Abstract
Early antiretroviral therapy (ART) initiation is essential, but linkage to care following community-based services is often poor, and inadequately understood. This study examined factors influencing linkage to care following home-based HIV-testing services (HBHTS) in a hyper-endemic setting in South Africa. HBHTS was offered to participants (N=10,236) enrolled in the second HIV Incidence Provincial Surveillance System survey (2015–2016), KwaZulu-Natal. Follow-up telephone surveys with 196 of the 313 individuals diagnosed HIV-positive through HBHTS were used to measure linkage to care (i.e., a clinic visit within 12 weeks) and ART-initiation. Among newly diagnosed individuals (N=183), 55% linked to care, and 21% of those who were ART-eligible started treatment within 12 weeks. Linkage to care was less likely among participants who had doubted their HIV-diagnosis (aOR:0.46, 95%CI: 0.23–0.93) and more likely among participants who had disclosed their HIV-status (aOR:2.31, 95%CI: 1.07–4.97). Reasons for not linking to care included no time (61%), only wanting to start treatment when sick (48%), fear of side-effects (33%), and not believing the HIV-diagnosis (16%). Results indicate that HBHTS needs to be paired with targeted interventions to facilitate early linkage to care. Interventions are required to counter denial of HIV status and facilitate early linkage to care among healthier individuals.
Keywords: community-based HIV testing services, home-based HIV testing services, linkage to care, antiretroviral therapy, HIV treatment cascade, Southern Africa
Introduction
Antiretroviral therapy (ART) has the potential to curb the effects of the HIV epidemic. ART reduces AIDS-related morbidity and mortality, with gains in clinical outcomes the greatest among individuals who start treatment early (Johnson et al., 2013; The INSIGHT START Study Group, 2015). ART is also effective in reducing onward HIV transmission (Cohen et al., 2011) and consequently for reducing HIV incidence. Accordingly, the World Health Organisation (WHO) recommends ART for all HIV-positive individuals (WHO, 2016), and South Africa adopted a ‘test-and-treat’ policy in September 2016 (Motsoaledi, 2016).
HIV diagnosis and ART initiation early in disease progression are key to the success of the treat-all approach. However, a substantial proportion of people living with HIV remain undiagnosed (UNAIDS, 2014), and many seek care only once their immune system is severely compromised (Carmona et al., 2018; Lahuerta et al., 2014). Home-based HIV Testing Services (HBHTS) are effective in reaching previously undiagnosed individuals (Lewis et al., 2019; Sharma, Ying, Tarr, & Barnabas, 2015), and an important component of the test-and-treat strategy (WHO, 2016). Poor rates of linkage to care subsequent to HBHTS could, however, undermine the potential benefits of early detection of HIV-positive cases.
In the absence of interventions to facilitate linkage to care, the proportion of individuals linking to care after HBHTS is low in many countries (Sharma et al., 2015). In Kenya, 15% of participants had seen a clinician within three years of diagnosis (Genberg et al., 2015). In Swaziland, 34% of individuals testing HIV-positive during HBHTS linked to HIV care within six months (Parker et al., 2015). In South Africa, the proportion linking to care following HBHTS was 62% within three months in one study (Naik et al., 2015), and 47.5% within six months in another (Iwuji et al., 2016). Important gaps remain in our understanding of poor linkages to care. A recent systematic review found that few studies have assessed the factors affecting linkage to care following HBHTS among newly diagnosed individuals (Ruzagira et al., 2017). As active case finding through HBHTS and subsequent referral to clinics for ART remains important for achieving universal treatment access, it is necessary to better understand how to effectively link newly diagnosed individuals living with HIV to care. Moreover, as most studies have focused on socioeconomic and demographic determinants of linkage to care, there is a gap in our understanding of the psychosocial factors acting as barriers to HIV care.
This study used prospective data – from men and women (15–49 years old) in a hyper-endemic setting – to assess the association between linkage to care following a first-time HIV-positive diagnosis through HBHTS and a range of demographic, socioeconomic and psychosocial factors.
Methods
Data
Data were collected from individuals testing HIV-positive during HBHTS offered as part of the second serial cross-sectional survey of the HIV Incidence Provincial Surveillance System (HIPSS) from July 2015 to June 2016 (Kharsany et al., 2015). HIPSS was conducted in two areas (Vulindlela (rural) and Greater Edendale (peri-urban)) in the uMgungundlovu District, KwaZulu-Natal province, South Africa.
Households were randomly selected using a two-stage random sampling of enumeration areas and households. One individual per household (15–49 years old) was randomly selected from a roster of eligible household members. The sample comprised 10,236 individuals. Fieldworkers collected venous blood samples from all participants for health screening (including laboratory HIV and CD4 cell count tests), administered a face-to-face questionnaire and then offered participants on-site HBHTS, as per national guidelines (Department of Health, 2015). The HBHTS protocol involved a rapid diagnostic test (Alere Determine HIV-1/2,Matsuodo, Japan) followed by a confirmatory rapid test (UniGold HIV, Trinity Biotech, Bray, Ireland) when the first test was reactive. Post-test counselling encouraged individuals to visit their nearest clinic and emphasized the benefits of early ART initiation. Eighty percent of HIPSS households were located within 2.5 kilometres of a health facility (range: 0.07–6.05km).
The sample for this linkage to care study consisted of HIPSS participants who consented to HBHTS and were newly diagnosed HIV-positive (i.e., no prior HIV test or the result from their most recent test was self-reported as negative/indeterminant). Follow-up surveys were conducted as close to 12 weeks after HBHTS as possible. Considerable follow-up efforts were made, including six contact attempts, with three outside working hours. The follow-up survey collected data on dates of clinic visits and ART initiation.
The HIPSS study was approved by the Biomedical Research Ethics Committee, University of KwaZulu-Natal (BF269/13), the Centers for Disease Control and Prevention, and the KwaZulu-Natal Provincial Department of Health (HRKM 08/14).
Measures
Linkage to care was measured during the follow-up telephone survey by asking participants the date of their first visit to an HIV clinic after their home-based HIV test. Consistent with the 3-month period commonly used in linkage to care studies (Fox, Larson, & Rosen, 2012), we created a binary variable to identify participants who linked to care within 12 weeks (i.e., 84 days).
