Abstract
A detailed protocol for the synthesis of North-methanocarba-thymidine (N-MCT), a potent antiviral nucleoside with a restricted bicyclo [3.1.0] hexane pseudosugar conformation, is presented. The process is described in two parts. The first basic protocol deals with the synthesis of the carbobicyclic pseudosugar precursor that can be utilized in the syntheses of other bicyclo [3.1.0] hexane nucleosides with natural and non-natural nucleobases. The second basic protocol describes the specific construction of the thymine base in a linear fashion from the carbobicyclic intermediate.
Keywords: carbocyclic nucleosides, bicyclo [3.1.0] hexane nucleosides, conformationally locked, antiviral
INTRODUCTION
North-methanocarba-thymidine (N-MCT, S.12) is a conformationally locked, carbocyclic nucleoside with potent anti-herpes activity (for a review, see Marquez et al., 2006). The efficacy of N-MCT against genital HSV-2 infection in mice and guinea pigs has been confirmed and the drug is a strong clinical candidate, which is currently under development (R.I. Glazer, pers. comm.). As a molecular probe, incorporation of N-MCT into DNA has been utilized to impart a conformational bias to the biopolymer (Wu et al., 2005; Madeira et al., 2007; Pallan et al., 2012). Incorporation of N-MCT into RNA, on the other hand, was found to be consistent with a strong siRNA interference activity while at the same time increasing serum stability (Terrazas et al., 2011).
This unit describes the synthesis of N-MCT on a preparative scale that represents a significant improvement relative to previously published small-scale syntheses (Marquez etal.,1996,1999; Ezzitounietal.,1997). The synthesis begins from the readily accessible cyclopentenol synthon (1S,2R)-2-[(benzyloxy) methyl] cyclopent-3-enol (S.1) (Biggadike et al., 1987, 1988). This material was prepared in large scale by Ash-Stevens, Inc. (Detroit, Michigan).
The process is divided into two parts (A and B). Part A, described in Basic Protocol 1, presents the synthesis of the carbobicyclic pseudosugar precursor (S.7), which can also be used for the syntheses of other nucleoside analog bearing different nucleobases. Part B, described in Basic Protocol 2, describes the specific construction of the thymine base.
CAUTION:
Carry out all operations involving organic solvents and reagents in a well-ventilated fume hood, wearing gloves and protective glasses.
Basic Protocol 1: SYNTHESIS OF CARBOBICYCLIC PSEUDOSUGAR PRECURSOR
The overall scheme for part A is shown below. Starting with 323 g (1.58 mol) of S.1, a total of ∼275 g (0.86 mol) of S.7 is obtained with an overall yield of 55% after 6 to 7 steps (Fig. 1.29.1). The first step is the protection of the free, secondary alcohol with the same robust benzyl protecting group as the primary alcohol. Subsequently, the double bond in S.2 reacts regio- and stereoselectively with PhSeCl/AgO2CCF3 to give the trans-2-(phenylseleno) cyclopentan-1-ol intermediate (S.3) after hydrolysis of the trifluoroacetate ester. Following oxidation of the (phenylseleno) group, unidirectional elimination of PhSeOH gives exclusively the corresponding allylic alcohol S.4. This allylic alcohol is inverted following a Mitsunobu esterification, which gives the intermediate benzoate S.5 and the allylic alcohol S.6 after ester hydrolysis. Finally, a hydroxyl-directed cyclopropanation under Simmons-Smith conditions gives the key carbobicyclic intermediate S.7.
Figure 1.29.1.
Synthetic scheme of the carbobycyclic pseudosugar S.7.
Materials
60% sodium hydride (mol. wt. 24.00; Aldrich)
N2 source
Tetrahydrofuran (THF, anhydrous, sieves; Pharmco-AAPER)
Compound S.1 (mol. wt. 204.26; Ash Stevens)
Tetra-n-butylammonium iodide (mol. wt. 369.37; Aldrich)
Benzyl bromide (mol. wt. 171.03; Aldrich)
Hexanes (Pharmco-AAPER)
Silica gel (70 to 230 mesh; Silicycle)
10% ethyl acetate (EtOAc; Pharmco-AAPER)
Dimethyl sulfoxide (DMSO, anhydrous, sieves; Acros)
Phenylselenyl chloride (mol. wt. 191.52; Aldrich)
Silver trifluoroacetate (mol. wt. 220.9; Aldrich)
95% ethanol/5% KOH solution
Ammonium chloride (half-saturated NH4Cl)
Brine
MgSO4
Methanol (MeOH; Pharmco-AAPER)
Sodium periodate (NaIO4, mol. wt. 213.89; Aldrich)
Dichloromethane (CH2Cl2, Pharmco-AAPER)
Triphenylphosphine (mol. wt. 262.29; Aldrich)
Benzoic acid (mol. wt. 122.12; Aldrich)
Benzene (anhydrous; Aldrich)
Diisopropyl azodicarboxylate (DIAD, mol. wt. 202.21; Aldrich)
Potassium carbonate (powdered, mol. wt. 138.21; Aldrich)
1 M diethylzinc ((Et)2Zn, heptanes; Aldrich) Diiodomethane (mol. wt. 267.84; Aldrich)
3-L, 5-L, and 12-L, 4-neck flasks with overhead stirrer, N2 inlet, type-J Teflon-covered thermocouple, and addition funnel
18°, 20°, and 25°C water baths
Sintered glass Buchner funnels (medium porosity)
Vacuum
Silica and thin layer chromatography equipment
Rotary evaporator
Celite pads
Perform benzyl protection of the secondary alcohol (see Fig. 1.29.1)
1. At 0°C on ice, equip a 5-L, 4-neck flask with an overhead stirrer, an N2 inlet, a type-J Teflon-covered thermocouple, and an addition funnel. Purge flask with N2 and charge with sodium hydride and 1.8 L of THF.
