Abstract
Sirtuin-1 (Sirt1), a member of the NAD-dependent sirtuin family of histone/protein deacetylases (HDAC), is an important target for immunotherapy due to its role in deacetylating the transcription factors Foxp3 and RORγt. Sirt1 inhibition can increase Foxp3 acetylation and promote the production and functions of Foxp3+ T-regulatory (Treg) cells, whereas the acetylation of RORγt decreases its transcriptional activity DNA binding and decreases the differentiation of pro-inflammatory Th17 cells. Pharmacologic inhibitors of Sirt1 increase allograft survival and decrease autoimmune colitis and experimental allergic encephalomyelitis. However, in contrast to its role in T cells, Sirt1 has anti-inflammatory effects in myeloid cells, and, context dependent, in Th17 cells. Here, inhibition of Sirt1 can have pro-inflammatory effects. In addition to effects arising from the central role of Sirt1 in cellular metabolism and NAD-dependent reactions, such pro-inflammatory effects further complicate the potential of Sirt1 for therapeutic immunosuppression. This review aims to reconcile the opposing literature on pro- and anti-inflammatory effects of Sirt1, provides an overview of the role of Sir1 in the immune system, and discusses the pros and cons associated with inhibiting Sirt1 for control of pro-inflammation and immunologic responses.
Keywords: T cells, Foxp3, Treg, p65, Immunosuppression
Introduction
Therapeutic immunosuppression is required for many different medical conditions, ranging from autoimmune diseases to transplantation. Current immunosuppressive drugs are limited by non-specificity and toxicities [1, 2], and developing less toxic and more precise drugs that suppress only the unwanted immune responses while preserving protective immunity is an important goal. Over the past 15 years, T-regulatory (Treg) cells have been recognized as a therapeutic target. Tregs express the transcription factor Forkhead box P3 (Foxp3) and are capable of restricting undesired immune responses against self-antigens, allergens, and commensal bacteria [3]. Tregs can be expanded ex-vivo and administered to patients, for example in the context of graft-versus-host disease [4]. However, this treatment approach is limited by Foxp3+ Treg instability once the expanded Tregs are adoptively transferred [5]. Pharmacologic interventions that favor stabilizing the Foxp3+ Treg phenotype and function in vivo are an unmet need, that could either supplement or substitute for adoptive Treg cell therapies. Foxp3 protein is post-translationally regulated by lysine acetylation through histone/protein deacetylases (HDACs) and histone acetyl transferases (HATs) [6]. Global HDAC inhibition [7-9] or deletion of HDAC6, 9, 11 and Sirtuin-1 [10-16] augments Treg function, whereas deleting HDAC3 or 5, Sirtuin-3, or targeting the HATs p300 or CBP impairs Treg [17-21].
Sirtuins are class III HDACs, and stand out from the other HDAC families by being dependent on nicotinamide adenine dinucleotide (NAD) as a co-factor (Figure 1). Sirtuins are highly conserved across eukaryotic species [22, 23]. Initial studies on Sirtuin-1 (Sirt1) in T cells utilized global Sirt1 deletion, and found that complete loss of Sirt1 led to profound autoimmunity [24]. To avoid confounding effects from Sirt1 deletion on thymic development, where Sirt1 plays a role on self-tolerance [25], we mated Sirt1fl/fl with CD4cre and Foxp3cre mice, generating mouse models bypassing the severe autoimmunity of global Sirt1 deletion, as well as using Sirt1 specific small molecule inhibitors [12]. We observed that Sirt1 deletion and pharmacologic inhibition augmented Foxp3+ Treg function [12-15]. In this review, we will discuss the role of Sirtuin-1 in the immune system and its mechanism of action. We will also address the practicality of therapeutically targeting Sirt1 for regulation of deleterious immune responses.
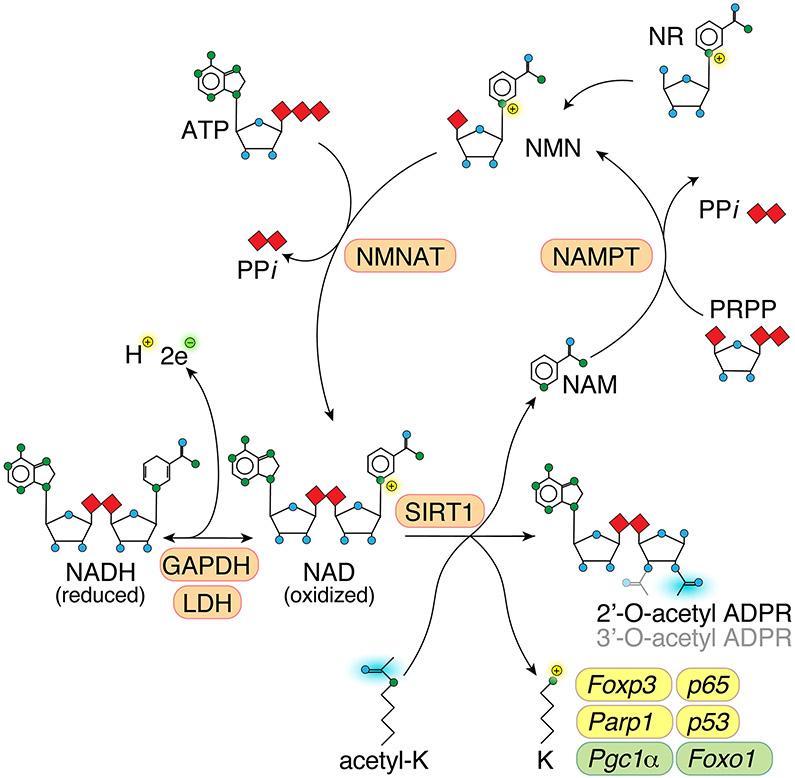
Figure 1: Sirtuin-1 reaction and NAD recycling.
