Y. Can persistence with cholinesterase inhibitor treatment lower mortality and health-care costs among patients with Alzheimer’s disease? a population-based study in Taiwan. Am J Alzheimers Dis Other Demen. 2018;33(2):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pariente A, Fourrier-Reglat A, Bazin F, et al. Effect of treatment gaps in elderly patients with dementia treated with cholinesterase inhibitors. Neurology. 2012;78(13):957–963. [DOI] [PubMed] [Google Scholar]
- 39.O’Regan J, Lanctot KL, Mazereeuw G, et al. Cholinesterase inhibitor discontinuation in patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry. 2015;76(11):e1424–e1431. [DOI] [PubMed] [Google Scholar]
- 40.Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366(10):893–903. [DOI] [PubMed] [Google Scholar]
- 41.Amuah JE, Hogan DB, Eliasziw M, et al. Persistence with cholinesterase inhibitor therapy in a population-based cohort of patients with Alzheimer’s disease. Pharmacoepidemiol Drug Saf. 2010;19(7):670–679. [DOI] [PubMed] [Google Scholar]
- 42.Parsons C, Briesacher BA, Givens JL, Chen Y, Tjia J. Cholinesterase inhibitor and memantine use in newly admitted nursing home residents with dementia. J Am Geriatr Soc. 2011;59(7):1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reeve E, Farrell B, Thompson W, et al. Deprescribing cholinesterase inhibitors and memantine in dementia: guideline summary. Med J Aust. 2019;210(4):174–179. [DOI] [PubMed] [Google Scholar]
- 44.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367–425. [Google Scholar]
- 45.Niznik JD, Zhang S, Mor MK, et al. Adaptation and initial validation of Minimum Data Set (MDS) mortality risk index to MDS version 3.0. J Am Geriatr Soc. 2018;66:2353–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porock D, Parker-Oliver D, Petroski GF, Rantz M. The MDS mortality risk index: the evolution of a method for predicting 6-month mortality in nursing home residents. BMC Res Notes. 2010;3:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