ART initiation within 12 weeks (binary indicator) was assessed in the follow-up survey by asking participants whether they had started ART and the date of initiation. Participants missing data on the date of initiation (n=8), were assumed to have initiated ART within 12 weeks if they had visited a clinic within 38 days after HBHTS (n=7). This assumption was based on the average time in our sample between first clinic visit and ART initiation of approximately one month.
Reasons for not linking to care were collected at follow-up using responses (agree/disagree) to eleven statements relating to different reasons for not visiting the clinic.
A list of potential determinants of linkage to care was based on factors that could theoretically influence linkage to care, or those found to be associated with linkage in previous studies (Bassett et al., 2010; Bogart et al., 2013; Govindasamy, Ford, & Kranzer, 2012; Govindasamy et al., 2013; Hachfeld et al., 2015; Katz et al., 2015; Mugglin et al., 2012; Plazy, Dray-Spira, Orne-Gliemann, Dabis, & Newell, 2014; Smith et al., 2013). Measures were created using two data sources (see Supplemental Online Material File 1 for details). First, from the HIPSS baseline survey, demographic and socioeconomic variables included gender, age, relationship status, highest level of education, household monthly income, and personal monthly income. Health-related measures included baseline laboratory CD4 cell count results; and self-reported HIV-testing history, depressive symptoms: CES-D scale (Radloff, 1977), and alcohol consumption (yes/no). Psychosocial measures based on single questionnaire items included perceived stigma (i.e., ‘My friends/family would disown me if I was to contract HIV’ [agree/disagree]); knowing someone who had died of AIDS; beliefs about AIDS (i.e., ‘AIDS is probably the worst disease I could get’ [agree/disagree]); and beliefs about ART (i.e., ‘I am not afraid of contracting HIV as there are effective drugs to treat it’ [agree/disagree]). Engagement in HIV activities in the previous 12 months was assessed using multiple response options to a list of 10 activities (e.g., having watched an HIV play), with a binary variable created to indicate any activity. A binary measure of emotional support was based on whether participants reported that they had received informational/emotional support in the last 12 months from biological father; biological mother; other family member; community member; or other. Measurement of stigmatising attitudes was based on the AIDS-Related Stigma Scale (Kalichman et al., 2005), with the eight scale items adapted to the local context through pilot surveys (e.g., ‘People with HIV/AIDS should be ashamed’ [strongly disagree to strongly agree]). The Cronbach’s alpha for the set of stigmatising attitudes items is 0.74, suggesting that the items have relatively high internal consistency. Latitude and longitude co-ordinates were used to measure distance from households to the nearest health facility.
Second, retrospective data on two further potential determinants of linkage to care were collected in the follow-up telephone survey. Uncertainty relating to the accuracy of their HIV-diagnosis was assessed with the question ‘Have you ever thought that the result from your last HIV test could have been wrong?’ HIV-status disclosure was assessed by asking participants whether they had told anyone about their HIV status. Twenty participants were missing data for these measures as a result of an initial error in the questionnaire.
Analysis
First, to assess how attrition might have affected results, we compared the baseline characteristics of the sample used for the prospective analysis, to the sample without follow-up telephone survey data. The proportion of participants visiting the clinic and initiating ART within 12 weeks was estimated, with gender differences assessed using chi-squared tests. We provide data on ART initiation by ART-eligibility (as determined at baseline and according to Department of Health Policy of CD4 ≤500 cells/μL at the time of the study).
As linkage to care is necessary for ART initiation, and a high proportion of patients who link to care have been shown to start treatment (Desai, Okal, Rose, Ndivo, & Oyaro, 2017; Iwuji et al., 2016), we focused the remaining analyses on factors associated with linkage to clinics. We examined the reasons for not visiting a clinic, with gender differences assessed (using chi-squared tests), given linkage to HIV services are often poorer among men (Dorward, Mabuto, Charalambous, Fielding, & Hoffmann, 2017). Recent study results indicate that self-perceived barriers to linkage to care have predictive power (Bassett et al., 2017). Multiple logistic regression models were used to examine the association between linkage to care and each of the potential covariates described above. All regression models used the following key demographic and socioeconomic indicators as control variables: age, gender, education, and monthly household income (logged). All models also included a variable to control for the time (in days) between the baseline and the follow-up telephone survey, which could influence recall of clinic visit dates as well as key independent measures collected during the follow-up survey, such as HIV-status disclosure. All analyses were conducted using Stata 15 (Stata Corporation LP, College Station, TX).
Results
Figure 1 displays the study profile. Thirty-seven percent of HIPSS participants agreed to the HBHTS, with already knowing their HIV-positive status being the most common reason for refusal. HIV-prevalence in the rapid test sample was 8% (n=313). Follow-up telephone surveys were completed with 63% (n=196) of the HIV-positive cohort. The main reasons for incomplete follow-up were not being reachable (77%); refusal (14%); and participants not believing their HIV-positive status (9%). In total, 183 participants were included in the analysis after excluding 13 participants: eight who self-reported an HIV-positive status at baseline, one reporting being on ART, and four individuals missing data on their most recent HIV test or on linkage to care.
Figure 1.
