Abstract
PURPOSE:
To compare clinical outcomes between low-dose-rate (LDR) brachytherapy and high-dose-rate (HDR) brachytherapy for cervical cancer patients.
METHODS AND MATERIALS:
All consecutive newly diagnosed cervical cancer patients undergoing pretreatment 18-fluorodeoxyglucose positron emission tomography imaging and treated with curative-intent definitive chemoradiation from 1997 to 2016 at a U.S. academic center were included. Brachytherapy boost was LDR or HDR 2D treatment planning from 1997 to 2005 and HDR with MR-based 3D planning from 2005 to 2016. Local control (LC), cancer-specific survival (CSS), and late bowel/bladder complications were evaluated.
RESULTS:
Tumor stages were International Federation of Gynecology and Obstetrics IB1-IIB (n = 457; 75%) and III-IVA (n = 152; 25%). Brachytherapy was LDR for 104 patients and HDR for 505 patients. Concurrent weekly cisplatin was administered to 536 patients (88%). With median followup of 9.4 years, there was no difference in LC (p = 0.24) or CSS (p = 0.50) between LDR and HDR brachytherapy. Cox multivariable regression showed that only International Federation of Gynecology and Obstetrics stage III-IVA (HR=2.4, p = 0.004) was associated with worse LC. A propensity-matched cohort (90 LDR vs. 90 HDR) was created, and the 5-year LC rates were 88% LDR and 82% HDR, p = 0.26; 5-year CSS rates were 66% LDR and 58% HDR, p = 0.19; 5-year grade ≥3 bowel/bladder toxicities were 23% LDR and 16% HDR, p = 0.44. For all patients, the 5-year late toxicity in stage III-IVA patients was higher with LDR 47% vs. HDR 15%, p = 0.03, with no difference in LC, 86% and 75%, respectively (p = 0.09).
CONCLUSIONS:
There was no difference in LC with either LDR or HDR brachytherapy. The late complication rate was reduced with HDR and 3D-planned brachytherapy compared to LDR and 2D-planned brachytherapy. © 2018 American Brachytherapy Society. Published by Elsevier Inc. All rights reserved.
Keywords: Low-dose-rate, High-dose-rate, Brachytherapy, Image-guided adaptive brachytherapy, Cervical cancer, Chemoradiation
Introduction
The adoption of high-dose-rate (HDR) brachytherapy over low-dose-rate (LDR) brachytherapy has increased in the United States over the last 2 decades (1,2), which will soon be near complete as Cesium-137 tubes are no longer manufactured. However, there is still controversy over whether HDR can adequately treat bulky stage IIIB tumors while limiting toxicity (3). There have been four single-institution prospective trials comparing outcomes of LDR and HDR brachytherapies for cervical cancer (4–7), but none were done in the United States, and all were completed before the era of concurrent chemoradiation. Two Japanese studies compared Cobalt-60 HDR to Cesium-137 LDR brachytherapy, both given with external beam radiation delivered with opposed anterior-posterior 10 MV beams with a central block after 20 Gy. LDR brachytherapy was given in 2–3 implants toward the end of treatment, and HDR was given once a week during external beam radiation. There were no differences in cause-specific survival (CSS), but late complications were higher with HDR in the study by Teshima et al. (4,6). A trial from India used similar techniques as the abovementioned studies and found similar local control (LC) and survival for LDR and HDR, but reduced grade 1–2 rectal complications with HDR (5). The most recent prospective trial from Thailand compared Iridium-192 HDR to Cesium-137 LDR brachytherapy, both given weekly during external beam radiation, and found no difference in disease control or late toxicity (7).
Since these trials were published, advanced diagnostic imaging, concurrent chemotherapy, intensity-modulated radiation therapy (IMRT), and 3-dimensional image-guided adaptive brachytherapy (3D-IGABT) with CT and/or MRI have developed during the period that HDR brachytherapy was displacing LDR for cervical cancer. Our institution began treating locally advanced cervical cancer with HDR brachytherapy in 1997. During the LDR to HDR transition period from 1997 to 2005, LDR brachytherapy accounted for about 40% of cases, and HDR brachytherapy accounted for 60% of cases. After 2005, all patients were treated with HDR brachytherapy and we transitioned from 2-dimensional (2D) brachytherapy planning to 3D-IGABT with CT/MRI. External beam radiation also transitioned from 2D planning to IMRT in 2005 (8,9). Previous analysis of clinical outcomes of 3D-IGABT, all delivered with HDR brachytherapy, showed excellent LC and CSS (10,11). As systemic control improves with chemotherapy (12), it is essential to reevaluate the value of HDR compared to LDR brachytherapy for LC. This study was undertaken to assess disease control and toxicity, comparing LDR to HDR brachytherapy, for a large American cohort treated with concurrent chemoradiation in the last 2 decades.
Methods and materials
Patients
The study population consisted of all consecutive newly diagnosed cervical cancer patients with pretreatment 18-fluorodeoxyglucose positron emission tomography (FDGPET) treated with curative-intent radiation from 1997 to 2016 at a U.S. academic center. Brachytherapy boost was LDR or HDR 2D treatment planning from 1997 to 2005 and HDR with MR-based 3D planning from 2005 to 2016. Patients alive at the last follow-up were required to have 2 years minimum follow-up. All patients underwent a complete pretreatment staging workup, including a history and physical examination, examination under anesthesia, cervical tumor biopsy, pelvic CT or MRI, and whole-body FDG-PET. Patients with International Federation of Gynecology and Obstetrics (FIGO) clinical stage IVB, clinically occult FDG-avid supraclavicular nodes, or imaging evidence of distant metastases were excluded. This retrospective analysis was approved by our institutional Human Research Protection Office with waiver of informed consent (IRB# 201808184).