2. To the stirring suspension at 0 ± 2°C, add a solution of 214 g (1.05 mol) S.1 in 0.8 L of THF over a period of 30 min.
There is no exothermal reaction. Off-gassing only occurs during the last third of the addition.
3. Raise the temperature to 20°C over 30 to 45 min by removing from ice, during which time a very controlled off-gassing continues. Stir the mixture 2 to 3 hr (1 hr after off-gassing has ceased) at 20°C.
4. Charge 3.7 g (0.01 mol) tetra-n-butylammonium iodide and add 195 g (1.14 mol) benzyl bromide (neat) over 15 min (no exotherm). Stir overnight at room temperature to complete the reaction.
5. Filter off the solids through a sintered glass funnel (medium porosity) and wash with∼1000 mL hexane.
CAUTION:
The filter cake contains some residual live NaH.
6. Concentrate the filtrate in vacuo and chromatograph the residue on 3 kg of silica.
7. Elute with ∼1000 mL hexane to remove some pink color (possibly I2) and excess benzyl bromide.
8. Elute with 10% EtOAc in hexanes to obtain the product (TLC: Rf 0.72, 9:1 hexane:EtOAc), UV, Hanessian’s stain [(NH4)6Mo7O244H2O; Ce(SO4)2; H2SO4].
9. Evaporate using a rotary evaporator the solvents to give 295 g (95%) of S.2 as a clear oil.
1H NMR (CDCl3) δ 7.40–7.20 (m, 10 H), 5.75 (m, 1 H), 5.65 (m, 1 H), 4.56 (s, 2 H), 4.51 (s, 2 H), 4.08 (irregular quintuplet, 1 H), 3.45 (dd, J = 9.2, 5.7 Hz, 1 H), 3.33 (dd, J = 9.2, 7.2 Hz, 1 H), 3.05 (br m, 1 H), 2.70 (m, 1, H), 2.42 (m, 2, H). Anal. Calcd. for C22H22O20.25H2O: C, 80.37; H, 7.58. Found: C, 80.15; H, 7.58.
Perform hydroxy-selenylation of the double bond (see Fig. 1.29.1)
10. Equip a 3-L, 4-neck flask with an overhead stirrer, an N2 inlet, a type-J Teflon-covered thermocouple, and an addition funnel. Purge the flask with N2 and charge with DMSO (400 mL) and 214 g (0.727 mol) of S.2.
11. To the stirring solution at 20° to 25°C (use a water bath to control mild exotherm), add 97.5 g (0.50 mol) of phenylselenyl chloride (solid) in portions over 30 min so that most is dissolved before adding the next portion. Stir the dark orange solution for 2 hr and then cool to 15° to 20°C (do not allow to freeze).
12. Add 104 g (0.47 mol) of silver trifluoroacetate (solid) in portions over 20 min and stir the mixture for 5 hr at 20°C.
13. After cooling to 15°C, add 625 mL of 95% EtOH/5% KOH solution over 30 min holding the temperature at 15◦C and stir for 20 min.
14. Add 1 L EtOAc and filter off the solids through a Celite pad and wash with EtOAc.
15. Concentrate the filtrate of EtOH and EtOAc in vacuo, dissolve the residue in 2 Lof EtOAc and wash with 2 L of water. Back extract the water wash with EtOAc (two times using 0.5 L each time), combine the organic layers, and wash with half-saturated NH4Cl (1 L, 0.5 L) and brine (0.5 L).
16. Dry the organic layer (MgSO4), filter off the solids using a sintered glass Büchner funnel (medium porosity), and evaporate the solvent to obtain 198 g of product S.3 as an oil that can be used directly in the next reaction.
No attempt is made to purify or analyze this material. It is either used immediately or stored overnight at 0° to 5°C and used without further purification.
Perform elimination of PhSeOH and rearrangement of the double bond (see Fig. 1.29.1)
17. Equip a 12-L, 4-neck flask with an overhead stirrer, an N2 inlet, and a type-J Teflon-covered thermocouple. Purge the flask with N2 and charge with methanol (4.7 L), water (0.52 L), and 194 g (0.415 mol) of S.3.
18. Stir the solution at 18°C and add 203 g (0.95 mol) of NaIO4 (solid) in one portion.
An exothermic reaction occurs from 18° to 24°C.
A large paddle must be used and placed close to the bottom of the flask; otherwise the periodate will not be stirred adequately. While the reaction appears to be fast, it may need to be stirred overnight to complete. This is probably due to slow dissolution of the last of the periodate in the mixture. Powdering the periodate is a potential improvement. A thick precipitate (possibly NaIO3) forms during the course of the reaction, and adequate stirring must be maintained.
19. Stir the yellow slurry overnight at room temperature.
20. Filter off the solids through a medium porosity sintered glass funnel and wash with 2 L of MeOH.
21. Evaporate the MeOH and partition the residue between 1.2 L of CH2Cl2 and 1 L of water to dissolve all of the solids. Back extract the water wash with CH2Cl2 (two times with 300 mL each time), and wash the combined organic layers with 0.5 L of brine, dry (using anhydrous MgSO4), filter through a sintered glass funnel (medium porosity), and evaporate the solvent.
There are indications of instability in the mixture. During the final evaporation before the chromatography, two new less polar TLC spots (same solvent system described above) have been observed and some solids formed. These are preferably filtered off before loading the material onto the column. No attempt was made to isolate or identify them.
22. Dissolve the residue (200 g) in 400 mL of 1:1 (v/v) hexane/CH2Cl2 (insolubles may be filtered off as needed) and apply to a 2-kg silica column. Elute using the following step gradient: hexanes, 10%, 20%, 30%, 50% EtOAc to finally elute all of the product.
Crossover fractions may need a second chromatography (TLC:Rf 0.5,6:4hexane:EtOAc).
23. Evaporate the product-containing fractions to give compound S.4 (81 g, 63%) as a clear oil that should be used immediately in the next step.