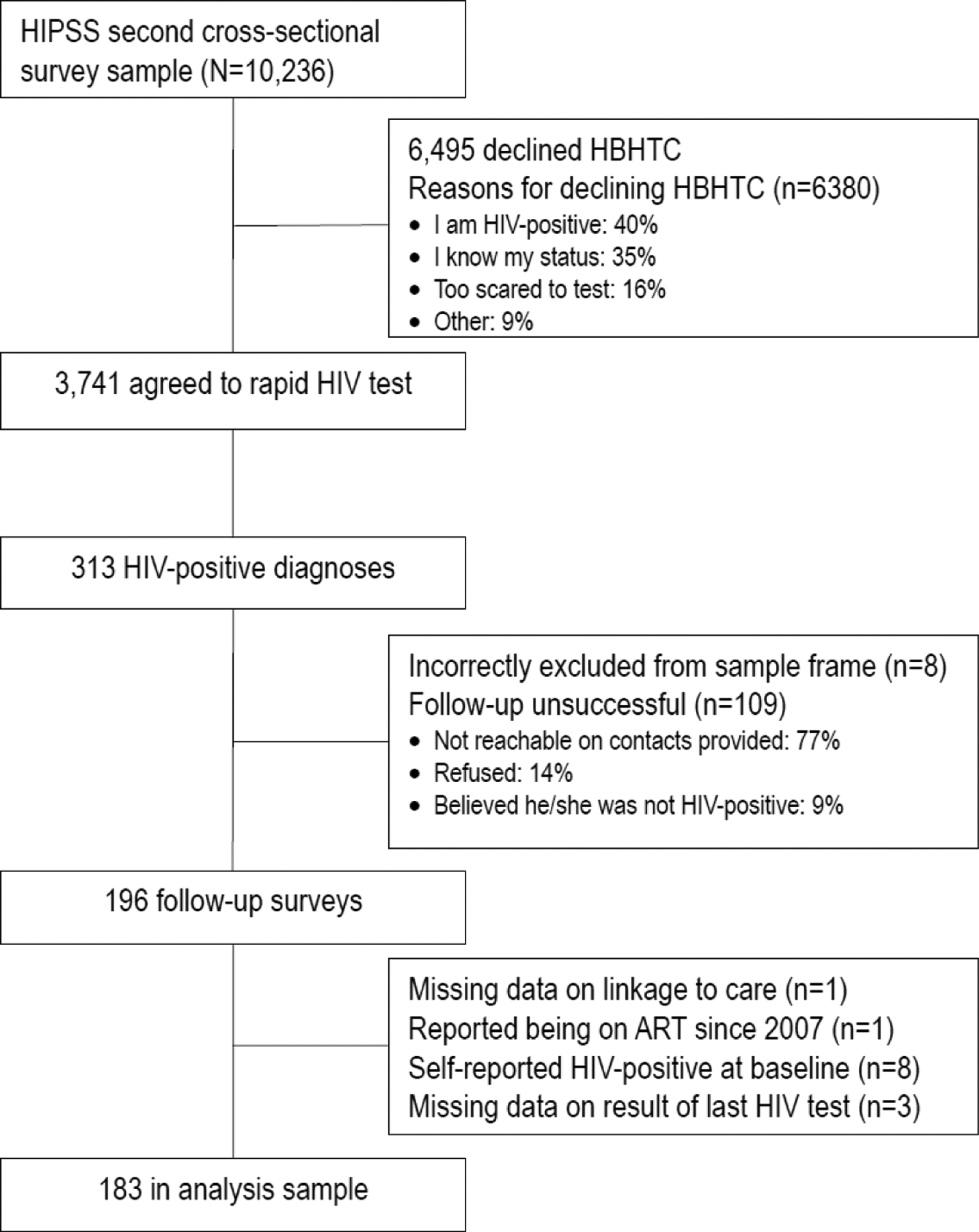
table in the linkage to care study
Our prospective study sample consisted of Black African individuals, who reported high levels of food insecurity and low levels of education (Table 1). Few were at an advanced stage of disease progression, with only 15% having a CD4 < 200 cells/μL. Differences in characteristics were generally small and statistically insignificant between participants who completed the follow-up linkage to care survey and those that did not.
Table 1.
Comparison of HIPSS participants newly diagnosed HIV-positive with linkage to care data and those who did not participate in the linkage to care survey
| (1) | (2) | (difference) | |
|---|---|---|---|
| Completed linkage survey N=183 | Did not participate in linkage survey N=109 | p-value† | |
| % (n) | % (n) | ||
| Demographics & SES | |||
| Female | 67 (123) | 61 (66) | 0.249 |
| Age | 0.153 | ||
| 15–24 | 26 (48) | 35 (38) | |
| 25–34 | 42 (77) | 41 (45) | |
| 35–44 | 22 (40) | 20 (22) | |
| 45–49 | 10 (18) | 4 (4) | |
| Black African | 100 (183) | 99 (108) | 0.194 |
| Married (or living together as if married) | 21 (39) | 9 (10) | 0.007 |
| In an on-going partnership | 88 (161) | 88 (96) | 0.981 |
| Education | |||
| Less than secondary | 7 (12) | 6 (7) | 0.964 |
| Secondary school incomplete | 55 (101) | 53 (58) | 0.742 |
| Grade 12 completed | 38 (70) | 40 (44) | 0.720 |
| Always lived in the community | 51 (93) | 54 (59) | 0.617 |
| No personal monthly income | 56 (102) | 57 (62) | 0.849 |
| Household monthly income <R2000‡ | 39 (71) | 42 (46) | 0.566 |
| Household ran out of money for food in past year | 45 (83) | 52 (55) | 0.251 |
| Health | |||
| Any TB symptoms in past 2 weeks | 8 (15) | 5 (5) | 0.238 |
| CD4 count <200 cells/μL | 15 (27) | 15 (16) | 0.986 |
| Drinks alcohol | 36 (65) | 39 (42) | 0.605 |
| Depressed (occasionally/always) | 16 (29) | 22 (24) | 0.186 |
| Psychosocial | |||
| Perceived no/low risk of HIV infection | 36 (65) | 32 (35) | 0.553 |
| Aware that ART is available at clinics | 92 (169) | 95 (103) | 0.483 |
| Agrees AIDS is worst disease you could get | 50 (91) | 48 (52) | 0.738 |
| Not afraid of contracting HIV due to ART | 42 (76) | 40 (44) | 0.845 |
| Know someone who died of AIDS | 32 (58) | 32 (33) | 0.909 |
| Perceived stigma | 10 (18) | 8 (9) | 0.652 |
| Stigmatising attitudes (any)^ | 62 (114) | 56 (61) | 0.286 |
Notes: Totals may not sum 100% due to rounding to the nearest integer.
N refers to total sample size. n refers to the size of the subset of the sample
p-value derived from chi-squared tests.
R2000 was equivalent to approximately US$129 on 1 January, 2016.
A binary variable was created to identify individuals who agreed to any of the items measuring stigmatising attitudes
Linkage to care and treatment
By 12 weeks, 55% (100/183) had visited a clinic (men:55%; women:54%). Similar proportions linked to care among the sample with a baseline CD4 count below 500 cells/μL (55% linked) and above 500 cells/μL (53% linked). The median CD4 count was 420 cells/μL (Interquartile Range (IQR):263–609) and 431 cells/μL (IQR: 261–613) among those that did, and did not, link to care respectively. Few men (12%, n=7) or women (12%, n=15) had linked to care within 4 weeks (see Figure 2). Among the sample with data on timing of ART initiation (N=174), 15% initiated onto ART within 12 weeks (men:18%, women:14%). Among ART-eligible individuals (N=106), 21% started treatment within 12 weeks (men:27%, women:17%).
Figure 2.