External beam radiation treatment
From 1997 to 2005, whole-pelvis radiation was delivered with AP-PA beams to a total dose of 50.4 Gy in 28 daily fractions. A midline step-wedge block was used after 20 Gy to allow the majority of dose to the cervix to come from brachytherapy (13). After 2005, patients were treated with PET-guided IMRT to emulate the step-wedge technique. Details of simulation and treatment planning were previously described (9,10). The metabolic tumor volume (MTV) was contoured at the 40% threshold and prescribed 20 Gy. The clinical target volume was defined as a 7 mm expansion around pelvic vessels, which were contoured from the bifurcation of the aorta to the medial circumflex arteries and included the internal iliac and obturator nodes. Para-aortic vessels were contoured up to the renal vessels only if para-aortic nodes were metabolically involved. Metabolically active lymph nodes were included in the clinical target volume but not routinely boosted with additional radiation dose. A planning target volume expansion of 5–7 mm was prescribed to 50.4 Gy (14). From 2001 to 2013, some patients (13%) also received a parametrial boost of 5.4–14.4 Gy.
Chemotherapy
Weekly bolus cisplatin (40 mg/m2) was given concurrently with radiation in 88% of patients. The median number of cycles given was 6 (0–7) with no difference in chemotherapy use or number of cycles of chemotherapy between LDR and HDR brachytherapy groups (p = 0.61).
Intracavitary brachytherapy
The LDR brachytherapy cohort received 2 weeks of external irradiation, their first LDR brachytherapy, two additional weeks of external irradiation, their second LDR brachytherapy, and then completed two final weeks of external irradiation. LDR brachytherapy was delivered using Cesium-137 intracavitary Fletcher-Suit-Delclos implants prescribed to 8000–9000 mgRaEq-h or total reference air kerma 57,810–65,036 cGy-cm2. The dose to point A was approximately 30–34 Gy delivered over 50 h for each fraction. HDR brachytherapy was delivered using an Iridium-192 source and tandem and ovoid intracavitary applicators administered in six weekly fractions during external irradiation. Each HDR implant was prescribed to 800–900 mgRaEq-h, or 5781–6504 cGy-cm2, and the dose to point A was 6.5–7.3 Gy for each weekly fraction. External irradiation was administered 4 days per week and HDR brachytherapy 1 day per week. The Iridium-192 source dwell positions and times were planned to mimic the LDR brachytherapy pear-shaped dose distribution.
In 2005, our institution changed from 2D planning to 3D-IGABT, first with CT and then MRI in 2007. CT and MRI were obtained for planning each HDR fraction. Treatment planning parameters with MRI were previously described (10,11). Briefly, the tumor was delineated based on T2-weighted and apparent diffusion coefficient MR images. The bladder, rectum, and sigmoid colon were contoured on the T2-weighted images. The weekly equivalent dose in 2 Gy fractions (EQD2) to the tumor (α/β = 10) and normal structures (α/β = 3) was tracked (15), and, if needed, the HDR brachytherapy dose was modified to maximize tumor control and limit late toxicities. After 2014, the goal dose to 90% of the gross tumor volume was EQD2 > 100 Gy or mean gross tumor volume dose > 260 Gy (10). The dose to ≥ 2 mL of the sigmoid colon, rectal, and bladder volume was limited to EQD2 < 75 Gy, < 75 Gy, and <90 Gy, respectively.
Outcomes
Patients were followed with clinical examinations approximately every 2 months for the first 6 months, every 3 months for the next 2 years, and then every 6 months. FDG-PET was performed 3 months after completion of treatment in most patients and then as indicated by clinical examination or symptoms. Local failure was defined as persistent disease or failure at the cervix. LC and CSS were measured from the date of their initial diagnostic PET scan. Common Terminology Criteria for Adverse Events Version 3.0 was used to score the maximum late toxicity.
Statistical analyses
The Fisher’s exact test was used to compare categorical data and the nonparametric Mann-Whitney U test was used for continuous variables to compare the LDR and HDR cohorts. Kaplan-Meier survival analyses were performed with statistical significance calculated by log-rank test. To reduce the possible influence of confounders in this retrospective study, a 1:1 propensity matching between LDR and HDR patient groups was performed using the nearest neighbor technique with a caliper distance of 0.15 of the SD of the logit of the propensity score. Matching variables included age, clinical stage, histology, PET lymph node status, concurrent chemotherapy, and external irradiation and brachytherapy planning. Balance between the matched groups was performed by comparing standardized mean differences for each of the matching variables and confirming that no standardized mean difference was greater than 0.20.
The cumulative hazard function was used to compare the rates at conditional times for the development of grade three and greater bowel and bladder toxicities between the LDR and HDR brachytherapy groups. Cox regression analysis was done for both univariable and multivariable modeling of LC and for the propensity-matched groups. Factors significant on univariable analysis (p < 0.1) were entered in a forward-conditional multivariable model. Final significance was defined as p ≤ 0.05, and all tests were two-tailed. Statistical analyses were done in SPSS, version 23 (IBM, Armonk, NY).
Results
Patient and tumor characteristics
The LDR brachytherapy group (n = 104) consisted of tumors with higher FIGO stage (p < 0.001) compared to the HDR brachytherapy group (n = 505). In the subset of patients with MTVs and PET maximum standard uptake value data available, the HDR group had smaller tumors (p < 0.002) with higher maximum standard uptake value (p = 0.002) than the LDR group. There was no difference in distribution of FDG-avid lymph nodes (p = 0.49) between the two groups. Nearly all (88%) patients were treated with concurrent chemotherapy. Patient and treatment characteristics are summarized in Table 1. The median length of treatment for LDR and HDR groups was 52 and 49 days, respectively.
Table 1.
