Abstract
The aim of this study was to study an antimicrobial peptide (AMP), aurein 1.2, which substantially increased protein delivery directly into multiple mammalian inner-ear cell types in vivo. Different concentrations of aurein 1.2 with superpositively charged GFP (+36 GFP) protein fused with Cre recombinase were delivered to postnatal day 1-2 (P1-2) and adult cochleae of Cre reporter transgenic mice with various delivery methods. By cochleostomy at different concentrations of aurein 1.2–+36 GFP (1 μM, 5 μM, 22.5 μM, and 50 μM, respectively), the tdTomato (tdT) expression was observed in outer hair cells (OHCs; 20.77%, 23.02%, 76.36%, and 92.47%, respectively) and inner hair cells (IHCs; 14.90%, 44.50%, 89.59%, and 96.13%, respectively) in the cochlea. The optimal concentration was 22.5 μM with the highest transfection efficiency and the lowest cytotoxicity. Wide-spread tdT signals were detected in the cochlear-supporting cells, utricular-supporting cells, auditory nerve, and spiral ligament in neonatal and adult mice. Compared to cochleostomy, injection through the round window membrane (RWM) also produced highly efficient tdT+ labeled cells with less cell loss. In summary, the peptide aurein 1.2 fused with +36 GFP dramatically expanded the target cells with increased efficiency in direct protein delivery in the inner ear. Aurein 1.2–+36 GFP has the potential to be developed as protein-based therapy in regeneration and genome editing in the mammalian inner ear.
Keywords: inner ear, antibacterial peptides, aurein 1.2, supercharged GFP, targeted delivery
Graphical Abstract
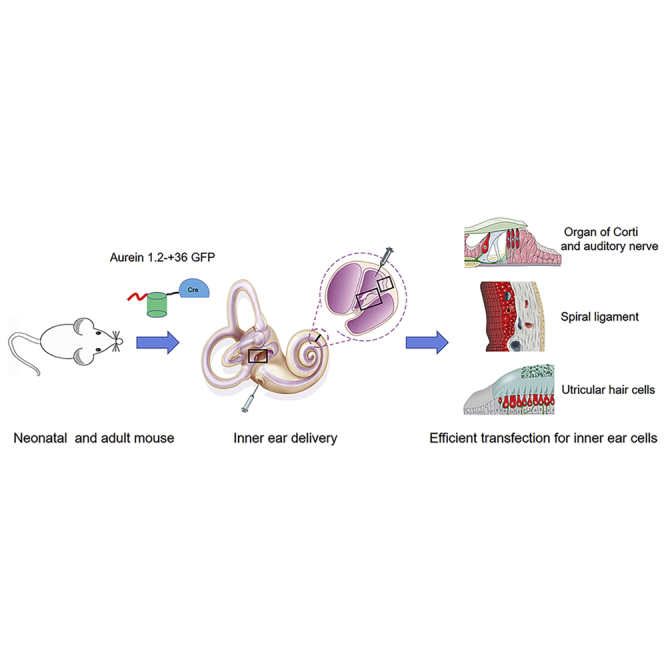
Inefficient intracellular delivery of functional proteins has been a persistent challenge for treatment of hearing loss and vestibular dysfunction. In this article, Shu and colleagues demonstrate that the aurein 1.2 fused with a supercharged protein can effectively deliver functional proteins into multiple mammalian inner ear cell types in vivo.