Schematic showing Sirt1 reaction and NAD recycling. Sirt1 deacetylates ε-amino groups of lysine side chains in histones and many non-histone proteins, creating a positive charge. The acetylgroup is transferred to NAD, which has nicotinamide cleaved off, generating 2’- or 3’-O-acetyl ADP-ribose [23]. Sirt1 can use oxidized NAD, but not reduced NADH as co-factor. Nicotinamide is recycled to NAD through NAMPT and NMNAT. Several Sirt1 client proteins relevant to the immune system are indicated, which are either activated and stabilized (yellow) or destabilized (green) when Sirt1-dependent deacetylation is impaired. Abbreviations: NAD, nicotinamide adenine dinucleotide; NAM, nicotinamide; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside; ATP, adenosine triphosphate; ADPR, adenosine diphosphate riboside; PRPP, phosphoribosyl pyrophosphate; PPi, inorganic pyrophosphate; K, lysine; NAMPT, Nicotinamide phosphoribosyltransferase; NMNAT, nicotinamide mononucleotide adenylyltransferase.
Immunosuppressive effects from Sirt1 inhibition in T cells
Foxp3 protein expression is regulated not only by gene transcription and translation, but also heavily depends upon post-translational modifications, including acetylation, phosphorylation, ubiquitination, and poly(ADP)ribosylation [7, 26-31]. Sirt1 has a key role in binding and deacetylating Foxp3, which increases the rate of Foxp3 turnover since acetylated, but not deacetylated Foxp3 is resistant to K48 poly-ubiquitination [32, 33]. Three lysine acetylation sites in murine Foxp3 are susceptible to Sirt1 mediated deacetylation, K31, K262 and K267 [34]. Foxp3 acetylation is not just important for the protein stability of Foxp3, but also for its ability to bind to DNA and exert transcriptional control [6, 9]. In addition to the post-translational control of Foxp3, deletion or pharmacologic inhibition with EX-527, a Sirt1 inhibitor, leads to increased Foxp3 mRNA production [12, 13, 15]. Foxp3 mRNA transcription from Sirt1 targeting could be the result of chromatin accessibility through histone acetylation, or Sirt1 affecting other regulators of Foxp3 gene expression. One such regulator is p65 (RelA), which is deacetylated by Sirt1 at lysine 310 (Figure 2) [35]. In our studies, we did not observe differences in chromatin accessibility with Sirt1 deletion but did find increased p65 K310 acetylation [12] as well as p65 nuclear translocation [13], consistent with the expected effects of Sirt1 deletion [35]. Although p65 often acts as a pro-inflammatory transcription factor, it can enhance Foxp3 gene expression together with c-Rel (like p65, a nuclear factor-κB (NF-κB) co-factor) and promote the formation of a Foxp3-specific enhanceosome, which increases Foxp3 gene expression (Figure 2) [36, 37]. Taken together, genetic deletion or pharmacologic inhibition of Sirt1 through EX-527 improves Foxp3+ Treg quantity and function through increased Foxp3 mRNA transcription and Foxp3 acetylation, which leads to decreased Foxp3 turnover from ubiquitination and poly(ADP)ribosylation, and increases the transcriptional efficiency of Foxp3. As a result, targeting Sirt1 increases both thymic and induced Foxp3+ Treg numbers and their suppressive functions.
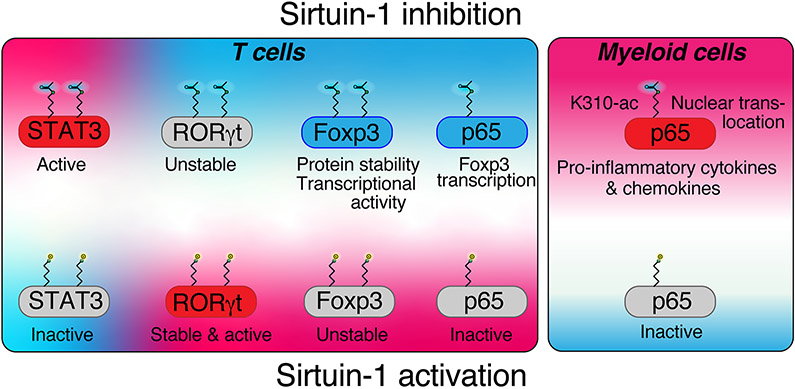
Figure 2: Sirtuin-1 targeting in T cells and myeloid cells.