1H NMR (CDCl3) δ 7.44–7.30 (m, 2 H), 6.03 (br s, 1 H), 5.10–5.04 (symmetrical m, 1 H), 4.90–4.84 (symmetrical m, 1 H), 4.67 (AB d, J = 11.9 Hz, 1 H); 4.64 (AB d, J = 11.7 Hz, 1 H), 4.61 (AB d, J = 11.9 Hz, 1 H), 4.52 (AB d, J = 11.7 Hz, 1 H), 4.26 (br s, 2 H), 2.38 (ddd, J = 14.4, 6.8, 3.2 Hz, 1 H), 2.09 (ddd, J = 14.1, 6.6, 3.2 Hz, 1 H), 1.91 (br s, 1 H); 13C-NMR (CDCl3) δ 145.54, 138.25, 138.03, 132.89, 128.25, 128.24, 127.57, 127.52, 127.48, 82.10, 75.28, 72.70, 71.28, 66.51, 41.16. Anal. calcd. for C20H22O3·0.25 H2O: C, 76.27, H, 7.21. Found: C, 76.40; H, 7.28.
Perform Mitsunobu esterification and inversion of configuration (see Fig. 1.29.1)
24. Equip a 12-L, 4-neck flask with an overhead stirrer, an N2 inlet, a type-J Teflon-covered thermocouple, and addition funnel. Purge the flask with N2 and charge it with 175 g (0.56 mol) S.4, 296 g (1.13 mol) triphenylphosphine, 104 g (0.85 mol) benzoic acid, and 5.3 L benzene.
25. Stir mixture at 18°C and add 226 g (1.12 mol) DIAD (neat) holding the temperature with a water bath (mild exotherm).
26. After 1 hr, evaporate the benzene.
TLC (8:2 hexane/EtOAc) shows total completion of the reaction after such time.
27. Triturate the residue with 2 L of 8:2 hexane/EtOAc. Filter the solids (Ph3PO, DIAD-H) through a sintered glass funnel (medium porosity) and wash with 8:2 hexane/EtOAc.
28. Evaporate the combined organic solvents and dissolve the residue (580 g) in 1.2 Lof 6:2 hexanes/CH2Cl2.
29. Apply the solution to a 5-kg column of silica and use a step gradient elution with hexanes, 5% and 10% EtOAc to elute the product (TLC Rf 0.8, 8:2 hexane/EtOAc, UV, Hanessian’s stain).
30. Evaporate the solvent of the product-containing fractions to give 231 g of S.5 as a stable thick oil.
1H NMR (CDCl3) δ 8.16–8.12 and 7.67–7.34 (2 m, 15 H), 6.17 (br s, 1 H), 5.92–5.82 (symmetrical m, 1 H), 4.75–4.60 (m, 5 H), 4.35 (br s, 2 H); 3.01 (dt, J = 14.2, 7.3 Hz, 1 H), 2.08 (dt, J = 14.4, 4.2 Hz, 1 H); 13C NMR (CDCl3) δ 166.46, 147.62, 138.48, 138.22, 133.07, 130.44, 129.82, 128.57, 128.55, 128.51, 128.47, 127.87, 127.85, 127.80, 80.80, 76.78, 73.11, 71.37, 66.73, 38.22. Anal. calcd. for C27H26O4: C, 78.24; H, 6.32. Found: C, 78.40; H, 6.72.
The benzoate appears to be pure by TLC. However, in the analysis after the next step, ∼5% of the epimer was detected. It is probable that the racemization occurs at this step but is not resolved by the analyses.
Perform ester hydrolysis (see Fig. 1.29.1)
31. Equip a 12-L, 4-neck flask with an overhead stirrer, an N2 inlet, and a type-J Teflon-covered thermocouple.
32. Purge the flask with N2 and charge with 242 g of S.5 (0.58 mol), 120 g (0.87 mol) potassium carbonate, and 3.8 L methanol.
33. Stir the solution for 2 hr at room temperature (HPLC complete).
HPLC conditions: Phenomenex Jupiter column, 5 μm, C18, 300 Å, 4.6 × 250–mm; 10:90 to 90:10 CH3CN/H2O in 15 min at room temperature. Hold at 90:10 for 2 min; 0.025% TFA buffer, 1.5 mL/min flow rate, UV at 220 nm.
34. Filter the solids through a sintered glass funnel (medium porosity) and wash withMeOH.
35. Evaporate the combined filtrates and partition the residue between 6 L of EtOAc and4 L of water.
36. Back extract the water with 2 L of EtOAc and wash the combined organic layerswith 2 L of water and1 L of brine.
37. Dry (MgSO4), filter through as intered glass funnel (medium porosity), and evaporate the solvent.
38. Take up the residue in 600 mL of hexanes with enough CH2Cl2 (∼100 mL) to achieve solution.
39. Apply to a 4-kg silica column and use a stepped gradient elution 5%, 10%, and20% EtOAc in hexanes to remove some fast running impurities. After reaching 60% EtOAc, the product begins to elute (TLC: Rf 0.5, 6:4 hexane/EtOAc, UV, Hanessian’s stain).
40. Evaporate solvent in the product-containing fractions to give 174 g (96%) of S.6 as a syrup.
The syrup will crystallize (seeds needed), but this has no practical utility since cryogenic crystallization from hexanes and methyl tert-butyl ether (MTBE) gives only a 65% recovery of pure material (melting point 42° to 44°C), and the major impurity present at this stage (epimer 4) can be removed at the penultimate step (when at levels of ∼5%). The significant impurity at this point is 4, suggesting that the Mitsunobu does not proceed with clean inversion. The epimeric alcohols of intermediate polarity can be seen by HPLC and barely by TLC, but the less polar benzoate shows no resolution by either technique.