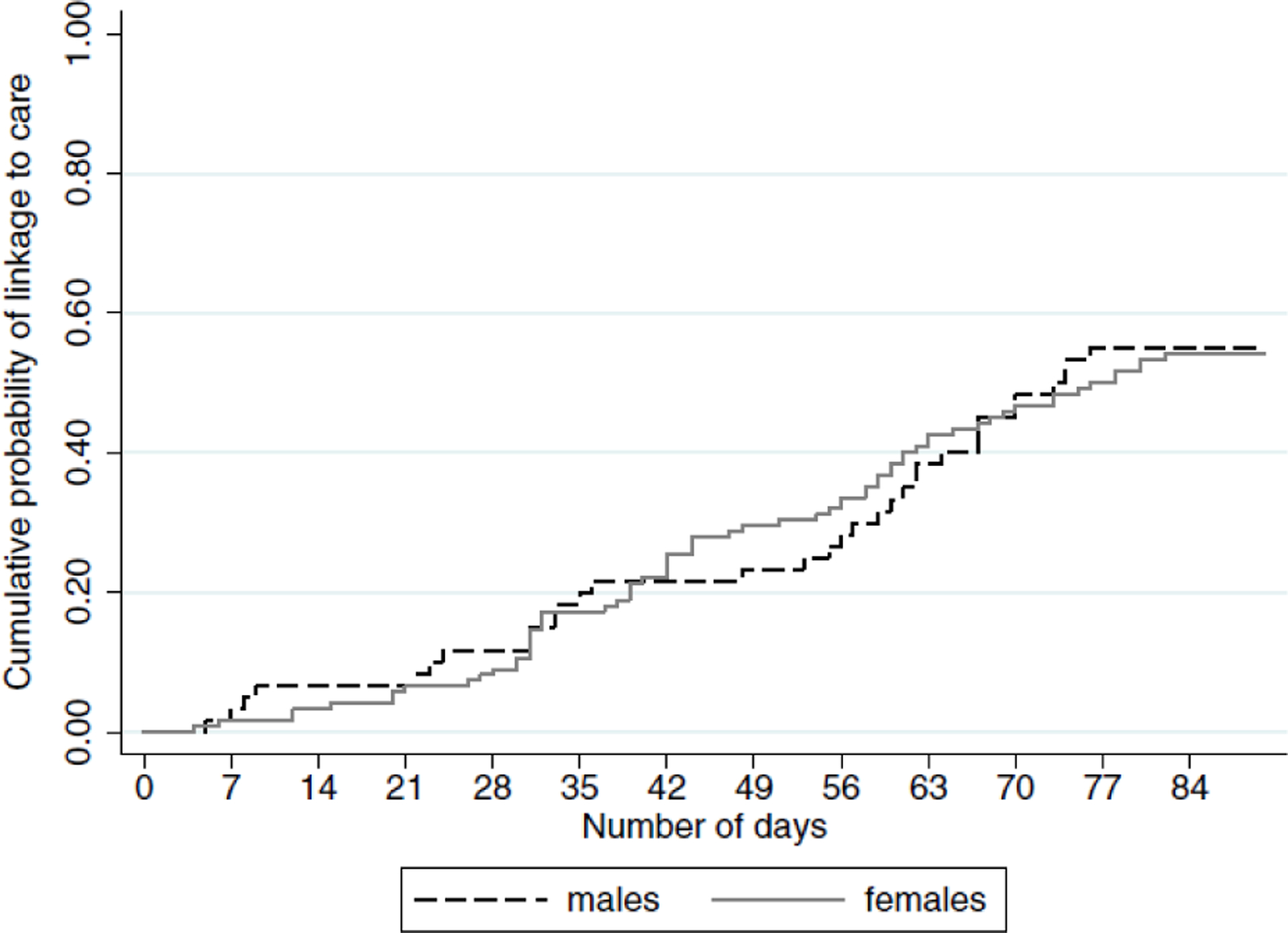
Cumulative probability of visiting a clinic within 12 weeks (linked to care) among men and women diagnosed HIV-positive though home-based HIV testing services provided to HIPSS participants
Self-reported reasons for not linking to care (Table 2)
Table 2.
Self-reported reasons for not having visited a clinic recorded in the linkage to care survey by HIPSS participants who had not attended a clinic since being diagnosed HIV-positive during home-based HIV testing services
| Reason | All (N=67) % | Female (N=44) % | Male (N=23) % | difference by gender p-value |
|---|---|---|---|---|
| I will only register for HIV treatment when I get sick | 48 | 41 | 61 | 0.120 |
| I don’t believe the HIV treatment works | 13 | 16 | 9 | 0.411 |
| I would rather go to a traditional healer | 3 | 2 | 4 | 0.636 |
| I won’t believe the HIV positive test result | 16 | 23 | 4 | 0.054 |
| I would seek treatment elsewhere | 5 | 5 | 4 | 0.970 |
| I am afraid my family/colleagues/friends will find out my HIV status | 15 | 16 | 13 | 0.755 |
| I am afraid of the side-effects of the HIV treatment | 33 | 34 | 30 | 0.762 |
| I don’t have the time to go to the clinic | 61 | 55 | 74 | 0.129 |
| I don’t have the money to go to the clinic | 21 | 18 | 26 | 0.122 |
| The medical staff at the clinic cannot be trusted | 9 | 9 | 9 | 0.957 |
| I won’t be treated with respect at the clinic | 13 | 16 | 9 | 0.450 |
Note: p-values based on chi-squared tests.
The three most common reasons were not having time (61%), only wanting to start treatment when sick (48%), and being afraid of side-effects of HIV medication (33%). Large gender differences were observed. Sixty-one percent of men (vs 41% of women, p=0.120) agreed that they will only register for treatment when sick. Twenty-three percent of women (vs 4% of men, p=0.054) did not believe the HIV positive test result. Additionally, a quarter of men reported lack of money as a barrier, and a quarter of women believed either that clinic staff cannot be trusted or that they would not be treated with respect at the clinic.
Determinants of linkage to care
Table 3 presents the analysis of factors associated with linkage to care. Participants who had ever thought that their HIV-diagnosis could be wrong were less likely to have linked to care (aOR:0.46, 95%CI:0.23–0.93, p=0.031). Linkage to care was more likely among participants who had disclosed their HIV status to someone (aOR:2.31, 95%CI:1.07–4.97, p=0.033). None of the other potential determinants were significantly associated with linkage to care.
Table 3.
