Abstract
There is evidence for a relationship between raised inflammatory markers, including high sensitivity C-reactive protein (hs-CRP), measured late in life, and an increased risk of cognitive decline and dementia. This study evaluates the association of midlife hs-CRP concentrations with late-life longitudinal trends in cognitive function. Data are from the Honolulu-Asia Aging Study (HAAS), a longitudinal community-based study of Japanese American men. hs-CRP levels were measured on average 25 years before cognitive testing began in 1991. Subjects were followed from up to three follow-up examinations (mean of 6.1 years). At each exam, cognitive function was measured with the Cognitive Abilities Screening Instrument (CASI). This analysis includes a sub-sample of 691 subjects dementia-free in 1991. With incident dementia cases included, those with the highest quartile of hs-CRP had significantly more cognitive decline than those in the lowest quartile, after adjustment for baseline CASI score, demographic and cardiovascular risk factors. When cases were removed, there was no difference in cognitive decline by CRP quartile. This relationship was not modified by the presence of apolipoprotein E ε4. These findings suggest that inflammatory mechanisms during midlife may reflect underlying processes contributing to dementia-related cognitive decline late in life.
Keywords: C-reactive protein, Cognition, Longitudinal studies
1. Introduction
There is a strong evidence that C-reactive protein (CRP) is associated with an increased risk for cardiovascular disease (Ridker, 2004). Inflammatory mechanisms have also been hypothesized to contribute to the neuropathophysiologic cascade leading to late-age cognitive decline and dementia. The association of inflammatory markers, including CRP, to these late-age changes in cognitive function has been studied in epidemiologic samples. Results are inconsistent, some showing a positive (Weaver et al., 2002; Yaffe et al., 2003, 2004) and some no association (Dik et al., 2005).
Based on prevalent and early incident dementia cases in the Honolulu-Asia Aging Study we found a significant relationship between high sensitivity C-reactive protein (hs-CRP) concentrations measured at midlife and the risk 25 years later of dementia, Alzheimer’s disease and vascular dementia (Schmidt et al., 2002). Here we evaluate whether hs-CRP measured in midlife is associated with late-life longitudinal trends in cognitive function. The modifying effect of apolipoprotein E (Apo E) genotype on this relationship was also investigated since carrying the ε4 allele is associated with the risk for Alzheimer’s disease and studies have reported that Apo E ε4 is associated with lower CRP concentrations (Eiriksdottir et al., 2006).
2. Methods
The HAAS is a community-based study of 3734 Japanese American men followed up since 1965 as part of the Honolulu Heart Program. Subjects have been evaluated up to eight times after baseline; data on hs-CRP are based on samples drawn in the second examination in 1968–1970. Prevalent cases of dementia were first diagnosed in 1991–1993 (examination 4); follow-up visits considered in the current analysis (examinations 5–7) took place in 1994–1996, 1997–1999, and 1999–2000, respectively [mean of 6.1 years, range from 2 to 9 years]. Dementia case finding was conducted in a multi-step procedure according to a previously described protocol that included screening and standardized clinical evaluations (White et al., 1996). Briefly, cognitive function of all participants was tested at baseline and all follow-up exams with the 100-point Cognitive Abilities Screening Instrument (CASI) (Teng et al., 1994). The CASI is a combination of the Hasegawa Dementia Screening Scale, the Folstein Mini-Mental State Examination, and the Modified Mini-Mental State Test. The CASI score was used to identify a subgroup for further evaluation that included a neurologic exam, neuropsychological testing, and an informant interview. In subjects with dementia, a brain image was performed and routine blood tests conducted. Based on these data, a consensus diagnosis for dementia was given by the study neurologist and two physicians with expertise in dementia, according to published criteria (American Psychiatric Association, 1987; McKhann et al., 1984; Chui et al., 1992).
Concentrations (mg/L) of hs-CRP were determined in nonfasting blood specimens stored at −70°C. hs-CRP levels were measured with an enzyme-linked immunosorbent assay, calibrated with World Health Organization hs-CRP Reference Material, at the Laboratory for Clinical Biochemistry Research, University of Vermont (Macy et al., 1997). The inter-assay coefficient of variation for this assay was 5.14%.