Sirtuin-1 targeting has contrasting effects in myeloid and in T cells: In myeloid cells, p65 acetylation at lysine 310 leads to increased transcription of pro-inflammatory cytokines. In T cells, Foxp3 is stabilized by Sirtuin-1 inhibition due to increased lysine acetylation, which increases transcriptional activity and reduces proteasomal turnover. Foxp3 transcription is augmented by p65. In contrast to Foxp3, Rorγt is stabilized by Sirtuin-1 deacetylation, and thus, impaired with Sirtuin-1 inhibition, which aids in tilting the balance between pro-inflammatory Th17 and suppressive Treg cells. STAT3 can also be deacetylated (and destabilized) by Sirt1, which may explain that in some models, especially autoimmune uveitis, Sirt1 activation can suppress Th17 differentiation.
In contrast to Treg, conventional T cells seem less Sirt1-dependent. While T cells from mice with germline Sirt1 deletion exhibit a phenotype of severe autoimmunity and co-stimulation independent activator protein-1 signaling [24], global Sirt1 deletion is detrimental to many different cell types causing a multitude of diseases, and only some strains are viable at all. For example, Sirt1 is known to control Aire, a key transcription factor in thymic T cell selection [25], which could explain the autoimmune phenotype seen in mice with global Sirt1 deletion. To assess the effects of Sirt1 deletion on CD4+ and CD8+ T cells independent of the effects of global Sirt1 deletion on thymic development, we used a CD4cre conditional Sirt1 knockout model [12]. We did not observe differences in effector and cytotoxic T cell function in vitro, or in vivo. Adoptive transfer of Sirt1fl/flCD4cre conventional T cells in a graft-vs-host disease parent-to-F1 model did not show any differences in T cell proliferation, viability, or cytokine production [12]. However, Sirt1fl/flCD4cre conventional T cells, when adoptively transferred to Rag1−/− recipients, did not induce colitis, and formed more Foxp3+ iTreg in vivo [15], matching the earlier observations by Kwon et al of increased Foxp3+ iTreg formation in vitro [34].
In addition to the beneficial effects of Sirt1 targeting on Foxp3+ iTreg formation, Lim et al. observed that Sirt1 can bind and deacetylate thymic retinoid acid receptor related orphan receptor gamma (RORγt, Figure 2) [38]. In contrast to Foxp3, acetylated RORγt has weaker transcriptional activity, leading to less IL-17 induction and IL-2 repression, and thus weakening the Th17 phenotype [38]. We later confirmed the observations by Lim et al. showing decreased Th17 polarization phenotype in Sirt1fl/flCD4cre conventional CD4+ T cells [14]. That said, Sirt1 also has been observed to interfere with Th17 development via deacetylation of signal transducer and activator of transcription (STAT)-3, which is required for RORγt transcription [39]. A number of studies have indicated, that augmenting rather than inhibiting Sirt1 can impair Th17 development, especially in the context of autoimmune uveitis [40-42]. In conclusion, Foxp3, p65 and RORγt are T cell transcription factors in which Sirt1 targeting leads to net immunosuppressive effects in T cells, while STAT3 can augment Th17 development (Figure 2).
Anti-inflammatory effects from Sirt1 augmentation
The immunosuppressive effects of Sirt1 deletion in T cells indicate that Sirt1 inhibition could be a promising option for immunosuppressive therapy. While this is the case with the Sirt1 inhibitor EX-527 extending allograft survival and alleviating colitis [12-15], there are a number of studies in which Sirt1 deletion or inhibition led to pro-inflammatory changes [43, 44], and in which Sirt1 activators like resveratrol or SRT1720 act in an anti-inflammatory fashion [45, 46]. Beyond the above mentioned ambivalent role of Sirt1 increasing Th17 differentiation [39-42], a common denominator of many inflammatory conditions is the prominent role of Sirt1 in myeloid cells.
As noted above, Sirt1 binds and deacetylates the p65 subunit of NF-κB (Figure 2). Schug et al. observed that deletion of Sirt1 in myeloid cells results in hyperacetylated NF-κB, causing increased transcriptional activation of genes that promote an inflammatory response [44]. This occurs through lysine K310, the same residue found to be more acetylated in Sirt1-deficeint T cells [12]. However, unlike in Treg cells, in myeloid cells, p65 K310 acetylation translates into the transcription of inflammatory response mRNAs controlled by NF-κB [47]. Thus, loss of Sirt1 in macrophages induces production of NF-κB-dependent pro-inflammatory cytokines such as TNF-α and IL-1β [43]. As a result, Sirt1’s ability to target acetylation of NF-κB allows regulation of the inflammatory response, and Sirt1 inhibition can lead to chronic inflammation through its direct deacetylation of p65 [48].