Introduction
Hearing loss is one of the most common sensory disorders in humans. About 466 million people suffer from hearing impairment worldwide, affecting 2 in every 1,000 newborns and approximately one-third of people over 65 years of age.1,2 Hearing loss is generally permanent as inner-ear hair cells and spiral ganglion cells cannot regenerate naturally after damage or death, and there is no pharmaceutical drug to treat hearing loss. Cochlear implants and hearing aids can help some people with hearing loss but have their own limitations.3,4
Gene therapy has emerged as a potential new treatment for some forms of hearing loss recently. The most popular types right now are achieved by introducing exogenous genes into the inner ear by adeno-associated virus (AAV) to compensate for the lost function due to gene mutations or by delivering gene-editing agents into the inner ear to edit out the mutations, which ultimately restore gene functions and recover hearing.5,6 Given that genome-editing agents can mediate targeted gene disruption or repair, direct delivery of genome-editing agents, such as RNPs (ribonucleoproteins), has the advantage over delivery of DNA vectors due to its superior safety and scalability. In addition, compared with direct introduction of exogenous DNA into host cells, intracellular delivery of functional proteins can avoid the possibility of permanent recombination into the genome, the potential damage of endogenous genes, and long-term exposure to the encoded agents.7 Meanwhile, protein therapy is also a promising option for the treatment of diseases, such as protein deficiency, mutations, and misfolding. Therapeutic proteins, including growth factors, cytokines, monoclonal antibodies, and recombinant proteins, have been placed on the medical market and transformed the pharmaceutical industry.8 To fully realize the therapeutic potential of protein biologics, it is necessary to ensure that it can access the intracellular targets.9,10 However, the low transfection efficiency of mammalian cells in vivo remains a major barrier to realize the therapeutic potential of functional proteins. There remains a challenging task to achieve intracellular delivery of proteins due to the intrinsic properties of proteins, such as large size, complex three-dimensional structure, uncertainty of molecular charge, and susceptibility to degradation.11 During the past decade, several methods have been explored for protein delivery, including peptides, liposome, nanoparticles, and polymers.12,13 Although these methods have promoted the development of protein delivery, the application is still limited by challenges, including cytotoxicity, low activity, and instability.14
According to the previous study, the variant of green fluorescent protein (GFP), a class of naturally occurring and engineered proteins with a theoretical net superpositive charge, can be a more effective way to deliver functional proteins than cell-penetrating peptides (CPPs) into cells.15,16 Whereas the superpositively charged proteins are highly efficiently endocytosed, only a small portion of functional protein can reach the cytosol because of the inefficiency of endosomal escape. It has been reported that the membrane-active peptides, such as influenza-derived hemagglutinin 2 (HA2), can be endosomolytic.17 However, many of them were cytotoxic at the concentrations necessary for protein delivery.18 Antimicrobial peptides (AMPs) as a series of membrane-active peptides can pass through microbial membranes to defend against exogenous pathogens.19 To address this difficulty in protein delivery, a screening was designed and performed to discover the aurein 1.2 as an AMP that enhanced the endosomal escape of a variety of proteins fused to superpositively charged GFP (+36 GFP) in vitro and shown elementary hair cell transfection in cochlea of neonatal in vivo.16 Based on the previous screening results of endosomal escape in vitro and preliminary in vivo data, here, we performed a systematic test by delivering functional proteins for the whole regions of the cochlea and vestibular system in vivo at different age stages with different delivery routes. We hypothesized that aurein 1.2–+36 GFP can efficiently deliver functional proteins in vivo at a whole age stage with different delivery routes that would contribute to future research on protein delivery in the treatment of hearing loss.
In this article, we performed the study to characterize transfection patterns of aurein 1.2–+36 GFP vectors by cochleostomy and by injection through the round window membrane (RWM), the two most used inner-ear injection routes. We compared how different concentrations affect transfection efficiency and target cell types and toxicity in the auditory organ and vestibular systems. We discovered the efficient delivery of functional proteins into a wide range of mammalian inner-ear cell types in vivo, including hair cells, supporting cells, auditory nerve, and spiral ligament, as well as utricular cells.