2.1. Analytical sample
To be included in the current analysis, subjects had to have participated in the second examination [when blood was drawn] and the fourth examination [when dementia assessment started]. There were 934 subjects with CRPs assayed for another study on the association of hs-CRP and stroke (Curb et al., 2003). An additional 116 were randomly selected assays, giving a sample of 1050 (Schmidt et al., 2002). The analytical sample was representative of the total examination 4 cohort (HAAS baseline) and included men with stroke, coronary heart disease, left ventricular hypertrophy and atrial fibrillation. From these, prevalent cases (n = 140) were excluded and 219 did not have follow-up data, leaving 691 subjects for analysis, including 110 incident cases of dementia. Of the 110 incident cases, 75 were included in the analysis of Schmidt et al. (2002) and 35 were newly identified in examination 6. Compared with subjects from the study sample, those not included were on average older (p < 0.001), had less education (p < 0.05), a lower baseline CASI score (p < 0.001), but had similar concentrations of hs-CRP.
We examined the mean differences in hs-CRP concentrations between non-demented and incident dementia cases. Additional analyses with the dementia outcome were not warranted because of the low number of new incident cases that developed subsequent to what was examined in the analysis of Schmidt et al. (2002).
For these analyses, subjects were grouped into quartiles of hs-CRP concentrations (<0.34, 0.34–0.56, 0.57–1.00, >1.00 mg/L) as defined by Schmidt et al. (2002). Mean cognitive impairment based on baseline CASI scores were computed per hs-CRP quartile, adjusting for potential confounders, including age at examination 2 [when blood was collected], years of education, midlife values of body mass index, smoking status, systolic blood pressure, total cholesterol concentration, and for Apo E ε4 genotype.
A mixed model was used to estimate the association of cognitive decline to follow-up time, hs-CRP concentrations, and whether the rate of decline differed among the quartiles. This difference is tested as an interaction between follow-up time and CRP quartile; quartile 1 was used as the reference. The mixed model takes into account the presence of correlation of within person measures and the fact that a different number of unequally spaced visits were available for each subject. In addition to baseline CASI score, models were adjusted for confounders listed above.
3. Results
Baseline characteristics of subjects included in the analytical sample are summarized in Table 1. The 691 subjects had a mean age of 77.0 years (S.D. 4.1), a mean baseline CASI score of 85.9 (S.D. 8.8), and a mean hs-CRP level of 1.12 mg/L (S.D. 2.58) (median 0.56 mg/L, interquartile range 0.31–1.00 mg/L). Compared to subjects remaining non-demented (n = 581), those who developed dementia (n = 110) were, on average, older (79.6 years vs. 76.6 years, p < 0.001), had lower mean CASI scores (80.4 vs. 87.0, p < 0.001), a lower body mass index (23.3 kg/m2 vs. 24.1 kg/m2, p < 0.01), and included less subjects in the reference quartile and more subjects in the third quartile of hs-CRP (p = 0.004).
Table 1.
Baseline characteristics of analytical samplea
| Whole sample n = 691 | Non-demented subjects n = 581 | Incident cases n = 110 | p-Value | |
|---|---|---|---|---|
| Age (years) | 77.0 (4.1) | 76.6 (3.9) | 79.6 (4.2) | <0.001 |
| CASI score | 85.9 (8.8) | 87.0 (8.4) | 80.4 (8.7) | <0.001 |
| hs-CRP (mg/L) (median, IR) | 0.56 (0.31–1.00) | 0.53 (0.30–1.00) | 0.60 (0.43–1.00) | 0.09 |
| hs-CRP quartiles (%) | ||||
| First (<0.34 mg/L) | 27.2 | 29.4 | 15.5 | 0.004 |
| Second (0.34–0.56 mg/L) | 23.0 | 22.7 | 24.6 | |
| Third (0.57–1.00 mg/L) | 25.2 | 23.1 | 36.4 | |
| Fourth (>1.00 mg/L) | 24.6 | 24.8 | 23.6 | |
| Education (years) | 10.6 (3.1) | 10.7 (3.0) | 10.1 (3.1) | 0.10 |
| Apo E ε4 carrier (%) | ||||
| No | 80.5 | 81.1 | 77.3 | 0.36 |
| Yes | 19.5 | 18.9 | 22.7 | |
| Smoking status (%) | ||||
| Never | 36.5 | 36.5 | 36.4 | 0.95 |
| Past | 31.7 | 31.3 | 33.6 | |
| Current | 26.6 | 26.9 | 25.5 | |
| Missing | 5.2 | 5.3 | 4.5 | |
| BMI (kg/m2) | 24.0 (2.8) | 24.1 (2.9) | 23.3 (2.5) | <0.01 |
| Systolic blood pressure tertiles (%) | ||||
| First (lowest) | 33.1 | 34.6 | 25.5 | 0.14 |
| Second | 31.6 | 31.3 | 32.7 | |
| Third | 35.3 | 34.1 | 41.8 | |
| Cholesterol (mg/dL) | 215 (31) | 215 (30) | 214 (33) | 0.94 |
p-Values of comparison between non-demented subjects and incident dementia cases were calculated using the Kruskal–Wallis test for continuous variables and the χ2-test for categorical variables. IR = interquartile range; kg/m2 = weight in kilograms divided by height in meters squared.