The spectral data that follows corresponds to a pure analytical sample: 1H NMR (CDCl3) δ 7.54 – 7.38 (m, 10 H), 6.03 (br s, 1 H), 4.75 – 4.55 (m, 4 H), 4.49 – 4.54 (dd, J = 6.8, 3.9 Hz, 1 H), 4.30 (br s, 2 H), 2.74 (dt, J = 14.2, 7.1 Hz, 1 H), 2.68 (br s, 1 H), 1.82 (dt, J = 14.2, 3.9 Hz, 1 H); 13C NMR (CDCl3) δ 144.86, 138.39, 138.20, 133.01, 128.44, 127.82, 127.78, 127.71, 80.99, 73.98, 72.84, 71.48, 66.62, 41.16. Anal. calcd. for C20H22O3·0.5H2O: C, 75.21; H, 7.26. Found: C, 75.46; H, 7.51.
Perform Simmons-Smith cyclopropanation (see Fig. 1.29.1)
41. Equip a 12-L, 4-neck flask with an overhead stirrer, an N2 inlet, a type-J Teflon-covered thermocouple, and two addition funnels.
42. Purge the flask with N2, charge with 118 g of S.6 (0.38 mol) and DCM (4.6 L) and cool the mixture to −4° to −8°C.
43. Holding at −4° to −8°C, add one-half (417 mL, 0.415 mol) of the (Et)2Zn solution and stir the mixture for 20 min.
44. Add one-half of the diiodomethane (111.5 g, 0.415 mol) and stir for 10 min.
45. Proceed to add the remaining (Et)2Zn solution, stir for 10 min, add the remaining diiodomethane, and stir for 10 min (the addition time is ∼20 min for each component).
46. Allow the mixture to reach room temperature and stir overnight.
47. Carefully pour the mixture into 3 L of half-saturated NH4Cl solution.
CAUTION:
There may remain some residual live (Et)2Zn.
48. Extract the aqueous layer with 1 L DCM and wash the combined organic layers with1 L half-saturated NH4Cl, 2 L of brine, dry (MgSO4), filter through a sintered glass funnel (medium porosity), and evaporate the solvent.
49. Dissolve residue in 500 mL of 10% EtOAc in hexanes with enough (<1 L) DCM added to achieve solution.
50. Apply to a 3-kg column of silica and elute with a step gradient of 25%, 40%, andthen 50% EtOAc in hexane to obtain a mixed fraction (23 g) of mostly S.7 plus some unreacted S.6.
51. After re-chromatography and re-reaction, a pure fraction of 98 g (HPLC >95%) of S.7 is obtained as an oil.
Total recovery: 121 g (98%); 1H NMR (CDCl3) δ 7.45–7.30 (m, 10 H), 4.65–4.40 (m, 5 H), 4.31 (t, J = 8.0 Hz, 1 H), 3.98 (d, J = 10.5 Hz, 1 H), 3.21 (d, J = 10.5 Hz, 1 H), 2.37 (dt, J = 12.9, 7.4 Hz, 1 H), 1.70–1.60 (m, 2 H), 1.40–1.20 (m, 2 H), 0.63 (dd, J = 7.4, 5.6 Hz, 1 H); 13C NMR (CDCl3) δ 138.84, 138.53, 128.49, 128.47, 127.88, 127.78, 127.72, 127.70, 73.01, 72.13, 71.87, 70.02, 35.59, 32.99, 28.20, 7.00. Anal. calcd. for C21H24O3: C, 77.74; H, 7.46. Found: C, 77.51; H, 7.40.
HPLC analysis of compound S.7 (Phenomenex Jupiter column 5 μm, C18, 300 Å, 4.6 × 250–mm; 10:90 to 90:10 CH3CN/H2O in 15 min at room temperature. Hold at 90:10 for 2 min; 0.025% TFA buffer, 1.5 mL/min flow rate, UV at 220 nm) revealed a purity of >95%.
BASIC PROTOCOL 2 LINEAR CONSTRUCTION OF THE THYMINE BASE
For the assembly of the nucleobase from S.7, the thymine base can be attached either directly or linearly, constructing the thymine ring in a stepwise fashion. As discussed previously (Ludek and Marquez, 2007), the linear protocol produces better yields.
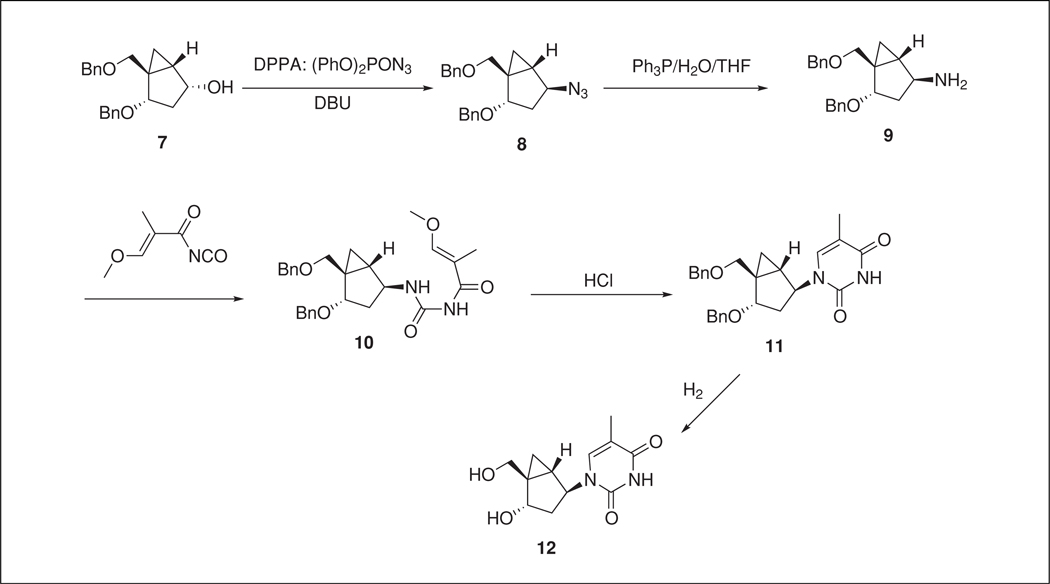
The overall scheme for part B is shown in Figure 1.29.2. It is necessary to overcome epimerization problems for the steps to convert alcohol S.7 into azide S.8 and removal of some chromatographically inseparable impurities (derivatives of S.4 and unknowns). The use of DPPA rectifies the first problem giving clean inversion to S.8. The azide S.8 is then quantitatively converted to the amine S.9 using the Staudinger reduction. The amine is subsequently reacted with the acylisocyanate S.13 in DMF that is prepared fresh from 3-methoxy-2-methylacryloyl chloride and silver isocyanate in benzene. Ring closure of urea S.10 under aqueous acidic conditions gives S.11. Crystallization of S.11 allows for the first clean-up purification in the sequence. Pure S.11 then allows for a quantitative conversion to pure N-MCT (S.12) without the need of a final purification.