Factors associated with visiting an HIV clinic within 12 weeks (linked to care) among HIPSS participants newly diagnosed HIV-positive during home-based HIV testing services
| Linked to care | Odds ratio from unadjusted logit models | Adjusted odds ratio from multivariable logit models^ | |||
|---|---|---|---|---|---|
| % (n/N) | OR | (95%CI) | aOR | (95%CI) | |
| Baseline independent variables | |||||
| Gender: female | 54 (67/123) | 1.00 | 1.00 | ||
| male | 55 (33/60) | 1.02 | (0.55 – 1.90) | 0.99 | (0.51 – 1.93) |
| Age: 15–24 | 56 (27/48) | 1.00 | 1.00 | ||
| 25–34 | 57 (44/77) | 1.04 | (0.50 – 2.15) | 1.06 | (0.48 – 2.30) |
| 35–44 | 48 (19/40) | 0.70 | (0.30 – 1.63) | 0.59 | (0.24 – 1.45) |
| 45–49 | 55 (10/18) | 0.97 | (0.33 – 2.89) | 0.75 | (0.24 – 2.35) |
| Education: less than secondary | 67 (8/12) | 1.00 | 1.00 | ||
| Some secondary | 58 (59/101) | 0.70 | (0.20 – 2.49) | 0.68 | (0.19 – 2.51) |
| Completed secondary | 42 (25/60) | 0.36 | (0.10 – 1.32) | 0.30* | (0.07 – 1.17) |
| Some tertiary | 80 (8/10) | 2.00 | (0.28 – 14.20) | 1.81 | (0.24 – 13.46) |
| Household monthly income (logged) | na | 1.16 | (0.90 – 1.50) | 1.18 | (0.90 – 1.55) |
| Any personal monthly income: no | 57 (58/102) | 1.00 | 1.00 | ||
| yes | 52 (42/81) | 0.82 | (0.45 – 1.47) | 0.86 | (0.44 – 1.67) |
| Married or living together: no | 57 (82/144) | 1.00 | 1.00 | ||
| yes | 46 (18/39) | 0.65 | (0.32 – 1.32) | 0.62 | (0.28 – 1.37) |
| Ever tested for HIV: no | 53 (18/34) | 1.00 | 1.00 | ||
| yes | 55 (82/149) | 1.09 | (0.52 – 2.30) | 1.03 | (0.44 – 2.38) |
| CD4 count (cells/μL): <200 | 52 (14/27) | 1.00 | 1.00 | ||
| ≥200 | 55 (86/156) | 0.88 | (0.39 – 1.99) | 0.98 | (0.40 – 2.40) |
| Consumes alcohol: no | 55 (65/118) | 1.00 | 1.00 | ||
| yes | 54 (35/65) | 0.95 | (0.52 – 1.75) | 0.76 | (0.36 – 1.63) |
| CES-D depression scale (0–15)a | na | 1.06 | (0.94 – 1.19) | 1.04 | (0.92 – 1.18) |
| Perceived HIV-related stigma: no | 55 (91/165) | 1.00 | 1.00 | ||
| yes | 50 (9/18) | 0.81 | (0.31 – 2.15) | 0.87 | (0.29 – 2.60) |
| Stigma attitudes (scale: 0–32)b | na | 1.02 | (0.94 – 1.10) | 1.01 | (0.93 – 1.10) |
| AIDS is the worst disease I could get: disagree | 60 (62/103) | 1.00 | 1.00 | ||
| agree | 48 (38/80) | 0.60 | (0.33 – 1.08) | 0.71 | (0.37 – 1.34) |
| Unafraid of HIV due to ART: disagree | 59 (67/114) | 1.00 | (0.36 – 1.15) | 1.00 | (0.28 – 1.01) |
| agree | 48 (33/69) | 0.64 | (0.35 – 1.17) | 0.54* | (0.28 – 1.05) |
| Emotional support: none | 58 (31/53) | 1.00 | 1.00 | ||
| Yes | 53 (69/130) | 0.80 | (0.42 – 1.53) | 0.77 | (0.39 – 1.54) |
| Exposure to HIV information (0–18) | na | 1.00 | (0.92 – 1.09) | 1.00 | (0.91 – 1.10) |
| Know someone who died of AIDS: no | 57 (71/125) | 1.00 | 1.00 | ||
| yes | 50 (29/58) | 0.76 | (0.41 – 1.42) | 0.92 | (0.47 – 1.79) |
| Involved in HIV-related activities: no | 52 (71/137) | 1.00 | 1.00 | ||
| yes | 63 (29/46) | 1.59 | (0.80 – 3.15) | 1.94* | (0.92 – 4.08) |
| Distance to nearest clinic: closest 33% | 57 (30/53) | 1.00 | 1.00 | ||
| Second closest third of sample | 54 (31/57) | 0.91 | (0.43 – 1.94) | 0.79 | (0.35 – 1.79) |
| Furthest third of sample | 53 (39/73) | 0.88 | (0.43 – 1.79) | 0.80 | (0.37 – 1.71) |
| Factors assessed at follow-up | |||||
| Ever thought HIV-diagnosis could be wrong: no | 66 (70/106) | 1.00 | 1.00 | ||
| yes | 51 (29/57) | 0.53* | (0.28 – 1.03) | 0.46** | (0.23 – 0.93) |
| Disclosed HIV-status to someone: no | 49 (22/45) | 1.00 | 1.00 | ||
| yes | 65 (76/117) | 1.94* | (0.97 – 3.89) | 2.31** | (1.07 – 4.97) |
Notes: OR: unadjusted odds ratio; CI: confidence interval;
p<0.05,
p<0.1
aOR: adjusted odds ratio
Multivariable logit models included the following control variables: age, gender, education, monthly household income (logged), and days between baseline and follow-up surveys.
The Cronbach’s alpha for the set of five items used to measure depression is 0.77, suggesting that the items have relatively high internal consistency.
The Cronbach’s alpha for the eight items used to measure stigmatising attitudes is 0.74.
Discussion
Linking individuals to HIV care and treatment following HBHTS is necessary to achieve the full therapeutic and prevention benefits of ART. Our study results demonstrate poor rates of early linkage to care following HBHTS in a high HIV prevalence region in South Africa. This finding is consistent with those from elsewhere within the KwaZulu-Natal province (Iwuji et al., 2016; Naik et al., 2015), and in sub-Saharan Africa (Genberg et al., 2015; Parker et al., 2015; Sharma et al., 2015). Study results underline the need for interventions to facilitate ART initiation following community-based HIV diagnosis, especially as HIV-testing with facilitated linkage to care has been demonstrated to improve uptake of HIV services (Barnabas et al., 2014).