Beyond p65, c-Jun (a member of the activator protein-1 complex) is an additional Sirt1 target relevant to myeloid cells. Sirt1 deacetylation of the c-Jun can reduce inflammation in peritoneal macrophages by decreasing cyclooxygenase-2 (COX2) mRNA expression and prostaglandin E2 production [49]. Decreased prostaglandin E2 production attenuates macrophage and tumoricidal functions, in another example of the anti-inflammatory effects of Sirt1 in myeloid cells.
Sirt1 and cellular metabolism
In contrast to the effects of Sirt on Foxp3, p65, Parp1, and p53, the key metabolic regulators transcription factor PGC1α (peroxisome proliferators-activated receptor-γ coactivator 1α) and FoxO1 are activated and stabilized by Sirt1-mediated deacetylation (Figure 1) [50, 51], whereas their acetylation by the HATs, GCN5 and p300 respectively [50, 51], impairs their transcriptional activity [52]. For these reasons, Sirt1 mediated deacetylation has marked effects on cellular metabolism and mitochondrial biogenesis.
Foxp3+ Treg rely on oxidative phosphorylation for their energy production [53, 54]. Perhaps unsurprisingly, Foxp3+ Treg deficient in PGC1α or FoxO1 show weaker suppressive function [19, 55] and phenotypic stability [56, 57]. Given the role of Sirt1 deacetylation in enhancing PGC1α and FoxO1, we had expected that Sirtuin-1 knockout Treg would have dysfunctional mitochondria and impaired oxidative phosphorylation. However, this was not observed [19, 58], leading us to speculate that the effect of absent Sirt1 on transcription factors such as Foxp3 [58-60] may overrule the potential impairment of mitochondrial biogenesis and function resulting from PGC1α acetylation. HATs that acetylate and inactivate PGC1α (GCN5) and FoxO1 (p300) both also acetylate (and activate) Foxp3, mirroring the role of Sirt1, with deletion of either GCN5 or p300 impairing Foxp3+ Treg [20, 61].
An additional metabolic (and immunologic) effect of Sirt1 targeting can arise from NAD+ availability. Sirt1 consumes NAD+ during its deacetylation reaction (Figure 1). Mice deficient in the enzyme PARP-1, which also utilizes NAD, have increased Sirt1 activation, improved metabolism and are leaner [62]. Hence, loss of Sirt1 activity may affect the NAD+ pool or other NAD consuming enzymes, which can have its own effects on the cellular redox state and immune function [30, 58, 63].
Sirt1 in other organ systems
Sirt1 is an important regulator of cellular metabolism, autophagy and chromatin accessibility, and is associated with a wide variety of pathophysiologic processes, including longevity, aging, obesity, heart disease, and cancer [64, 65]. The effects of targeting Sirt1 are thus very widely spread and hard to accurately predict. For example, with regard to Sirt1 and cancer, Sirt1 can both suppress or favor the formation of tumors, depending upon its targets in individual signaling pathways or in specific cancers, such as p53, p65, or metabolic and autophagy regulatory circuits [66]. This is especially relevant when Sirt1 inhibition is considered. For example, when using the Sirt1 inhibitor EX-527 in MHC-mismatched murine renal allografts, we noted that prolongation of allograft survival and function was present, though to a lesser extent than in mice with conditional deletion of Sirt in their T cells (Sirt1fl/flCD4cre), and was worsened upon increasing the dose of EX-527 from 1 to 10 mg/kg/d [14]. In addition to pro-inflammatory effects from innate immune cells [44, 67], Sirt1 can aid protecting from ischemic injury [68, 69], which is practically unavoidable during transplantation, and thus, the immunosuppressive treatment with EX-527 may augment such injury. Furthermore, Sirt1 has been shown to alleviate lipopolysaccharide-induced injury, and mitigate oxidative stress arising in diabetic nephropathy [70-72], all of which would be common exposures in kidney transplant recipients. These broad side effects make Sirt1 targeting for immunosuppression less practical [6].
The role of other sirtuins in in the immune system
In addition to Sirt1, other class III histone/protein deacetylases (Sirt2-7) have effects on immune responses. While all sirtuins share the NAD-dependent deacetylation reaction (Figure 1), each of these sirtuins has individual binding partners, and different subcellular localizations that affect their role in the immune system. Sirt1, Sirt6, and Sirt7 are predominantly nuclear, Sirt2 is present in the cytosol, while Sirt3-5 are located in the mitochondria. Sirt2, like Sirt1, regulates p65 K310 acetylation [73]. Sirt2 did not have any notable effect on T-effector or Treg cell functions in vitro [6], perhaps because of its cytosolic localization. In myeloid cells, Sirt2 deletion improved bacterial phagocytosis and augmented host responses against staphylococcal infections [74]. On the flip side, Sirt2−/− mice had increased susceptibility to dextran sodium sulfate induced colitis [75]. Sirt3 deletion did not have any overt effect on myeloid and lymphocyte development [19, 76], however, Sirt3−/− Treg function was noted to be impaired [19]. The role of Sirt4 in the immune system is relatively unknown, through Sirt4 has been suggested to be important to recover monocyte function during sepsis [77]. Sirt5 has an interesting effect on inflammation by competing with Sirt2 and blocking deacetylation of p65 at K310, the same amino acid targeted by Sirt1, in turn causing cytokine production and inducing an inflammatory response in vivo [78]. In fact, Sirt5 had notably opposing expression levels and effects when compared to Sirt1 and Sirt2 on inflammation arising from peritoneal macrophages [78]. While reduction of Sirt1/2 expression increased IL-6 mRNA expression, Sirt5 targeting also downregulated IL-6 mRNA. Accordingly, Sirt5−/− mice show a reduced inflammatory cytokine response during sepsis [78]. Sirt6 is important for TNF-α production [79] and dendritic cell function [80]. Sirt7−/− mice show a shortened lifespan and due to cardiac failure, cardiac myocyte apoptosis mediated by p53 hyperacetylation, with myocardial infiltrates of granulocytes and T cells [81]. In addition to cardiac myocyte apoptosis, these findings also raised the question if Sirt7 may have immunoregulatory functions. However, Burg et al. did not observe Sirt7 to affect outcomes in experimental autoimmune encephalomyelitis aside from a minor reduction in CD8+ T cell IFN-γ production [82].