Figure 1.29.2.
Final, linear assembly of the thymine nucleobase to give N-MCT (S.12).
Materials
N2 source
S.7 (see Basic Protocol 1)
Tetrahydrofuran (THF; Pharmco-AAPER)
Diphenyl phosphoryl azide (Sigma-Aldrich)
1,8-Diazabicyclo [5.4.0] undec-7-ene (DBU; Sigma-Aldrich)
Methyl tert-butyl ether (MTBE; Pharmco-AAPER)
Hydrochloric acid (37% aqueous HCl solution; Pharmco-AAPER)
Sodium sulfate (Na2SO4; Dawn Scientific Inc.)
Biotage SNAP 340 g column (Biotage)
Ethyl acetate (Pharmco-AAPER)
Hexanes (Fisher)
Sodium hydroxide (NaOH; Acros)
Triphenylphosphine (Acros)
Diethyl ether (Et2O)
Dichloromethane (CH2Cl2; Fisher)
Benzene (anhydrous; Aldrich)
Silver cyanate (Aldrich)
3-Methoxy-2-methylacryloyl chloride (Aldrich)
DMF (anhydrous; Aldrich)
Silica gel (70 to 230 mesh; Sylicycle)
Ethyl acetate (EtOAc; Pharmco-AAPER)
Hexanes (Pharmco-AAPER)
Ethanol
NaHCO3 (saturated; Pharmco-AAPER)
MgSO4
Brine
Methanol (MeOH; Pharmco-AAPER)
HPLC-grade water (Pharmco-AAPER)
Acetone (Pharmco-AAPER)
1-L, 4-neck flasks with an overhead stirrer, an N2 inlet, a type-J-Teflon covered thermocouple, and an addition funnel
Stir plate
Sintered glass funnels (medium porosity)
Rotary evaporator
3-L, 3-neck flasks with an overhead stirrer, a type-J Teflon-covered thermocouple, and an addition funnel
3-L, 4-neck flasks with an overhead stirrer, an N2 inlet, a type-J Teflon-covered thermocouple, a reflux condenser, and an addition funnel
Ceramic Buchner (Whatman no. 1)
Krapcho funnel
5-L, 4-neck flasks with an overhead stirrer, an N2 inlet, a type-J Teflon-covered thermocouple, and an addition funnel
Vacuum
2-L hydrogenation bottle
Shaker hydrogenation apparatus (Parr)
Celite pad (1-cm; Aldrich)
Perform conversion of alcohol to azide with inversion of configuration (see Fig. 1.29.2)
1. Equipa1-L,4-neck flask with an overhead stirrer, an N2 inlet, a type-J Teflon covered thermocouple and an addition funnel.
2. Purge the flask with N2 and charge with a mixture of 33 g of alcohol S.7 (102 mol), dry THF (165 mL), and 26.4 mL diphenyl phosphoryl azide (122.4 mol).
3. Cool the mixture to 0°C on ice under N2, and add 18.15 mL (122.4 mol) of neat 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU).
4. Stir the reaction mixture 2 hr at 0°C, then 2 hr at 25°C, and finally 16 hr at 60°C. At this time, run a HPLC analysis to show that the reaction is complete.
This sequence of increasingly higher reaction temperatures was the only successful way to complete the conversion of S.7 to S.8.
HPLC analysis of azide S.8 (Phenomenex Jupiter column 5-μm, C18, 300 Å, 4.6 × 250–mm; 10:90 to 90:10 CH3CN/H2O in 15 min at room temperature. Hold at 90:10 for 2 min; 0.025% TFA buffer, 1.5 mL/min flow rate, UV at 220 nm) revealed a purity of 99.68%.
Attempts to heat the reaction mixture at the outset causes decomposition of the azide reagent before it can react with alcohol S.7.
5. Dilute the reaction mixture with methyl tert-butyl ether (500 mL), and wash the organic layer with H2O (two times with 400 mL each time) and 5% HCl (400 mL).
6. Dry the organic layer (Na2SO4), filter through a sintered glass funnel (medium porosity), and concentrate on a rotary evaporator to give crude product S.8 as a yellow oil.
7. Apply to a 2-kg column of silica and elute with 1:9 ethyl acetate/hexanes to obtain33.6 g (95%) of azide S.8 (Rf = 0.3 in the same solvent system) as a colorless oil; IR (neat) 2926, 2095 (N3), 1099 cm−1, 1H NMR (CDCl3) δ 7.32–7.51 (m, 10 H), 4.57–4.71 (m, 5 H), 4.00 (d, J = 6.1Hz, 1 H), 3.94 (AB d, J =10.7 Hz, 1 H), 3.44 (AB d, J = 10.7 Hz, 1 H), 2.17 (dd, J = 14.4, 7.5 Hz, 1 H), 1.56–1.67 (m, 2 H), 0.96 (dd, J = 5.6, 4.1 Hz, 1 H), 0.78 (dd, J= 8.3, 5.8 Hz, 1 H); 13C NMR (CDCl3) δ 138.7, 138.6, 128.5, 128.4, 127.8, 127.7, 127.6, 79.6, 72.7, 72.5, 71.4, 62.3, 35.2, 32.4, 26.7, 10.1; FAB MS m/z (relative intensity) 350 (MH+, 10), 91 (100); HRMS (FAB, MH+) calcd. for C21H24N3O2: 350.1869; found: 350.1874.