Results indicate that HIV-status denial is a major barrier to linkage to care. Participants (approximately one third) who had doubted their HIV diagnosis were less likely to link to care; and almost a quarter of women reported not visiting a clinic because they did not believe their HIV diagnosis. Furthermore, 9% of eligible study participants did not complete the follow-up survey because they did not believe that they had HIV. While denial has been cited by participants as a reason for delayed linkage to care (Paz-Bailey et al., 2013), and several qualitative studies have identified denial as a barrier to HIV services (Bogart et al., 2013; Horter et al., 2017; Nakigozi et al., 2013; Stinson & Myer, 2012; Wringe et al., 2009), few cohort studies have assessed denial as a predictor of linkage to care. Our study therefore adds to the quantitative evidence that denial of status is common and can both impede linkage to care after community-based HIV testing (Medley et al., 2013; Naik et al., 2015), and undermine ART readiness (Maughan-Brown, Smith, et al., 2018b). Denial of an HIV diagnosis may be common because self-perceived risk of HIV-infection is often inaccurate (Maughan-Brown & Venkataramani, 2018). In the treat-all era it is likely that HIV-status denial will remain an impediment to ART initiation as efforts are made to diagnose individuals earlier in disease progression, while they are asymptomatic.
Success in diagnosing people living with HIV before they are symptomatic, a demonstrated advantage of HBHTS (Suthar et al., 2013), is also coupled with the challenge of linking individuals to care because many people do not want to initiate onto ART while feeling healthy. In our study, 48% of all participants (61% of men) who had not visited a clinic reported that they intended starting treatment only once they are sick. Health status has also been positively associated with delayed ART initiation (Hatcher et al., 2012; Katz et al., 2011; Plazy et al., 2014), and there is the perception that ART is reserved for sick individuals (Katz et al., 2015). Asymptomatic individuals may delay ART initiation because they perceive ART as more of an immediate risk/cost than benefit. The high costs (real and perceived) associated with linkage to care are reflected by our study findings that insufficient time or money, and fear of discrimination were common reasons reported for not having visited a clinic, which is consistent with findings from elsewhere in South Africa (Bogart et al., 2013; Maughan-Brown, Kuo, et al., 2018a; Smith et al., 2013). Fear of ART side-effects was also common, adding to the growing evidence that anxiety relating to drug toxicity is a leading psychosocial barrier to treatment (Fox et al., 2010; Naik et al., 2015). While modern ART has a much improved side-effect profile, previous eras with inferior drugs may have resulted in a general perception that ART results in illness (Katz & Maughan-Brown, 2017).
Our findings also add to the evidence that people who disclose their HIV status are more likely to link to care (Govindasamy et al., 2013; 2011; Hatcher et al., 2012; Medley et al., 2013). While it is unknown whether disclosure led to, or resulted from, a supporting environment, our results further highlight the importance of social support for linkage to HIV services (Kelly, Hartman, Graham, Kallen, & Giordano, 2014). Our study did not find evidence of an association between linkage to care and several factors previously reported to affect linkage, such as gender, age, stigma, depressive symptoms, and distance from clinic.
Study results should be considered along with the methodological limitations. Self-reporting bias might have influenced the accuracy of the study measures. In particular, self-reported linkage to care has been documented to be higher than verified linkage to care in some studies (Hoffmann et al., 2017; MacKellar et al., 2016), although this is not always the case (Govindasamy et al., 2011). There is uncertainty around the extent of discrepancies between self-reports and clinic records as matching study participants to clinic records is difficult (Hoffmann et al., 2017), and there is often inadequate record keeping in clinics (Dorward et al., 2017). We could not create a verified linkage to care measure as participants had not consented to a clinic record review. Sample selection bias may also have been introduced via study attrition. Study attrition might have resulted in overestimated rates of linkage to care since participants who were not reached for the telephonic interview might have been less likely to link to care, particularly as denial of an HIV-positive status was a reason for attrition and as attrition was associated with not being married (a potential proxy for less social support). In addition, other important determinants may not have been detected due to the sample size. Finally, it is unclear how our findings would apply in other settings, such as regions with lower HIV-prevalence.
Overall, our findings highlight a significant challenge in linking individuals to HIV care post HBHTS and point to the need to pair HBHTS with targeted interventions to facilitate this linkage. Multifaceted interventions involving follow-up support appear to be effective (Barnabas et al., 2014; Van Rooyen et al., 2013). Same-day home-based ART initiation following HBHTS also show promise (Labhardt et al., 2018). In particular, our results indicate that post-test counselling and supporting interventions need to be specifically designed to counter HIV status-denial; and that targeted interventions are needed to encourage early ART initiation among individuals whose inclination is to initiate on ART only once they are ill.
Supplementary Material
Acknowledgements
We thank all the participants of HIV Incidence Provincial Surveillance System (HIPSS), as well as HIPSS co-investigators and members of the HIPSS study team from the following organisations: Epicentre, CAPRISA, HEARD, NICD and CDC. We thank the HIPSS collaborating partners: The National Department of Health, Provincial KwaZulu-Natal Department of Health, uMgungundlovu Health District, the uMgungundlovu District AIDS Council, local municipal and traditional leaders, and community members for all their support throughout the HIPSS study. We are extremely grateful to Kassahun Ayalew, Ehimario Igumbor and Mary Glenshaw for valuable feedback on previous versions of this manuscript.
Funding statement
HIV Incidence Provincial Surveillance System (HIPSS) is funded by a cooperative agreement (3U2GGH000372) between Epicentre and the Centers for Disease Control and Prevention (CDC). Support was provided to BMB by the National Research Foundation, South Africa, through the Research Career Advancement Fellowship. ABMK is supported by a joint South Africa-U.S. Program for Collaborative Biomedical Research, National Institutes of Health grant (R01HD083343). The findings and conclusions in this paper are those of the author(s) and do not necessarily represent the official views of any of the funders in this study.
Footnotes
Declaration of interest statement
No potential conflict of interest was reported by the authors.