Patient/tumor and treatment factors
| Baseline variables | LDR (n = 104) | HDR (n = 505) | p value | Matched LDR (n = 90) | Matched HDR (n = 90) |
|---|---|---|---|---|---|
| Median age | 49 (27–87) | 50 (23–90) | 0.40 | 50 (28–87) | 49 (26–81) |
| Histology | 0.02 | ||||
| Squamous | 96 (92%) | 437 (86.5%) | 82 (91%) | 79 (88%) | |
| Adenocarcinoma | 4 (4%) | 60 (12%) | 4 (4.5%) | 4 (4%) | |
| Adenosquamous | 4 (4%) | 8 (1.5%) | 4 (4.5%) | 7 (8%) | |
| FIGO stage | <0.001 | ||||
| Ib1 | 4 (4%) | 77 (15%) | 4 (4%) | 9 (10%) | |
| Ib2 | 13(12.5%) | 125 (25%) | 13 (14%) | 16 (18%) | |
| IIa | 0 (0%) | 9 (2%) | 0 (0%) | 3 (3%) | |
| IIb | 45 (43%) | 187 (37%) | 44 (49%) | 35 (39%) | |
| IIIa | 2 (2%) | 8 (1.5%) | 0 (0%) | 0 (0%) | |
| IIIb | 38 (36.5%) | 91 (18%) | 27 (30%) | 27 (30%) | |
| IVa | 2 (2%) | 8 (1.5%) | 2 (2%) | 0 (0%) | |
| Median metabolic tumor volume (cc) | 57 (9–216)a | 34 (1–536)b | <0.001 | Missing data, not matched | Missing data, not matched |
| Median cervix SUVmax | 11 (2.3–36)c | 13 (2.0–60)d | 0.002 | Missing data, not matched | Missing data, not matched |
| PET lymph nodes | 0.49 | ||||
| None | 47 (45%) | 224 (44%) | 42 (46.5%) | 40 (44%) | |
| Pelvic | 47 (45%) | 211 (42%) | 41 (45.5%) | 36 (40%) | |
| Para-aortic | 10 (10%) | 70 (14%) | 7 (8%) | 14 (16%) | |
| Brachytherapy planning | <0.001 | ||||
| 2D | 104 (100%) | 196 (39%) | 90 (100%) | 90 (100%) | |
| 3D CT | 0 (0%) | 51 (10%) | 0 (0%) | 0 (0%) | |
| 3D MRI | 0 (0%) | 258 (51%) | 0 (0%) | 0 (0%) | |
| External beam planning | <0.001 | ||||
| 2D | 104 (100%) | 174 (33%) | 90 (100%) | 90 (100%) | |
| IMRT | 0 (0%) | 331 (67%) | 0 (0%) | 0 (0%) | |
| Chemotherapy | 91 (88%) | 445 (88%) | 0.86 | 78 (87%) | 83 (92%) |
FIGO = International Federation of Gynecology and Obstetrics; HDR = high-dose-rate; LDR = low-dose-rate; IMRT = intensity-modulated radiation therapy.
Incomplete data:
N = 37 (36%).
N = 357 (71%).
N = 57 (55%).
N = 425 (84%).
Local control and cancer-specific survival of LDR vs. HDR brachytherapy
The median follow-up was 9.4 years (2.1–19.7) for patients alive at the time of last follow-up. There were 266 (44%) deaths with a median time to death of 23 months (2–210). In the whole cohort, the 5-year LC rates were 89% LDR and 86% HDR, p = 0.24; the 5-year CSS rates were 66% LDR and 69% HDR, p = 0.50 (Fig. 1). FIGO stage III-IVA tumors, larger MTV, PET-positive para-aortic nodes, and no chemotherapy use were significantly associated with worse LC in univariable Cox regression (p < 0.05). Adjusted for these factors, only FIGO stage III-IVA (HR=2.4, 95% CI 1.3–4.4) was associated with worse LC in a multivariable model (Table 2).
Fig. 1.
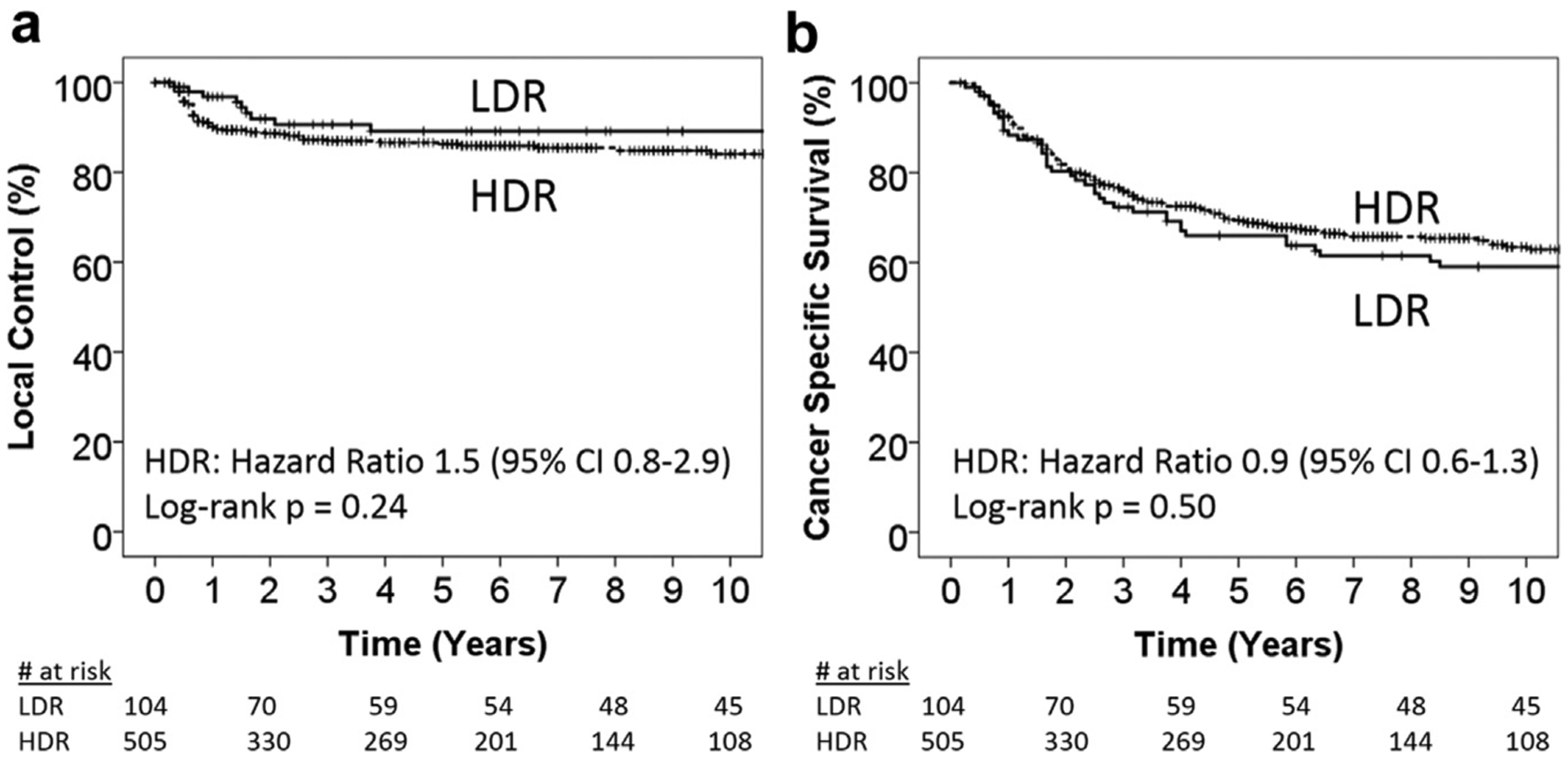
Kaplan-Meier plots comparing LDR vs. HDR outcomes for (a) local control (LC) and (b) cancer-specific survival (CSS) in the whole cohort. HDR = high-dose-rate; LDR = low-dose-rate.