Concluding Remarks
Pharmacologic Sirt1 inhibition can produce immunosuppressive effects, but these are context dependent and vary upon the cells type(s) involved in the immune response that is being targeted. This is in addition to other non-immune effects of Sirt1 inhibition. Together, these limitations make Sirt1, in contrast to other HDACs, such as HDAC6, less suitable as a pharmacologic target to achieve immunosuppression.
Acknowledgments
Funding
Supported by grants from the National Institutes of Health to U.H.B. (AI095353), W.W.H. (AI073489 and AI095276), as well as the Laffey McHugh foundation and American Society of Nephrology (to U.H.B.).
Abbreviations
- COX
Cyclooxygenase
- Foxp3
Forkhead box P3
- NAD
Nicotinamide adenine dinucleotide
- HAT
Histone/protein acetyltransferase
- HDAC
Histone/protein deacetylase
- NF-κB
Nuclear factor-κB
- PGC1α
Peroxisome proliferators-activated receptor-γ coactivator 1α
- RORγt
Thymic retinoid acid receptor related orphan receptor gamma
- Sirt
Sirtuin
- STAT
signal transducer and activator of transcription
- Treg
Regulatory T cells
Footnotes
Conflict of Interest Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Naesens M, Kuypers DR, Sarwal M (2009) Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4, 481–508. [DOI] [PubMed] [Google Scholar]
- 2.Fishman JA (2007) Infection in solid-organ transplant recipients. N Engl J Med 357, 2601–14. [DOI] [PubMed] [Google Scholar]
- 3.Feuerer M, Hill JA, Mathis D, Benoist C (2009) Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol 10, 689–95. [DOI] [PubMed] [Google Scholar]
- 4.Beres AJ and Drobyski WR (2013) The role of regulatory T cells in the biology of graft versus host disease. Front Immunol 4, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh K, Stempora L, Harvey RD, Kirk AD, Larsen CP, Blazar BR, Kean LS (2014) Superiority of rapamycin over tacrolimus in preserving nonhuman primate Treg half-life and phenotype after adoptive transfer. Am J Transplant 14, 2691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Beier UH, Akimova T, Dahiya S, Han R, Samanta A, Levine MH, Hancock WW (2018) Histone/protein deacetylase inhibitor therapy for enhancement of Foxp3+ T-regulatory cell function posttransplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 18, 1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, Wells AD, Hancock WW (2007) Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med 13, 1299–307. [DOI] [PubMed] [Google Scholar]
- 8.Akimova T, Ge G, Golovina T, Mikheeva T, Wang L, Riley JL, Hancock WW (2010) Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin Immunol 136, 348–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Wang L, Han R, Beier UH, Hancock WW (2012) Two lysines in the forkhead domain of foxp3 are key to T regulatory cell function. PloS one 7, e29035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias P, Hancock WW (2011) Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Molecular and cellular biology 31, 2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Zoeten EF, Wang L, Sai H, Dillmann WH, Hancock WW (2010) Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology 138, 583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beier UH, Wang L, Bhatti TR, Liu Y, Han R, Ge G, Hancock WW (2011) Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Molecular and cellular biology 31, 1022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beier UH, Wang L, Han R, Akimova T, Liu Y, Hancock WW (2012) Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Science signaling 5, ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine MH, Wang Z, Xiao H, Jiao J, Wang L, Bhatti TR, Hancock WW, Beier UH (2016) Targeting Sirtuin-1 prolongs murine renal allograft survival and function. Kidney Int 89, 1016–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akimova T, Xiao H, Liu Y, Bhatti TR, Jiao J, Eruslanov E, Singhal S, Wang L, Han R, Zacharia K, Hancock WW, Beier UH (2014) Targeting sirtuin-1 alleviates experimental autoimmune colitis by induction of Foxp3+ T-regulatory cells. Mucosal immunology 7, 1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Wang L, Dahiya S, Beier UH, Han R, Samanta A, Bergman J, Sotomayor EM, Seto E, Kozikowski AP, Hancock WW (2017) Histone/protein deacetylase 11 targeting promotes Foxp3+ Treg function. Sci Rep 7, 8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Liu Y, Han R, Beier UH, Bhatti TR, Akimova T, Greene MI, Hiebert SW, Hancock WW (2015) FOXP3(+) regulatory T cell development and function require histone/protein deacetylase 3. J Clin Invest 125, 3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao H, Jiao J, Wang L, O'Brien S, Newick K, Wang LC, Falkensammer E, Liu Y, Han R, Kapoor V, Hansen FK, Kurz T, Hancock WW, Beier UH (2016) HDAC5 controls the functions of Foxp3(+) T-regulatory and CD8(+) T cells. International journal of cancer. Journal international du cancer 138, 2477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beier UH, Angelin A, Akimova T, Wang L, Liu Y, Xiao H, Koike MA, Hancock SA, Bhatti TR, Han R, Jiao J, Veasey SC, Sims CA, Baur JA, Wallace DC, Hancock WW (2015) Essential role of mitochondrial energy metabolism in Foxp3(+) T-regulatory cell function and allograft survival. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 29, 2315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Wang L, Predina J, Han R, Beier UH, Wang LC, Kapoor V, Bhatti TR, Akimova T, Singhal S, Brindle PK, Cole PA, Albelda SM, Hancock WW (2013) Inhibition of p300 impairs Foxp3(+) T regulatory cell function and promotes antitumor immunity. Nature medicine 19, 1173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Wang L, Han R, Beier UH, Akimova T, Bhatti T, Xiao H, Cole PA, Brindle PK, Hancock WW (2014) Two histone/protein acetyltransferases, CBP and p300, are indispensable for Foxp3+ T-regulatory cell development and function. Molecular and cellular biology 34, 3993–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frye RA (1999) Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun 260, 273–9. [DOI] [PubMed] [Google Scholar]
- 23.Jackson MD and Denu JM (2002) Structural identification of 2'- and 3'-O-acetyl-ADP-ribose as novel metabolites derived from the Sir2 family of beta -NAD+-dependent histone/protein deacetylases. J Biol Chem 277, 18535–44. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Lee SM, Shannon S, Gao B, Chen W, Chen A, Divekar R, McBurney MW, Braley-Mullen H, Zaghouani H, Fang D (2009) The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest 119, 3048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuprin A, Avin A, Goldfarb Y, Herzig Y, Levi B, Jacob A, Sela A, Katz S, Grossman M, Guyon C, Rathaus M, Cohen HY, Sagi I, Giraud M, McBurney MW, Husebye ES, Abramson J (2015) The deacetylase Sirt1 is an essential regulator of Aire-mediated induction of central immunological tolerance. Nat Immunol 16, 737–45. [DOI] [PubMed] [Google Scholar]
- 26.Zaiss DMW and Coffer PJ (2018) Forkhead box transcription factors as context-dependent regulators of lymphocyte homeostasis. Nat Rev Immunol. [DOI] [PubMed] [Google Scholar]
- 27.Beier UH, Akimova T, Liu Y, Wang L, Hancock WW (2011) Histone/protein deacetylases control Foxp3 expression and the heat shock response of T-regulatory cells. Curr Opin Immunol 23, 670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morawski PA, Mehra P, Chen C, Bhatti T, Wells AD (2013) Foxp3 protein stability is regulated by cyclin-dependent kinase 2. J Biol Chem 288, 24494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Loosdregt J, Fleskens V, Fu J, Brenkman AB, Bekker CP, Pals CE, Meerding J, Berkers CR, Barbi J, Grone A, Sijts AJ, Maurice MM, Kalkhoven E, Prakken BJ, Ovaa H, Pan F, Zaiss DM, Coffer PJ (2013) Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity 39, 259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo X, Nie J, Wang S, Chen Z, Chen W, Li D, Hu H, Li B (2016) Poly(ADP-ribosyl)ation of FOXP3 protein mediated by PARP-1 regulates the function of regulatory T cells. The Journal of biological chemistry 291, 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Kumar S, Dahiya S, Wang F, Wu J, Newick K, Han R, Samanta A, Beier UH, Akimova T, Bhatti TR, Nicholson B, Kodrasov MP, Agarwal S, Sterner DE, Gu W, Weinstock J, Butt TR, Albelda SM, Hancock WW (2016) Ubiquitin-specific Protease-7 Inhibition Impairs Tip60-dependent Foxp3+ T-regulatory Cell Function and Promotes Antitumor Immunity. EBioMedicine 13, 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, Brenkman AB, Hijnen DJ, Mutis T, Kalkhoven E, Prakken BJ, Coffer PJ (2010) Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood 115, 965–74. [DOI] [PubMed] [Google Scholar]
- 33.van Loosdregt J, Brunen D, Fleskens V, Pals CE, Lam EW, Coffer PJ (2011) Rapid temporal control of Foxp3 protein degradation by sirtuin-1. PLoS One 6, e19047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon HS, Lim HW, Wu J, Schnolzer M, Verdin E, Ott M (2012) Three novel acetylation sites in the Foxp3 transcription factor regulate the suppressive activity of regulatory T cells. J Immunol 188, 2712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23, 2369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH (2009) Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity 31, 932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruan Q and Chen YH (2012) Nuclear factor-kappaB in immunity and inflammation: the Treg and Th17 connection. Adv Exp Med Biol 946, 207–21. [DOI] [PubMed] [Google Scholar]