References
- Barnabas RV, Van Rooyen H, Tumwesigye E, Murnane PM, Baeten JM, Humphries H, et al. (2014). Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. The Lancet HIV, 1(2), e68–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett IV, Coleman SM, Giddy J, Bogart LM, Chaisson CE, Ross D, et al. (2017). Barriers to Care and 1-Year Mortality Among Newly Diagnosed HIV-Infected People in Durban, South Africa. Journal of Acquired Immune Deficiency Syndromes, 74(4), 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett IV, Regan S, Chetty S, Giddy J, Uhler LM, Holst H, et al. (2010). Who starts antiretroviral therapy in Durban, South Africa?… not everyone who should. Aids, 24 Suppl 1, S37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart LM, Chetty S, Giddy J, Sypek A, Sticklor L, Walensky RP, et al. (2013). Barriers to care among people living with HIV in South Africa: Contrasts between patient and healthcare provider perspectives. AIDS Care, 25(7), 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona S, Bor J, Nattey C, Maughan-Brown B, Maskew M, Fox MP, et al. (2018). Persistent High Burden of Advanced HIV Disease Among Patients Seeking Care in South Africa’s National HIV Program: Data From a Nationwide Laboratory Cohort. Clinical Infectious Diseases, 66(suppl_2), S111–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. (2011). Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine, 365(6), 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health. (2015). National HIV Testing Services: Policy and Guidelines 2015. Pretoria. Retrieved from https://www.nacosa.org.za/wp-content/uploads/2016/05/HTS-Policy-guideline.pdf [Google Scholar]
- Desai MA, Okal DO, Rose CE, Ndivo R, & Oyaro B (2017). Effect of point-of-care CD4 cell count results on linkage to care and antiretroviral initiation during a home-based HIV testing campaign: a non-blinded, cluster-randomised trial. The Lancet HIV, 4(9), e393–e401. [DOI] [PubMed] [Google Scholar]
- Dorward J, Mabuto T, Charalambous S, Fielding KL, & Hoffmann CJ (2017). Factors Associated With Poor Linkage to HIV Care in South Africa: Secondary Analysis of Data From the Thol’impilo Trial. The Journal of Acquired Immune Deficiency Syndromes, 76(5), 453–460. [DOI] [PubMed] [Google Scholar]
- Fox MP, Larson B, & Rosen S (2012). Defining retention and attrition in pre-antiretroviral HIV care: proposals based on experience in Africa. Tropical Medicine & International Health, 17(10), 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MP, Mazimba A, Seidenberg P, Crooks D, Sikateyo B, & Rosen S (2010). Barriers to initiation of antiretroviral treatment in rural and urban areas of Zambia: a cross-sectional study of cost, stigma, and perceptions about ART. Journal of the International AIDS Society, 13(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genberg BL, Naanyu V, Wachira J, Hogan JW, Sang E, Nyambura M, et al. (2015). Linkage to and engagement in HIV care in western Kenya: an observational study using population-based estimates from home-based counselling and testing. The Lancet HIV, 2(1), e20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy D, Ford N, & Kranzer K (2012). Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. Aids, 26(16), 2059–2067. [DOI] [PubMed] [Google Scholar]
- Govindasamy D, Kranzer K, van Schaik N, Noubary F, Wood R, Walensky RP, et al. (2013). Linkage to HIV, TB and Non-Communicable Disease Care from a Mobile Testing Unit in Cape Town, South Africa. PLoS ONE, 8(11), e80017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy D, van Schaik N, Kranzer K, Wood R, Mathews C, & Bekker L-G (2011). Linkage to HIV care from a mobile testing unit in South Africa by different CD4 count strata. The Journal of Acquired Immune Deficiency Syndromes, 58(3), 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachfeld A, Ledergerber B, Darling K, Weber R, Calmy A, Battegay M, et al. (2015). Reasons for late presentation to HIV care in Switzerland. Journal of the International AIDS Society, 18(1), 20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher AM, Turan JM, Leslie HH, Kanya LW, Kwena Z, Johnson MO, et al. (2012). Predictors of Linkage to Care Following Community-Based HIV Counseling and Testing in Rural Kenya. AIDS and Behavior, 16(5), 1295–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann CJ, Mabuto T, Ginindza S, Fielding KL, Kubeka G, Dowdy DW, et al. (2017). Strategies to Accelerate HIV Care and Antiretroviral Therapy Initiation After HIV Diagnosis: A Randomized Trial. The Journal of Acquired Immune Deficiency Syndromes, 75(5), 540–547. [DOI] [PubMed] [Google Scholar]
- Horter S, Thabede Z, Dlamini V, Bernays S, Stringer B, Mazibuko S, et al. (2017). “Life is so easy on ART, once you accept it”: Acceptance, denial and linkage to HIV care in Shiselweni, Swaziland. Social Science & Medicine (1982), 176, 52–59. [DOI] [PubMed] [Google Scholar]
- Iwuji CC, Orne-Gliemann J, Larmarange J, Okesola N, Tanser F, Thiebaut R, et al. (2016). Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial. PLoS Medicine, 13(8), e1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, et al. (2013). Life Expectancies of South African Adults Starting Antiretroviral Treatment: Collaborative Analysis of Cohort Studies. PLoS Medicine, 10(4), e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman S, Simbayi L, Jooste S, Toefy Y, Cain D, Cherry C, & Kagee A (2005). Development of a brief scale to measure AIDS-related stigma in South Africa. AIDS and Behavior, 9(2), 135–143. [DOI] [PubMed] [Google Scholar]
- Katz IT, & Maughan-Brown B (2017). Improved life expectancy of people living with HIV: who is left behind? The Lancet HIV, 4(8), e324–e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz IT, Dietrich J, Tshabalala G, Essien T, Rough K, Wright AA, et al. (2015). Understanding treatment refusal among adults presenting for HIV-testing in Soweto, South Africa: a qualitative study. AIDS and Behavior, 19(4), 704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz IT, Essien T, Marinda ET, Gray GE, Bangsberg DR, Martinson NA, & de Bruyn G (2011). Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. Aids, 25(17), 2177–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JD, Hartman C, Graham J, Kallen MA, & Giordano TP (2014). Social Support as a Predictor of Early Diagnosis, Linkage, Retention, and Adherence to HIV Care: Results From The Steps Study. The Journal of the Association of Nurses in AIDS Care: JANAC, 25(5), 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharsany AB, Cawood C, Khanyile D, Grobler A, Mckinnon LR, Samsunder N, et al. (2015). Strengthening HIV surveillance in the antiretroviral therapy era: rationale and design of a longitudinal study to monitor HIV prevalence and incidence in the uMgungundlovu District, KwaZulu-Natal, South Africa. BMC Public Health, 15, 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labhardt ND, Ringera I, Lejone TI, Klimkait T, Muhairwe J, Amstutz A, & Glass TR (2018). Effect of Offering Same-Day ART vs Usual Health Facility Referral During Home-Based HIV Testing on Linkage to Care and Viral Suppression Among Adults With HIV in Lesotho. Journal of the American Medical Association, 319(11), 1103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahuerta M, Wu Y, Hoffman S, Elul B, Kulkarni SG, Remien RH, et al. (2014). Advanced HIV Disease at Entry into HIV Care and Initiation of Antiretroviral Therapy During 2006–2011: Findings From Four Sub-Saharan African Countries. Clinical Infectious Diseases, 58(3), 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L, Maughan-Brown B, Grobler A, Cawood C, Khanyile D, Glenshaw M, & Kharsany ABM (2019). Impact of Home-Based HIV Testing Services on Progress Toward the UNAIDS 90–90-90 Targets in a Hyperendemic Area of South Africa. The Journal of Acquired Immune Deficiency Syndromes, 80(2), 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKellar DA, Williams D, Storer N, Okello V, Azih C, Drummond J, et al. (2016). Enrollment in HIV Care Two Years after HIV Diagnosis in the Kingdom of Swaziland: An Evaluation of a National Program of New Linkage Procedures. PLoS ONE, 11(2), e0150086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan-Brown B, & Venkataramani A (2018). Accuracy and determinants of perceived HIV risk among young women in South Africa. BMC Public Health, 18, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan-Brown B, Kuo C, Galárraga O, Smith P, Lurie MN, Bekker L-G, & Harrison A (2018a). Stumbling Blocks at the Clinic: Experiences of Seeking HIV Treatment and Care in South Africa. AIDS and Behavior, 22(3), 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan-Brown B, Smith P, Kuo C, Harrison A, Lurie M, Bekker L-G, & Galárraga O (2018b). Readiness for Antiretroviral Therapy: Implications for Linking HIV-Infected Individuals to Care and Treatment. AIDS and Behavior, 22(3), 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medley A, Ackers M, Amolloh M, Owuor P, Muttai H, Audi B, et al. (2013). Early Uptake of HIV Clinical Care After Testing HIV-Positive During Home-Based Testing and Counseling in Western Kenya. AIDS and Behavior, 17(1), 224–234. [DOI] [PubMed] [Google Scholar]
- Motsoaledi A (2016). Health Department Budget Vote Speech 2016/17. Department of Health, South Africa: Retrieved from http://www.gov.za/speeches/debate-health-budget-vote-national-assembly-10-may-2016-dr-aaron-motsoaledi-minister-health [Google Scholar]
- Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T, et al. (2012). Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Tropical Medicine & International Health, 17(12), 1509–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik R, Doherty T, Jackson D, Tabana H, Swanevelder S, Thea DM, et al. (2015). Linkage to care following a home-based HIV counselling and testing intervention in rural South Africa. Journal of the International AIDS Society, 18(1), 19843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakigozi G, Atuyambe L, Kamya M, Makumbi FE, Chang LW, Nakyanjo N, et al. (2013). A qualitative study of barriers to enrollment into free HIV care: perspectives of never-in-care HIV-positive patients and providers in Rakai, Uganda. BioMed Research International, 2013(4), 470245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Jobanputra K, Rusike L, Mazibuko S, Okello V, Kerschberger B, et al. (2015). Feasibility and effectiveness of two community-based HIV testing models in rural Swaziland. Tropical Medicine & International Health, 20(7), 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Bailey G, Pham H, Oster AM, Lansky A, Bingham T, Wiegand RE, et al. (2013). Engagement in HIV Care Among HIV-Positive Men Who Have Sex with Men From 21 Cities in the United States. AIDS and Behavior, 18(S3), 348–358. [DOI] [PubMed] [Google Scholar]
- Plazy M, Dray-Spira R, Orne-Gliemann J, Dabis F, & Newell M-L (2014). Continuum in HIV care from entry to ART initiation in rural KwaZulu-Natal, South Africa. Tropical Medicine & International Health, 19(6), 680–689. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Ruzagira E, Baisley K, Kamali A, Biraro S, Grosskurth H, the Working Group on Linkage to HIV Care. (2017). Linkage to HIV care after home-based HIV counselling and testing in sub-Saharan Africa: a systematic review. Tropical Medicine & International Health, 22(7), 807–821. [DOI] [PubMed] [Google Scholar]
- Sharma M, Ying R, Tarr G, & Barnabas R (2015). Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature, 528(7580), S77–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LR, Amico KR, Shuper PA, Christie S, Fisher WA, Cornman DH, et al. (2013). Information, motivation, and behavioral skills for early pre-ART engagement in HIV care among patients entering clinical care in KwaZulu-Natal, South Africa. AIDS Care, 25(12), 1485–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson K, & Myer L (2012). Barriers to initiating antiretroviral therapy during pregnancy: a qualitative study of women attending services in Cape Town, South Africa. African Journal of AIDS Research, 11(1), 65–73. [DOI] [PubMed] [Google Scholar]
- Suthar AB, Ford N, Bachanas PJ, Wong VJ, Rajan JS, Saltzman AK, et al. (2013). Towards Universal Voluntary HIV Testing and Counselling: A Systematic Review and Meta-Analysis of Community-Based Approaches. PLoS Medicine, 10(8), e1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The INSIGHT START Study Group. (2015). Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. New England Journal of Medicine, 373(9), 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. (2014). THE GAP REPORT. UNAIDS. [Google Scholar]
- Van Rooyen H, Barnabas RV, Baeten JM, Phakathi Z, Joseph P, Krows M, et al. (2013). High HIV Testing Uptake and Linkage to Care in a Novel Program of Home-Based HIV Counseling and Testing With Facilitated Referral in KwaZulu-Natal, South Africa. The Journal of Acquired Immune Deficiency Syndromes, 64(1), e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2016). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: WHO. [PubMed] [Google Scholar]
- Wringe A, Roura M, Urassa M, Busza J, Athanas V, & Zaba B (2009). Doubts, denial and divine intervention: understanding delayed attendance and poor retention rates at a HIV treatment programme in rural Tanzania. AIDS Care, 21(5), 632–637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