Values are means (S.D.) unless otherwise stated.
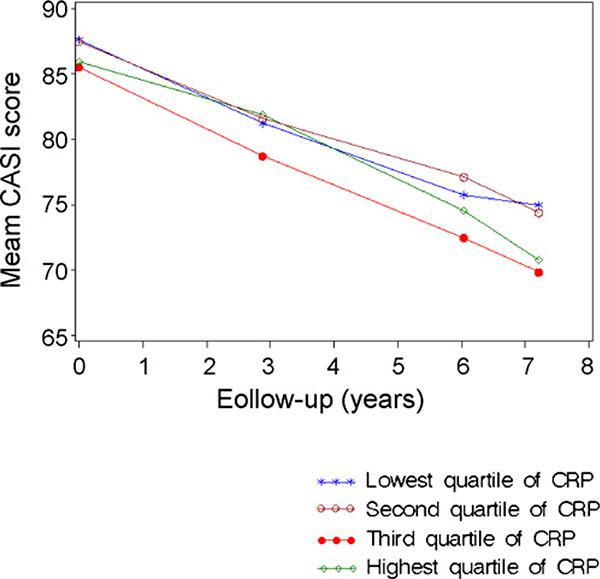
At baseline, CASI scores were not significantly different by hs-CRP quartile. Adjusted mean CASI scores by hs-CRP quartile, per year, are shown in Fig. 1. As expected, all quartiles demonstrated significant cognitive decline over time (p < 0.001). If the dementia cases remain in the analysis, there was a moderately significant difference (p < 0.05) in the slope of cognitive decline between the first and fourth quartiles. This comparison was no longer significant after the demented were removed from the analysis.
Fig. 1.
Mean CASI score over time by quartiles of hs-CRP—the HAAS, 1991–2000. Results presented in this figure were adjusted for age at examination 2, education, baseline CASI score, midlife values of body mass index, smoking status, systolic blood pressure, total cholesterol concentration, and Apo E ε4 genotype.
The study sample included 135 (19.7%) subjects with Apo E ε4. Subjects with Apo E ε4 had lower mean concentrations of hs-CRP compared to subjects without Apo E ε4 (0.78 mg/L vs. 1.20 mg/L, p < 0.01). Consequently, fewer subjects with Apo E ε4 were included in the highest quartile of the hs-CRP distribution (% of subjects with Apo E ε4 in ascending order of quartiles of hs-CRP: 30.4, 28.9, 25.9, and 14.8%). There was no global effect modification of Apo E ε4 on the relation of hs-CRP to cognitive decline (p-value for interaction term was 0.53).
4. Discussion
We examined the trajectory of cognitive decline associated with levels of hs-CRP, measured over 30 years before the first measure of global cognitive function. We found a modestly significant difference in decline between those in the lowest compared to highest quartile of hs-CRP. However, differences were no longer significant after the incident dementia cases were removed from the analysis. This association was not modified by Apo E ε4.
Our results extend the previous findings of inflammatory markers and cognition function in two ways. First, hs-CRP was measured during midlife, instead of late in life, when these measures would be less likely to be influenced by the prodromal manifestations of disease. Second we ascertained both dementia and cognitive decline in the same cohort. However, as in other studies, these findings are based on a single measure of hs-CRP. Although hs-CRP levels spike in the presence of acute inflammatory conditions, the average level may change little with time (Macy et al., 1997). Nevertheless caution is needed in interpreting results.
Experimental and neuropathologic evidence strongly suggests inflammation as part of the pathogenic pathways to cognitive disorders and dementia (Peila and Launer, 2006). Few studies have investigated the relation of inflammatory markers to cognitive decline, with inconsistent results. In the Health, Aging, and Body Composition (Health ABC) Study, higher hs-CRP concentrations (>2.5 mg/L) were associated with a greater risk of 2-year cognitive decline in a well-functioning African-American and white older population (Yaffe et al., 2003). Further analysis on that cohort suggested persons with a high level of inflammation and the metabolic syndrome were significantly more likely to develop cognitive impairment over 4 years compared to those with high levels of inflammatory proteins and no syndrome (Yaffe et al., 2004). No association between the metabolic syndrome and risk of cognitive impairment in subjects with low inflammation was observed. Other studies have found no association between levels of hs-CRP and cognitive decline (Dik et al., 2005) or cognitive function (Weuve et al., 2006).