Table 2.
Univariable and multivariable cox regression for local control in the whole cohort
| Tested variables | UVA HR (95% CI) | p value | MVA HR (95% CI) | p value |
|---|---|---|---|---|
| Age | 1.004 (0.99–1.02) | 0.66 | ||
| Histology | ||||
| Squamous | Ref | |||
| Adenocarcinoma | 1.05 (0.50–2.19) | 0.90 | ||
| Adenosquamous | 0.72 (0.10–5.21) | 0.75 | ||
| FIGO stage | ||||
| I-IIb | Ref | Ref | ||
| III-IVa | 1.99 (1.23-3.22) | 0.005 | 2.40 (1.32-4.36) | 0.004 |
| Metabolic tumor volume (cc) | 1.004 (1.000-1.007) | 0.03 | NS | |
| Cervix SUVmax | 1.03 (0.99–1.05) | 0.065 | NS | |
| PET LNs | ||||
| None | Ref | NS | ||
| Pelvic | 1.25 (0.76–2.06) | 0.38 | ||
| Aortic | 2.07 (1.06-4.05) | 0.03 | ||
| Brachytherapy | ||||
| LDR | Ref | |||
| HDR | 1.50 (0.76–2.94) | 0.24 | ||
| Brachytherapy planning | ||||
| 2D | Ref | |||
| 3D CT | 0.50 (0.15–1.62) | 0.25 | ||
| 3D MRI | 1.30 (0.81–2.08) | 0.29 | ||
| External beam planning | ||||
| 2D | Ref | |||
| IMRT | 1.005 (0.632–1.599) | 0.98 | ||
| Chemotherapy | ||||
| Yes | Ref | |||
| No | 1.82 (1.03-3.22) | 0.04 | NS | |
| Treatment length (days) | 0.92 (0.47–1.8) | 0.80 |
FIGO = International Federation of Gynecology and Obstetrics; PET = positron emission tomography; LNs = lymph nodes; IMRT = intensity-modulated radiation therapy; Ref = Reference; NS = Not significant; UVA = univariable; MVA = multivariable.
Hazard ratio (HR) and 95% confidence interval (CI) are shown for each variable.
Bolded values were statistically significant variables.
Late toxicities of LDR vs. HDR brachytherapy