- 38.Lim HW, Kang SG, Ryu JK, Schilling B, Fei M, Lee IS, Kehasse A, Shirakawa K, Yokoyama M, Schnolzer M, Kasler HG, Kwon HS, Gibson BW, Sato H, Akassoglou K, Xiao C, Littman DR, Ott M, Verdin E (2015) SIRT1 deacetylates RORgammat and enhances Th17 cell generation. J Exp Med 212, 607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Limagne E, Thibaudin M, Euvrard R, Berger H, Chalons P, Vegan F, Humblin E, Boidot R, Rebe C, Derangere V, Ladoire S, Apetoh L, Delmas D, Ghiringhelli F (2017) Sirtuin-1 Activation Controls Tumor Growth by Impeding Th17 Differentiation via STAT3 Deacetylation. Cell Rep 19, 746–759. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Zhao C, Kong P, Bian G, Sun Z, Sun Y, Guo L, Li B (2016) Methylene blue alleviates experimental autoimmune encephalomyelitis by modulating AMPK/SIRT1 signaling pathway and Th17/Treg immune response. J Neuroimmunol 299, 45–52. [DOI] [PubMed] [Google Scholar]
- 41.Gardner PJ, Joshi L, Lee RW, Dick AD, Adamson P, Calder VL (2013) SIRT1 activation protects against autoimmune T cell-driven retinal disease in mice via inhibition of IL-2/Stat5 signaling. J Autoimmun 42, 117–29. [DOI] [PubMed] [Google Scholar]
- 42.Gardner PJ, Yazid S, Chu CJ, Copland DA, Adamson P, Dick AD, Calder VL (2015) TNFalpha Regulates SIRT1 Cleavage during Ocular Autoimmune Disease. Am J Pathol 185, 1324–33. [DOI] [PubMed] [Google Scholar]
- 43.Caruso R, Marafini I, Franze E, Stolfi C, Zorzi F, Monteleone I, Caprioli F, Colantoni A, Sarra M, Sedda S, Biancone L, Sileri P, Sica GS, MacDonald TT, Pallone F, Monteleone G (2014) Defective expression of SIRT1 contributes to sustain inflammatory pathways in the gut. Mucosal Immunol 7, 1467–79. [DOI] [PubMed] [Google Scholar]
- 44.Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X (2010) Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol 30, 4712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A (2014) Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res 34, 837–43. [DOI] [PubMed] [Google Scholar]
- 46.Chen YX, Zhang M, Cai Y, Zhao Q, Dai W (2015) The Sirt1 activator SRT1720 attenuates angiotensin II-induced atherosclerosis in apoE(−)/(−) mice through inhibiting vascular inflammatory response. Biochem Biophys Res Commun 465, 732–8. [DOI] [PubMed] [Google Scholar]
- 47.Chen LF, Mu Y, Greene WC (2002) Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J 21, 6539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu TF and McCall CE (2013) Deacetylation by SIRT1 Reprograms Inflammation and Cancer. Genes Cancer 4, 135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang R, Chen HZ, Liu JJ, Jia YY, Zhang ZQ, Yang RF, Zhang Y, Xu J, Wei YS, Liu DP, Liang CC (2010) SIRT1 suppresses activator protein-1 transcriptional activity and cyclooxygenase-2 expression in macrophages. J Biol Chem 285, 7097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434, 113–8. [DOI] [PubMed] [Google Scholar]
- 51.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–5. [DOI] [PubMed] [Google Scholar]
- 52.Finck BN and Kelly DP (2006) PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116, 615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186, 3299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, Haeberli L, Huck C, Turka LA, Wood KC, Hale LP, Smith PA, Schneider MA, MacIver NJ, Locasale JW, Newgard CB, Shinohara ML, Rathmell JC (2015) Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. The Journal of clinical investigation 125, 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO (2010) Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nature immunology 11, 618–27. [DOI] [PubMed] [Google Scholar]
- 56.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, Chan P, Ma Q, Mo Y, Meijer D, Zhao K, Rudensky AY, Atwal G, Zhang MQ, Li MO (2012) Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature 491, 554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch'en IL, Stockmann C, Katayama CD, Hedrick SM (2010) Foxo transcription factors control regulatory T cell development and function. Immunity 33, 890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angelin A, Gil-de-Gomez L, Dahiya S, Jiao J, Guo L, Levine MH, Wang Z, Quinn WJ 3rd, Kopinski PK, Wang L, Akimova T, Liu Y, Bhatti TR, Han R, Laskin BL, Baur JA, Blair IA, Wallace DC, Hancock WW, Beier UH (2017) Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab 25, 1282–1293 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, Warmoes MO, de Cubas AA, MacIver NJ, Locasale JW, Turka LA, Wells AD, Rathmell JC (2016) Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nature immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Howie D, Cobbold SP, Adams E, Bokum AT, Necula AS, Zhang W, Huang W, Roberts DJ, Thomas B, Hester SS, Vaux DJ, Betz AG, Waldmann H (2017) Foxp3 drives oxidative phosphorylation and protection from lipotoxicity. JCI Insight 2, e89160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bao C, Liu Y, Wang L, Han R, Dent S, Hancock WW (2013) Epigenetic Mechanisms Underlying Allograft Rejection: Role of the Histone Acetyltransferase, GCN-5 in T Cell Activation, Proliferation and Cytokine Production [abstract]. Am J Transplant (suppl 5). [Google Scholar]