Perform Staudinger reduction of azide to amine (see Fig. 1.29.2)
8. Equip a 3-L, 4-neck flask with an overhead stirrer, an type-J Teflon-covered thermocouple, and an addition funnel.
9. Charge the flask with 38.9 g of azide S.8 (111 mol), THF (1.1 L), and H2O (65 mL).
10. To the stirring solution of azide S.8, add 49 mL of aqueous 5 M NaOH followed by 106.8 g of triphenylphosphine (407 mol).
11. Stir the mixture 18 hr at 25°C under air.
12. Separate the aqueous layer and wash the organic layer with 0.1 M HCl (six times with 200 mL each time). Check the pH value of the aqueous layer every time. If pH is not <2, add concentrated HCl dropwise to bring the pH value to between 1 and 2.
13. Combine all the aqueous layers and acidify to pH ∼2.
14. Wash the combined aqueous layer with Et2O (four times with 200 mL each time) and adjust the pH to ∼12 with aqueous NaOH.
15. Extract the basified aqueous layer with CH2Cl2 (one time with 400 mL, then three times with 200 mL each time).
16. Dry the combined CH2Cl2 layers (Na2SO4), filter through a sintered glass funnel (medium porosity), and concentrate to obtain amine S.9 (28 g, 78%) as a colorless oil with a purity calculated by HPLC-UV of 74%.
1H NMR (CDCl3) δ 7.39 (br s, 10 H), 4.54–4.69 (m, 5 H), 4.00 (AB d, J =10.2 Hz, 1 H), 3.42 (d, J = 5.8 Hz, 1 H), 3.28 (AB d, J = 10.2 Hz, 1 H), 1.66 (br s, 2 H), 1.79 (dd, J = 14.0, 8.0 Hz, 1 H), 1.55 (ddd, J = 14.4, 8.7, 6.3 Hz, 1 H), 1.31 (dd, J = 8.0, 4.1 Hz, 1 H), 0.91(apparent t, J = 5.1 Hz, 1 H), 0.63 (dd, J = 8.0, 5.8 Hz, 1 H); 13C NMR (CDCl3) δ 139.0, 138.7, 128.3 (x 2), 127.7, 127.6, 127.5 (x 2), 79.3, 72.8, 72.1 (x 2), 52.0, 38.4, 32.0, 30.8, 10.7. FAB MS m/z (relative intensity) 324 (MH+, 79), 91 (100); HRMS (FAB, MH+) calcd. for C21H26NO2 324.1964; found 324.1952.
The actual purity was much better since a small amount of Ph3PO impurity with a larger extinction coefficient distorts the value when determined by HPLC (Phenomenex Jupiter column 5-μm, C18, 300 Å, 4.6 × 250–mm; 10:90 to 90:10 CH3CN:H2O in 15 min at room temperature. Hold at 90:10 for 2 min; 0.025% TFA buffer, 1.5 mL/min flow rate, UV at 220 nm).
Perform reaction of amine with acylisocyanate (see Fig. 1.29.2)
17. Equipa3-L,4-neck flask with an overhead stirrer, an N2 inlet, a type-J Teflon-covered thermocouple, a reflux condenser, and an addition funnel.
18. Purge the flask with N2 and charge with benzene (2.3 L) at 20°C.
19. To the stirring benzene at 20°C, add 98 g (0.65 mol) of silver cyanate at such a rate to produce a good slurry without clumping (1 to 2 min).
20. To the stirring solution, add 44g (0.326 mol) of 3-methoxy-2-methylacryloylchlorideneat over 10 min.
A mild exothermic reaction occurs during the addition, raising the internal temperature to 27°C.
21. Heat the mixture 1 hr to 80°22.C, and then cool to room temperature.
22. Taking precautions to reduce atmospheric moisture, filter off the solids by pouring there action mixture from the reaction flask (with a slow flow from a connected nitrogen line) into a ceramic Buchner (Whatman no. 1) topped with a Krapcho funnel and wash with ∼200 mL of benzene.
A Krapcho funnel is a plastic laboratory funnel (wider than the Büchner) that is inverted closely over the Büchner. A stream of nitrogen is introduced via the small end, and a section of the body is cut out to allow the mixture to be poured into the Buchner. Thus, the mixture is kept under a nitrogen flow during the transfer and filtration.
23. Use this filtrate directly in the following step.
HPLC results indicated that acylisocyanate formation was incomplete in this run. One of the impurity peaks was the amide resulting from reaction of S.9 with the acid chloride (∼5%). This is of no consequence here other than to lower the yield, as the amide is removed during chromatography. The AgCNO reaction should be heated to full reflux for 90 min.
The acylisocyanate solution is unstable and should be used immediately upon preparation. A precipitate forms over a few hours on standing. There was no apparent problem with the reaction if the precipitate was added; it is unreactive and later removed by the subsequent chromatography.
24. Equipa5-L,4-neck flask with an overhead stirrer, an N2 inlet, a type-J Teflon-covered thermocouple, and an addition funnel.
25. Purge with N2 and charge with 75.9 g amine S.9 and 700 mL DMF.
26. To the stirring DMF solution at 0° ± 2°C, add the above benzene solution of acylisocyanate at such a rate (∼40 min) to maintain the temperature at ∼5°C.
Upon completion of the addition, the temperature increases to 15° to 20°C and HPLC shows the reaction to be complete.