Overall, there were 87 (14%) cases of late grade ≥3 bowel/bladder complications (LDR 21 [20%] vs. HDR 66 [13%], Fisher’s exact p = 0.06); the cumulative incidence is shown in Fig. 2a. However, 13 cases of late toxicity coincided with local tumor recurrence in the HDR group and could be considered a consequence of continued tumor invasion rather than a radiation-induced complication. Adjusting for coinciding tumor recurrence, Fig. 2b shows the 5-year cumulative incidence of late grade ≥3 bowel/bladder complications was 27% LDR vs. 12% HDR, p = 0.007.
Fig. 2.
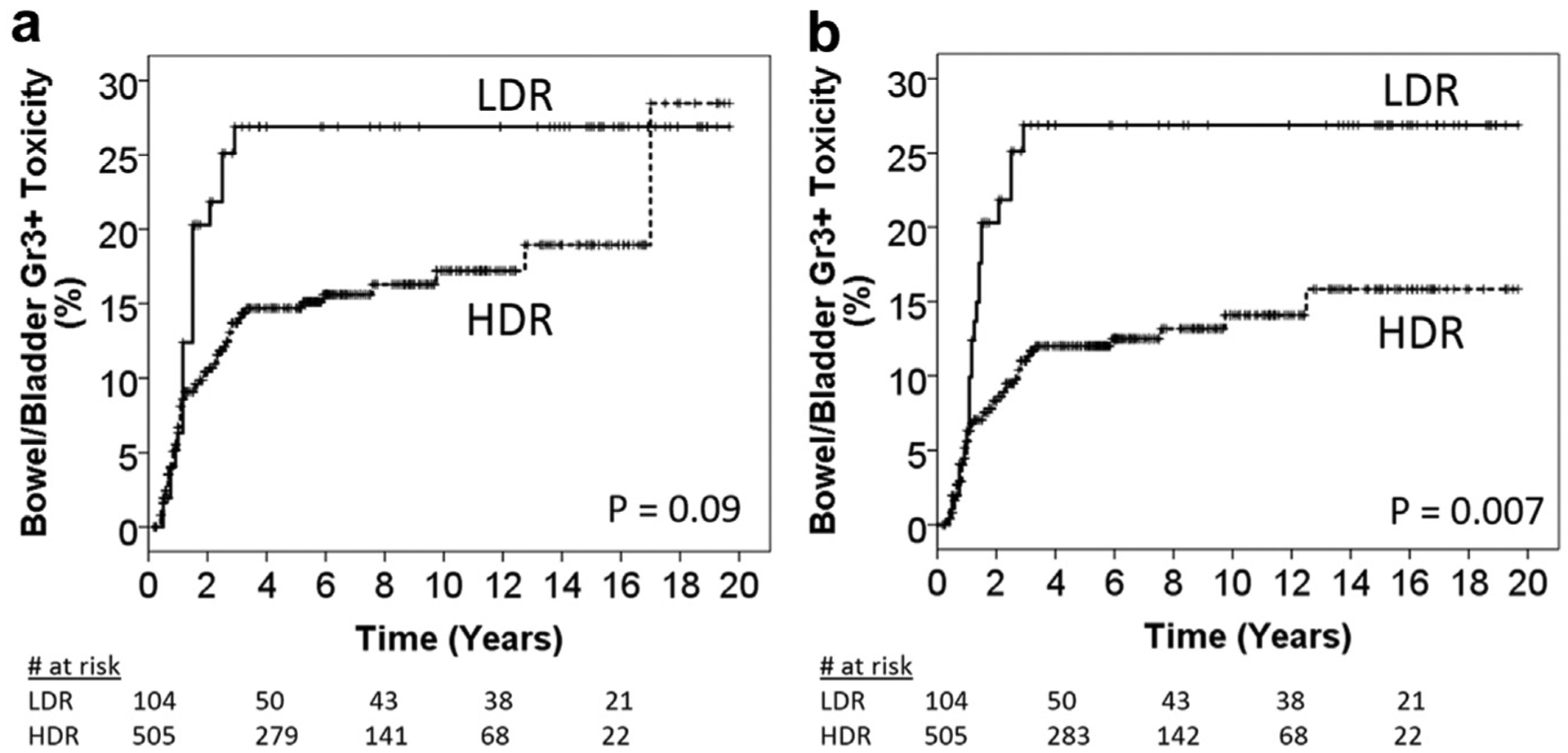
(a) Cumulative hazard plot of grade three or higher bowel/bladder late toxicities in the LDR and HDR groups. (b) Cumulative hazard plot of grade three or higher bowel/bladder late toxicities in the LDR and HDR groups without censoring for patients with coinciding tumor recurrence. HDR = high-dose-rate; LDR = low-dose-rate.
Local control and toxicity for FIGO III-IVA tumors
To address the question of whether LDR or HDR brachytherapy was more effective in controlling large primary tumors, we did a subset analysis of the FIGO stage III-IVA tumors. Figure 3a shows there was no difference in LC between LDR and HDR brachytherapy for the FIGO stage I-II tumors (p = 0.52). There was a statistically nonsignificant higher rate of 5-year LC for LDR (86%) compared to HDR (75%) brachytherapy in the FIGO stage III-IVA subgroup (p = 0.09). Late bowel/bladder toxicity was also significantly higher in the FIGO stage III-IVA LDR subgroup, with a 5-year cumulative incidence rate of 47% LDR vs. 15% HDR (p = 0.03) after adjusting for local recurrences (Supplemental Fig. 1).
Fig. 3.
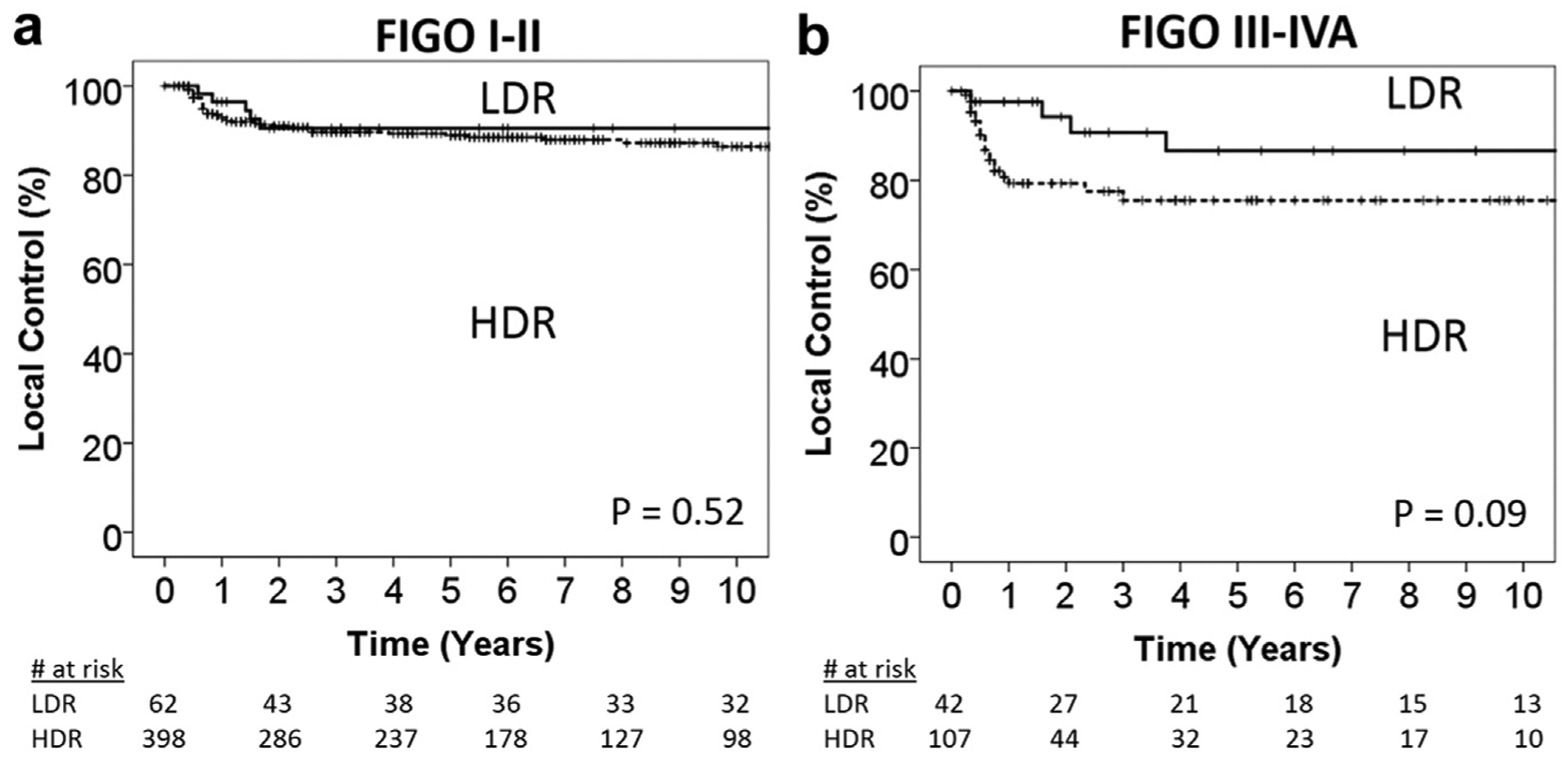
Kaplan-Meier plots of local control comparing LDR vs. HDR for patients with (a) stage I-II disease and (b) stage III-IVA disease. FIGO = International Federation of Gynecology and Obstetrics; HDR = high-dose-rate; LDR = low-dose-rate.