- 62.Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J (2011) PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grahnert A, Grahnert A, Klein C, Schilling E, Wehrhahn J, Hauschildt S (2011) Review: NAD +: a modulator of immune functions. Innate Immun 17, 212–33. [DOI] [PubMed] [Google Scholar]
- 64.Guarente L and Picard F (2005) Calorie restriction--the SIR2 connection. Cell 120, 473–82. [DOI] [PubMed] [Google Scholar]
- 65.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R (2012) Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov 11, 443–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin Z and Fang D (2013) The Roles of SIRT1 in Cancer. Genes Cancer 4, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeda-Watanabe A, Kitada M, Kanasaki K, Koya D (2012) SIRT1 inactivation induces inflammation through the dysregulation of autophagy in human THP-1 cells. Biochemical and biophysical research communications 427, 191–6. [DOI] [PubMed] [Google Scholar]
- 68.Khader A, Yang WL, Kuncewitch M, Jacob A, Prince JM, Asirvatham JR, Nicastro J, Coppa GF, Wang P (2014) Sirtuin 1 activation stimulates mitochondrial biogenesis and attenuates renal injury after ischemia-reperfusion. Transplantation 98, 148–56. [DOI] [PubMed] [Google Scholar]
- 69.Fan H, Yang HC, You L, Wang YY, He WJ, Hao CM (2013) The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney international 83, 404–13. [DOI] [PubMed] [Google Scholar]
- 70.Gao R, Chen J, Hu Y, Li Z, Wang S, Shetty S, Fu J (2014) Sirt1 deletion leads to enhanced inflammation and aggravates endotoxin-induced acute kidney injury. PloS one 9, e98909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, Hosoya K, Komatsu M, Kaneko Y, Kanda T, Kubota E, Tokuyama H, Hayashi K, Guarente L, Itoh H (2013) Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nature medicine 19, 1496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM (2010) Sirt1 activation protects the mouse renal medulla from oxidative injury. The Journal of clinical investigation 120, 1056–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO (2010) SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 123, 4251–8. [DOI] [PubMed] [Google Scholar]
- 74.Ciarlo E, Heinonen T, Theroude C, Herderschee J, Mombelli M, Lugrin J, Pfefferle M, Tyrrell B, Lensch S, Acha-Orbea H, Le Roy D, Auwerx J, Roger T (2017) Sirtuin 2 Deficiency Increases Bacterial Phagocytosis by Macrophages and Protects from Chronic Staphylococcal Infection. Front Immunol 8, 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lo Sasso G, Menzies KJ, Mottis A, Piersigilli A, Perino A, Yamamoto H, Schoonjans K, Auwerx J (2014) SIRT2 deficiency modulates macrophage polarization and susceptibility to experimental colitis. PLoS One 9, e103573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ciarlo E, Heinonen T, Lugrin J, Acha-Orbea H, Le Roy D, Auwerx J, Roger T (2017) Sirtuin 3 deficiency does not alter host defenses against bacterial and fungal infections. Sci Rep 7, 3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tao J, Zhang J, Ling Y, McCall CE, Liu TF (2018) Mitochondrial Sirtuin 4 Resolves Immune Tolerance in Monocytes by Rebalancing Glycolysis and Glucose Oxidation Homeostasis. Front Immunol 9, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin K, Han C, Zhang H, Li T, Li N, Cao X (2017) NAD(+) dependent deacetylase Sirtuin 5 rescues the innate inflammatory response of endotoxin tolerant macrophages by promoting acetylation of p65. J Autoimmun 81, 120–129. [DOI] [PubMed] [Google Scholar]
- 79.Van Gool F, Galli M, Gueydan C, Kruys V, Prevot PP, Bedalov A, Mostoslavsky R, Alt FW, De Smedt T, Leo O (2009) Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med 15, 206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lasiglie D, Boero S, Bauer I, Morando S, Damonte P, Cea M, Monacelli F, Odetti P, Ballestrero A, Uccelli A, Mostoslavsky R, Poggi A, Nencioni A (2016) Sirt6 regulates dendritic cell differentiation, maturation, and function. Aging (Albany NY) 8, 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E (2008) Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res 102, 703–10. [DOI] [PubMed] [Google Scholar]
- 82.Burg N, Bittner S, Ellwardt E (2018) Role of the epigenetic factor Sirt7 in neuroinflammation and neurogenesis. Neurosci Res 131, 1–9. [DOI] [PubMed] [Google Scholar]