HPLC conditions: 10:90 to 90:10 CH3CN/H2O (0.025% TFA buffer) over 10 min, hold at 90:10 (10 min); flow 0.50 mL/min; UV = 220 nm; Phenomenex Jupiter column 5-μm C18 300 Å, 4.6 × 250–mm (23°C). Retention time: 14.51 min, >99A%
27. Remove both solvents under vacuum (80°C water bath, 5 mm Hg).
The solution at this point is stable and can stand overnight if needed. If the amine has not been consumed, an additional batch of acylisocyanate can be prepared and added as needed.
28. Take up the residue in 200 mL of CH2Cl2 and apply to a 1.5-kg column of silica made up with 20% EtOAc in hexanes.
29. Begin a sequential elution with 30%, 50%, and 60% EtOAc to obtain the product(TLC: Rf 0.5, 1:1 hexane/EtOAc).
The column may or may not separate an unknown front impurity (mass = 478). It is not necessary to remove here, as it is removed in the next step.
30. Remove the solvents to give 106 g (97%) of S.10, which is used in the next step.
Perform ring closure to thymine (see Fig. 1.29.2)
31. Equip a 5-L, 4-neck flask with an overhead stirrer, an N2 inlet, a reflux condenser, and a type-J Teflon-covered thermocouple.
32. Purge with N2 and charge with 106 g (0.228 mol) of S.10, 2.9 L of ethanol, and 300 mL of 3 N HCl.
33. Stir and heat the mixture overnight (18 hr) to 78°C. After such time, perform HPLC analysis to confirm complete conversion.
HPLC conditions: 10:90 to 90:10 CH3CN/H2O (0.025% TFA buffer) over 10 min, hold at 90:10 (10 min); flow 0.50 mL/min; UV = 220 nm; Phenomenex Jupiter column 5-μm C18 300 Å, 4.6 × 250–mm (23°C). Retention time: 12.83 min, >99 A% (both crops).
34. Allow the mixture to cool to room temperature and evaporate the solvents to reduce to dryness.
35. Partition the residue between 1 L EtOAc and 500 mL saturated NaHCO3. Add more of either solvent if necessary to dissolve all solids.
36. Re-extract the aqueous layer with 0.5 L EtOAc, combine the organics, wash with brine, and dry (MgSO4).
37. Concentrate the solution (product begins to precipitate) to a slurry (∼500 mL) and proceed to cool to −10°C.
38. Filter off the solids and wash with minimal cold EtOAc (∼100 mL) and air dry the product to obtain 72 g (73%) of S.11.
39. Evaporate the filtrate to dryness and crystallize the residue (20 g) from MeOH to give an additional 8 g (total 80 g, 80%) of S.11.
1H NMR (CDCl3) δ 8.54 (s, 1 H), 7.80 (s, 1 H), 7.24–7.40 (m, 10 H), 5.00 (d, J = 7.2 Hz, 1 H), 4.60 (t, J = 8.5 Hz, 1 H), 4.40–4.56 (m, 4 H), 4.20 (d, J =10.0 Hz, 1 H), 3.16 (d, J = 10.0 Hz, 1 H), 1.96–2.04 (m, 1 H), 1.74–1.82 (m, 1 H), 1.48 (s, 3 H), 1.36 (dd, J = 8.6, 3.7 Hz, 1 H), 1.00 (dd, J = 5.9, 3.9 Hz, 1 H), 0.74 (distorted triplet, 1 H).
This is a critical step in the sequence. The crystallization of S.11 allows removal of epi-11 and other impurities that could not be removed by chromatography. Pure S.11 enables running the final step and isolating pure N-MCT (S.12) directly without a purification step.
40. The above mother liquor contains more S.11 as well as its epimer (epi-11) that was generated during the Mitsunobu reaction and carried forward. When the MeOH is evaporated off, re-chromatograph the residue to give only fractions enriched in one or the other.
No pure fractions could be obtained due to tailing. The purest epi-11 (second spot, was ∼50% pure).
Perform final deprotection (see Fig. 1.29.2)
41. Charge a 2-L hydrogenation bottle with 15 g catalyst and 1 L methanol.
42. Use a shaker hydrogenation apparatus (Parr) to achieve 20 psi and hydrogenate overnight at room temperature (30° to 37°C).
43. Disconnect the hydrogenation bottle following the manufacturer’s instructions and allow the catalyst to settle for 2 hr.
44. Decant the supernatant and filter through a Celite pad.
45. Leach the remaining catalyst by stirring 15 min with 500 mL of HPLC-grade water at 50°C.
Over half of the product precipitates out of the MeOH, onto the catalyst, necessitating the water treatment.
46. Filter off the catalyst through the same Celite pad, combine the filtrates, and begin evaporating the solvent.
The product starts to crystallize as the last of the water is removed.
47. Free the solid product from the walls of the flask by scraping and slurrying with acetone.
48. Stir the slurry for 15 min, filter off the solid, wash with acetone, and air dry to give 55 g of white crystalline S.12 (N-MCT).
mp 229° - 231°C; [α]25D = +47° (c 0.28, MeOH); 1H NMR (Me2SO-d6) δ 11.20 (s, 1 H, NH), 7.91 (s, 1 H, H-6), 5.01 (t, J = 4.8 Hz, 1 H, OH), 4.70 (d, J = 6.8 Hz, 1 H, H-4), 4.65–4.50 (m, 2 H, OH, H-2), 4.07 (dd, J = 11.2, 4.9 Hz, 1 H, CHHOH), 3.05 (dd, J = 11.2, 4.5 Hz, 1 H, CHHOH), 1.80–1.45 (m, 2 H, H-3’a,b), 1.22 (dd, J = 8.4, 3.5 Hz, 1 H, H-5’), 0.78 (irregular t, 1 H, H-6endo), 0.54 (dd, J = 8.4, 5.3 Hz, 1 H, H-6exo); 13C NMR (CH3OH-d4) δ 10.58, 12.39, 26.09, 37.80, 39.19, 57.56, 63.52, 71.27, 111.22, 139.82, 152.93, 167.23; FAB MS (m/z, rFel intensity) 253 (MH+, 100), 127 (b +2 H, 40). Anal. calcd. for C12H16N2O4: C, 57.13; H, 6.30; N, 11.10. Found: C, 57.18; H, 6.60; N, 11.03.