Local control, survival, and toxicity for propensity-matched cohort
The propensity-matched analysis was performed with 90/104 (86.5%) of LDR patients who were matched to 90/505 (17.8%) of the HDR patients (Table 1). All patients received 2D-planned external beam and 2D-planned brachytherapy radiation. In the propensity-matched cohort, 5-year LC was 88% LDR and 82% HDR, p = 0.26; 5 year CSS was 66% LDR and 58% HDR, p = 0.19 (Fig. 4a and 4b). The 5-year rate of grade three or higher bowel/bladder late toxicities after accounting for coinciding local recurrences was 23% LDR and 16% HDR, p = 0.44 (Fig. 4c).
Fig. 4.
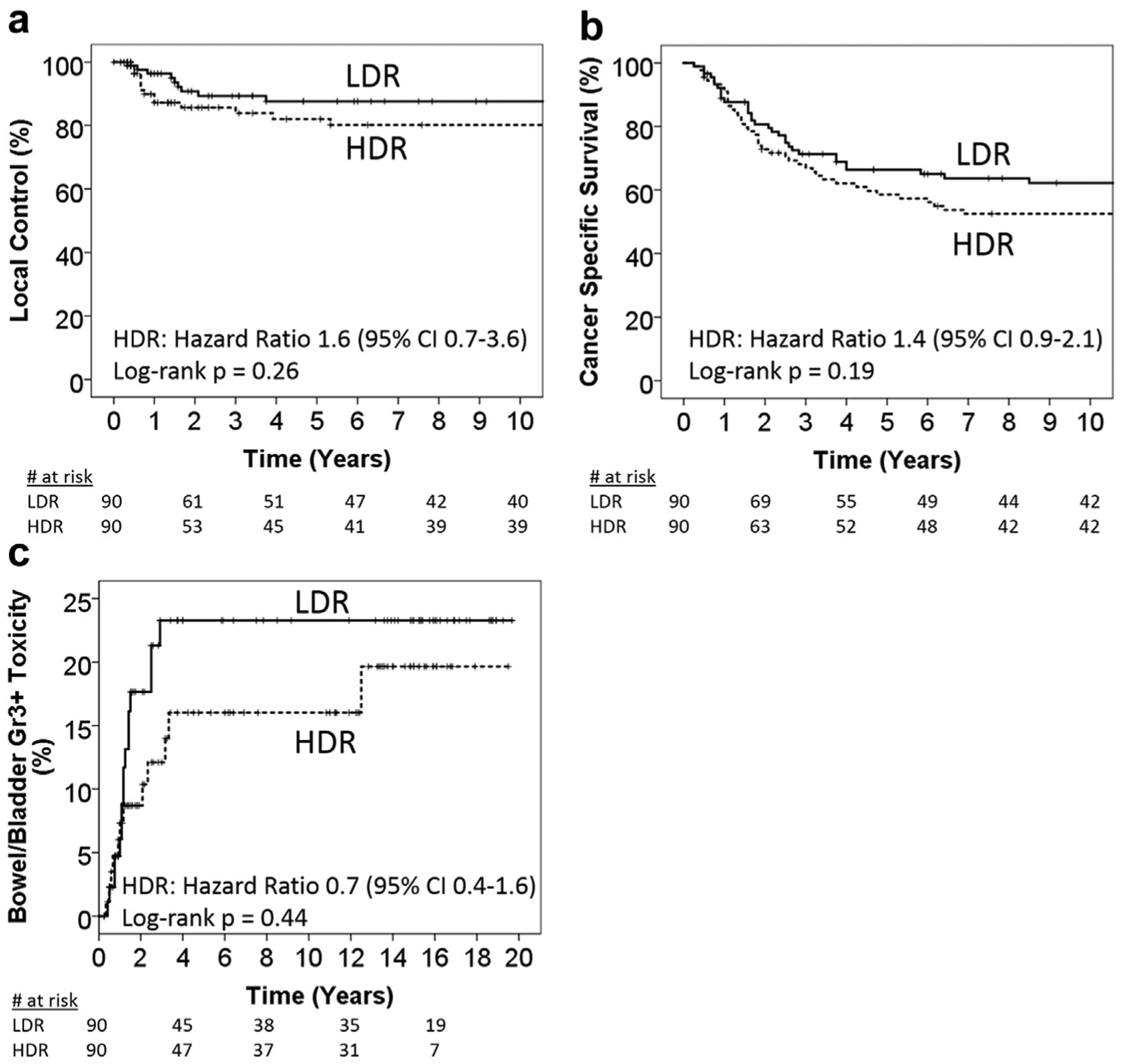
Propensity-matched cohort. Kaplan-Meier plots comparing LDR vs. HDR outcomes for (a) local control, (b) cancer-specific survival, and (c) cumulative hazard plot of grade three or higher bowel/bladder late toxicities in the LDR and HDR groups without censoring for patients with coinciding tumor recurrence. HDR = high-dose-rate; LDR = low-dose-rate.