It is possible that differences across these studies is related to the degree of cardiovascular disease across the cohorts, or the interval between the age at time of blood draw for the hs-CRP assays and age of cognitive assessment. However, extant studies did not ascertain dementia in the respective cohorts. As we find in this study, after excluding dementia cases, the association of hs-CRP and cognitive decline is no longer significant.
Our finding that the risk for cognitive decline is significant when dementia cases are included and not significant when the dementia cases were excluded is of interest. The non-significant results may reflect the fact that the exclusion resulted in a lower proportion of individuals at high risk for cognitive decline. Compared to non-demented subjects, there was three times more dementia cases with a low CASI score at baseline, and two times more who were older than 85 years. An alternative hypothesis may be that inflammatory processes contributing to late-life dementia begin early, and those related to a more ‘benign’ form of cognitive impairment become detectable closer to the beginning of the decline. Differences across studies, as well as across inflammatory markers, need to be tested in future studies.
Acknowledgments
The Honolulu-Asia Aging Study is supported by the Intramural Research Program of the NIH, the National Institute on Aging (grants U01 AG019349 and R01 AG0–17155 S1), and the National Heart, Lung, and Blood Institute (grant N01 HC05102). Dr. Laurin is a chercheur-boursier from the Fonds de la Recherche en Santé du Québec.
Footnotes
Conflict of interest
None.
References
- American Psychiatric Association, 1987. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. revised American Psychiatric Association, Washington, DC. [Google Scholar]
- Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R, 1992. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology 42, 473–480. [DOI] [PubMed] [Google Scholar]
- Curb JD, Abbott RD, Rodriguez BL, Sakkinen P, Popper JS, Yano K, Tracy RP, 2003. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation 107, 2016–2020. [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P, 2005. Serum inflammatory proteins and cognitive decline in older persons. Neurology 64, 1371–1377. [DOI] [PubMed] [Google Scholar]
- Eiriksdottir G, Aspelund T, Bjarnadottir K, Olafsdottir E, Gudnason V, Launer LJ, Harris TB, 2006. Apolipoprotein E genotype and statins affect CRP levels through independent and different mechanisms: AGES-Reykjavik Study. Atherosclerosis 186, 222–224. [DOI] [PubMed] [Google Scholar]
- Macy EM, Hayes TE, Tracy RP, 1997. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin. Chem 43, 52–58. [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM, 1984. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- Peila R, Launer LJ, 2006. Inflammation and dementia: epidemiologic evidence. Acta Neurol. Scand 185 (Suppl.), 102–106. [DOI] [PubMed] [Google Scholar]
- Ridker PM, 2004. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. Am. Heart J 148, S19–S26. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Curb J, Masaki K, White LR, Launer LJ, 2002. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann. Neurol 52, 168–174. [DOI] [PubMed] [Google Scholar]
- Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D, White LR, 1994. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int. Psychogeriatr 6, 45–58 (discussion 62). [DOI] [PubMed] [Google Scholar]
- Weaver J, Huang M, Albert M, Harris T, Rowe JW, Seeman TE, 2002. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 59, 371–378. [DOI] [PubMed] [Google Scholar]
- Weuve J, Ridker PM, Cook NR, Buring JE, Grodstein F, 2006. High-sensitivity C-reactive protein and cognitive function in older women. Epidemiology 17, 183–189. [DOI] [PubMed] [Google Scholar]
- White L, Petrovitch H, Ross G, Masaki KH, Abbott RD, Teng EL, Rodriguez BL, Blanchette PL, Havlik RJ, Wergowske G, Chiu D, Foley DJ, Murdaugh C, Curb JD, 1996. Prevalence of dementia in older Japanese-American men in Hawaii: the Honolulu-Asia Aging Study. JAMA 276, 955–960. [PubMed] [Google Scholar]
- Yaffe K,Kanaya A,Lindquist K,Simonsick EM,Harris T,Shorr RI, Tylavsky FA, Newman AB, Penninx BW, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, 2004. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 292, 2237–2242. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T, 2003. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 61, 76–80. [DOI] [PubMed] [Google Scholar]