The acetone filtrate should be evaporated to dryness to check for missing product. If all the water was not previously removed, the filtrate should contain the missing product.
Crystalline N-MCT (S.12) was difficult to redissolve, but is stable to 50°C water, so heating and sonicating should help dissolution. It appears to be notably less soluble in methanol and therefore, presumably ethanol.
COMMENTARY
Background Information
The synthesis of N-MCT described here represents a practical, large-scale process. The synthetic route, which is divided into two parts, opens an important door for accessing bicyclo [3.1.0] hexane nucleosides with different nucleobases, natural and non-natural. The first part of the synthesis (part A) describes the preparation of the pivotal intermediate S.7, which by using well-known methods for constructing the nucleobase, can provide chosen target nucleosides, either by linear or convergent approaches. Part B is specific for the thymine analog and describes the incorporation of this base via a linear approach. The product, N-MCT, is a potent and target-specific antiviral agent capable of inhibiting DNA synthesis through its 5’-triphosphate metabolite produced in cells expressing a virally-encoded thymidine kinase. The compound is stable and active in vivo and has a favorable pharmacokinetic profile. In this unit, a synthetic approach that has been tested in a preparative scale, which produced enough material (55 g) to perform all preclinical studies, is described.
Critical Parameters
During the synthesis, it is important to keep reaction vessels dry and under a nitrogen atmosphere, except when indicated otherwise. Throughout the description of the process critical parameters have been added as separate notes, which should allow the operator to make adjustments and improvements.
Anticipated Results
The process described in Basic Protocol 1 is a well-proven method that has been used in several publications (Marquez et al., 1999). This unit adds the ability to scale-up the process for the purposes of obtaining the larger amounts that is necessary for biological studies, including in vivo studies. The user can easily adapt the process for preparing smaller quantities if so desired. Basic Protocol 2 is a representative method specific for making the thymine analog. A similar linear approach will work just as well for the uracil and cytosine analog. For the purines, a convergent method as previously published is recommended (Marquez et al., 1999).
Time Considerations
If these reactions are processed on the same scale as indicated, it would take ∼3 months to perform Basic Protocol 1 and it would take 2 months to perform Basic Protocol 2.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Literature Cited
- Biggadike K, Borthwick AD, Exall AM, Kirk BE, Roberts SM, Youds P, Slawin AMZ, and Williams DJ 1987. Synthesis of fluorinated carbocyclic nucleosides: Preparation of carbocyclic 1-(2’-deoxy-6’-fluororibofuranosyl)-5-iodouracils. J. Chem. Soc. Chem. Commun 255–256. [Google Scholar]
- Biggadike K, Borthwick AD, Evans D, Exall AM, Kirk BE, Roberts SM, Stephenson L, and Youds P. 1988. Use of diethylaminosulphur trifluoride (DAST) in the preparation of carobycyclic nucleosides. J. Chem. Soc. Perkin Trans. 1:549–554. [Google Scholar]
- Ezzitouni A, Russ P, and Marquez VE 1997. (1S,2R)-[(Benzyloxy) methyl] cyclopent-3-enol. A versatile synthon for the preparation of 4’,1’ a-methano- and 1’,1’a-methanocarbocyclic nucleosides. J. Org. Chem 62:4870–4873. [Google Scholar]
- Ludek OR and Marquez VE 2007. Convergent or linear? A challenging question in carbocyclic nucleoside chemistry. Synthesis 3451–3460. [Google Scholar]
- Madeira M, Shenoy S, Van QN, Marquez VE, and Barchi JJ Jr. 2007. Biophysical studies of DNA modified with conformationally constrained nucleotides: Comparison of 2-exo (north) and 3-exo (south) ‘locked’ templates. Nucleic Acids Res. 35:1978–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez VE, Siddiqui MA, Ezzitouni A, Russ P, Wang J, Wagner RW, and Matteucci MD 1996. Nucleosides with a twist. Can fixed forms of sugar ring pucker influence biological activity in nucleosides and oligonucleotides? J. Med. Chem 39:3739–3747. [DOI] [PubMed] [Google Scholar]
- Marquez VE, Russ P, Alonso R, Siddiqui MA, Hernandez S, George C, Nicklaus MC, Dai F, and Ford H Jr. 1999. Synthesis of conformationally restricted carbocyclic nucleosides: The role of the O(4’)-atom in the key hydration step of adenosine deaminase. Helv. Chim. Acta 82:2119–2129. [Google Scholar]
- Marquez VE, Hughes SH, Sei S, and Agbaria R. 2006. The history of N-methanocarbathymidine: The investigation of a conformational concept leads to the discovery of a potent and selective nucleoside antiviral agent. Antiviral Res. 71:268–275. [DOI] [PubMed] [Google Scholar]
- Pallan PS, Marquez VE, and Egli M. 2012. The conformationally constrained N-methanocarba-dT analogue adopts an unexpected C4’-exo sugar pucker in the structure of a DNA hairpin. Biochemistry 51:2639–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrazas M, Ocampo SM, Perales JC, Marquez VE, and Eritja R. 2011. Effect of North bicyclo [3.1.0] hexane 2-deoxysugars on RNA interference: A novel class of siRNA modification. Chem Bio Chem 12:1056–1065. [DOI] [PubMed] [Google Scholar]
- Wu Z, Madeira M, Barchi JJ Jr., Marquez VE, and Bax A. 2005. Changes in DNA bending induced by restricting nucleotide ring pucker studied by weak alignment NMR spectroscopy. Proc. Natl. Acad. Sci. U.S.A 102:24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]