Discussion
This study reports the results of an American cohort comparing LC and late complications after LDR or HDR brachytherapies when most patients received concurrent cisplatin. Overall, there was no difference in LC or CSS. The 2D-planned LDR brachytherapy was associated with higher rates of late bowel and bladder toxicities, especially in FIGO stage III-IVB tumors.
Our results are consistent with four randomized trials before standard concurrent chemotherapy use, showing no overall difference in local failure between LDR and HDR brachytherapies (4–7). The local failure rate for LDR in those four trials ranged from 11 to 24%, and the local failure rate for HDR ranged from 12% to 27%. A metaanalysis of these four published trials, including unpublished data from Tata Memorial Hospital, found no statistically significant difference in LC, mortality, or late grade 3–4 bladder or bowel complications (16). In treating stage III tumors, Teshima et al. (4) had better LC with HDR (67% vs. 54%) but a higher rate of moderate-severe complications in the HDR arm (10% vs. 4%). Interestingly, as the trials modernized, the LC rates for stage III tumors also improved. The most recent trial showed LC rates of 93% for stage IIIB patients regardless of LDR or HDR brachytherapy (7). Our rates of LC for stage III-IVA tumors are comparable to these trials. We report no significant difference in LC with LDR compared to HDR, but the rates of late toxicity were significantly higher in the LDR cohort compared to HDR brachytherapy.
Late bowel and bladder toxicities were relatively high in our LDR cohort (27%) compared to other reports. The combined rate of late grade 3–4 rectal and bladder toxicities in the prospective randomized trials was only 4.3% (16), but concurrent chemotherapy was not used in these trials. Other retrospective series comparing LDR to HDR brachytherapy have reported late bowel and bladder complication rates from LDR brachytherapy ranging from 5% to 21% (17–21). The University of Virginia reported a 10% rate of late toxicity in cervical cancer patients treated with chemoradiation and LDR brachytherapy (21). The combination of larger tumor volume in the LDR group (median MTV 57 cc) and concurrent chemotherapy could have contributed to higher late complication rates in our study.
Advanced imaging and 3D-IGABT likely reduced the rate of late complications in our HDR group. When comparing LDR to HDR brachytherapy in the 2D planning era of treatment, there was no significant difference in late complications. Visualizing normal structures in relation to the brachytherapy implant can prevent treating small bowel in a clinically occult perforated uterus (22), and the geometry of the implant may be better optimized to reduce hot spots in the rectum and bladder. RetroEMBRACE, a multicenter study from Europe, found 3D-IGABT improved LC compared to historical controls, especially for larger tumors, while the 5-year rate of late complications was a very favorable 11% (23) (our 3D-IGABT HDR 5-year complication rate was 10%). EMBRACE has prospectively validated The Groupe Européen de Curiethérapie and the European Society for Radiotherapy & Oncology (GEC-ESTRO) approach to 3D-IGABT, reporting 3-year late bowel and bladder toxicities of 5% and 5.3%, respectively (24,25). Because no LDR patients were planned with 3D-IGABT in our study, we can only conclude that HDR brachytherapy with 3D-IGABT resulted in fewer late complications.
This retrospective study has some limitations. Brachytherapy was cotransitioned from LDR to HDR with a number of other changes in treatment planning, including IMRT and 3D-IGABT, which could have confounded our analysis. Notably, these other factors did not impact LC in multivariable analysis or in our propensity-matched cohort, although the patient numbers were limited for comparison. Second, we did not undertake a dosimetric analysis to see if mean doses to tumor were different between LDR and HDR brachytherapy plans, which could affect tumor control (10). However, all patients were treated according to standardized institutional guidelines that did not significantly change during the study period. Groups in Europe (26) and the United States (27) have used interstitial techniques in addition to intracavitary brachytherapy to improve dosimetric coverage of bulky tumors and achieved excellent LC. None of the patients in this study were treated with interstitial needles, so the brachytherapy implant dosimetry between LDR and HDR should be consistent for comparison. Third, late toxicity was not assessed prospectively, which may have resulted in underestimation of complication rates. Finally, these results are unique to the Mallinckrodt Institute of Radiology treatment paradigm and may not be generalizable to other clinics.
Conclusions
In conclusion, our study shows the increased adoption of HDR brachytherapy is safe in a large American cohort, even with concurrent chemotherapy. More work is needed to be done to improve LC while minimizing toxicity in the treatment of bulky and FIGO stage III-IVA tumors.
Supplementary Material
Acknowledgments
P.W.G. is supported by NIH R21 CA223799-01. J.S. is supported by NIH R01 CA181745-01. S.M. is supported by NIH K12 CA167540. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial disclosure: The authors declared no financial disclosures.
Conflicts of interest: P.S. reports a grant from Varian, outside the submitted work. J.Z. reports stock in ViewRay and being an invited speaker for Varian on topics outside the submitted work. In addition, J.Z. has a U.S. Patent 9,248,310 issued. J.F.W. reports grants from NIH, personal fees and other from American Association of Physicists in Medicine, personal fees and other from Chinese Society of Medical Physics, personal fees and other from Peking University, personal fees and other from Hong Kong Polytechnic University, grants and other from Varian Medical Systems, outside the submitted work.
Footnotes
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.brachy.2018.11.008.
References
- [1].Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy: A survey of the American brachytherapy society. Int J Radiat Oncol Biol Phys 2010;76:104–109. [DOI] [PubMed] [Google Scholar]
- [2].Patankar SS, Tergas AI, Deutsch I, et al. High versus low-dose rate brachytherapy for cervical cancer. Gynecol Oncol 2015;136:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stewart AJ, Viswanathan AN. Current controversies in high-dose-rate versus low-dose-rate brachytherapy for cervical cancer. Cancer 2006; 107:908–915. [DOI] [PubMed] [Google Scholar]
- [4].Teshima T, Inoue T, Ikeda H, et al. High-dose rate and low-dose rate intracavitary therapy for carcinoma of the uterine cervix. Final results of osaka university hospital. Cancer 1993;72:2409–2414. [DOI] [PubMed] [Google Scholar]
- [5].Patel FD, Sharma SC, Negi PS, et al. Low dose rate vs. high dose rate brachytherapy in the treatment of carcinoma of the uterine cervix: A clinical trial. Int J Radiat Oncol Biol Phys 1994;28:335–341. [DOI] [PubMed] [Google Scholar]
- [6].Hareyama M, Sakata KI, Oouchi A, et al. High-dose-rate versus low-dose-rate intracavitary therapy for carcinoma of the uterine cervix: A randomized trial. Cancer 2002;94:117–124. [DOI] [PubMed] [Google Scholar]
- [7].Lertsanguansinchai P, Lertbutsayanukul C, Shotelersuk K, et al. Phase III randomized trial comparing LDR and HDR brachytherapy in treatment of cervical carcinoma. Int J Radiat Oncol Biol Phys 2004;59:1424–1431. [DOI] [PubMed] [Google Scholar]
- [8].Macdonald DM, Lin LL, Biehl K, et al. Combined intensity-modulated radiation therapy and brachytherapy in the treatment of cervical cancer. Int J Radiat Oncol Biol Phys 2008;71:618–624. [DOI] [PubMed] [Google Scholar]
- [9].Kidd EA, Siegel BA, Dehdashti F, et al. Clinical outcomes of definitive intensity-modulated radiation therapy with fluorodeoxyglucose-positron emission tomography simulation in patients with locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2010;77: 1085–1091. [DOI] [PubMed] [Google Scholar]
- [10].Dyk P, Jiang N, Sun B, et al. Cervical gross tumor volume dose predicts local control using magnetic resonance imaging/diffusion-weighted imaging d guided high-dose-rate and positron emission tomography/computed tomography d guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2014;90:794–801. [DOI] [PubMed] [Google Scholar]
- [11].Zoberi JE, Garcia-Ramirez J, Hu Y, et al. Clinical implementation of multisequence MRI-based adaptive intracavitary brachytherapy for cervix cancer. J Appl Clin Med Phys 2016;17:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of Radiation Therapy Oncology Group Trial (RTOG) 90–01. J Clin Oncol 2004;22:872–880. [DOI] [PubMed] [Google Scholar]
- [13].Perez CA, Kavanaugh B. In: Perez CA, Luther L, Halperin E, editors. Principle and Practice of Radiation Oncology: Uterine Cervix. 4th ed Philadelphia: Lipincott, Williams & Williams; 2003. p. 1800–1915. [Google Scholar]
- [14].Santanam L, Esthappan J, Mutic S, et al. Estimation of setup uncertainty using planar and MVCT imaging for gynecologic malignancies. Int J Radiat Oncol Biol Phys 2008;71:1511–1517. [DOI] [PubMed] [Google Scholar]
- [15].Sun B, Yang D, Esthappan J, et al. Three-dimensional dose accumulation in pseudo-split-field IMRT and brachytherapy for locally advanced cervical cancer. Brachytherapy 2015;14:481–489. [DOI] [PubMed] [Google Scholar]
- [16].Viani GA, Manta GB, Stefano EJ, et al. Brachytherapy for cervix cancer : Low-dose rate or high-dose rate brachytherapy e a metaanalysis of clinical trials. J Exp Clin Cancer Res 2009;28:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sarkaria JN, Petereit DG, Stitt JA, et al. A comparison of the efficacy and complication rates of low dose-rate versus high dose-rate brachytherapy in the treatment of uterine cervical carcinoma. Int J Radiat Oncol Biol Phys 1994;30:75–82. [DOI] [PubMed] [Google Scholar]
- [18].Falkenberg E, Kim RY, Meleth S, et al. Low-dose-rate vs. high-dose-rate intracavitary brachytherapy for carcinoma of the cervix: The University of Alabama at Birmingham (UAB) experience. Brachytherapy 2006;5:49–55. [DOI] [PubMed] [Google Scholar]
- [19].Ferrigno R, Nishimoto IN, Ribeiro Dos Santos Novaes PE, et al. Comparison of low and high dose rate brachytherapy in the treatment of uterine cervix cancer. Retrospective analysis of two sequential series. Int J Radiat Oncol Biol Phys 2005;62:1108–1116. [DOI] [PubMed] [Google Scholar]
- [20].Orton CG, Seyedsadar M, Somnay A. Comparison of high and low dose rate remote afterloading for cervix and the importance of fractionation. Int J Radiat Oncol Biol Phys 1991;21:1425–1434. [DOI] [PubMed] [Google Scholar]
- [21].Romano KD, Pugh KJ, Trifiletti DM, et al. Transition from LDR to HDR brachytherapy for cervical cancer: Evaluation of tumor control, survival, and toxicity. Brachytherapy 2017;16:378–386. [DOI] [PubMed] [Google Scholar]
- [22].Barnes EA, Thomas G, Ackerman I, et al. Prospective comparison of clinical and computed tomography assessment in detecting uterine perforation with intracavitary brachytherapy for carcinoma of the cervix. Int J Gynecol Cancer 2007;17:821–826. [DOI] [PubMed] [Google Scholar]
- [23].Sturdza A, Pötter R, Fokdal LU, et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol 2016;120:428–433. [DOI] [PubMed] [Google Scholar]
- [24].Boje N, Jensen K, Pötter R, et al. Bowel morbidity following radio-chemotherapy and image-guided adaptive brachytherapy for cervical cancer : Physician- and patient reported outcome from the EMBRACE study. Radiother Oncol 2018;127:431–439. [DOI] [PubMed] [Google Scholar]
- [25].Fokdal L, Pötter R, Kirchheiner K, et al. Physician assessed and patient reported urinary morbidity after radio-chemotherapy and image guided adaptive brachytherapy for locally advanced cervical cancer. Radiother Oncol 2018;127:423–430. [DOI] [PubMed] [Google Scholar]
- [26].Pötter R, Georg P, Dimopoulos JCA, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 2011; 100:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tinkle CL, Weinberg V, Chen LM, et al. Inverse planned high-dose-rate brachytherapy for locoregionally advanced cervical cancer: 4-year outcomes. Int J Radiat Oncol Biol Phys 2015;92:1093–1100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