Results
To evaluate the ability of aurein 1.2 combined with +36 GFP to increase the efficacy of functional protein delivery in vivo, we delivered aurein-fused Cre recombinase protein (aurein 1.2–+36 GFP-Cre) to the inner ear of a Cre-mediated tdTomato (tdT) reporter mice. For Cre recombinase to work, it must enter the cytoplasm, escape the endosomes, and finally enter the nucleus to catalyze generation of tdT fluorescence. We injected aurein 1.2–+36 GFP-Cre and +36 GFP-Cre solutions to transfect the neonatal and adult mice inner-ear cells via cochleostomy or RWM injection (Figure 1). 5 days after injection, the cochleae were harvested for immunofluorescence staining and imaged for tdT+ florescence. We tested four working concentrations, 1 μM, 5 μM, 22.5 μM, and 50 μM, for the transfection of aurein 1.2–+36 GFP-Cre or 50 μM for +36 GFP-Cre without aurein 1.2 as a control.
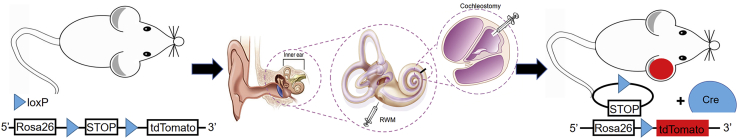
Figure 1.
Schematic Structure of Delivery Techniques via Round Window Membrane (RWM) Injection and Cochleostomy
Targeting Inner-Ear Hair Cells of Neonatal Mice via Cochleostomy
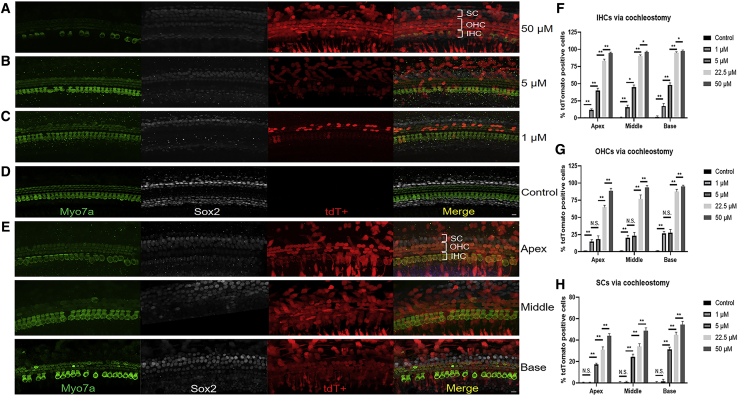
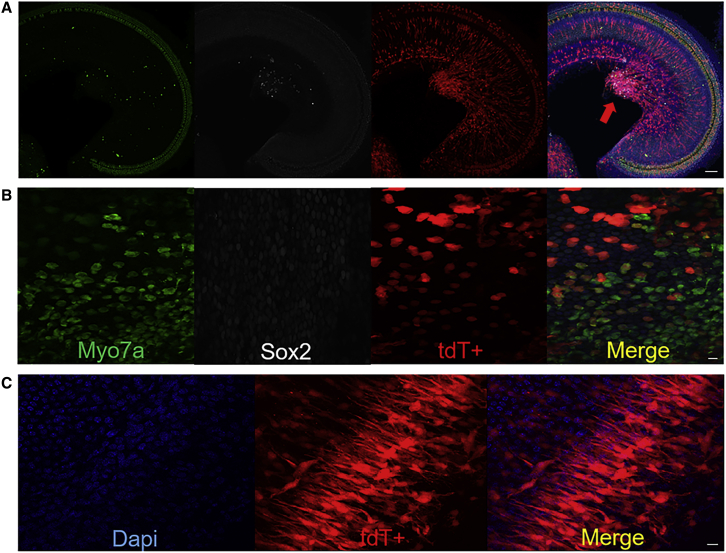
After injection of aurein 1.2–+36 GFP-Cre in neonatal mice via cochleostomy, the strong tdT signal was observed in both outer hair cells (OHCs) and inner hair cells (IHCs). Overall, the number of tdT+ OHCs and IHCs per cochlea was dose dependent. For four concentrations tested (1 μM, 5 μM, 22.5 μM, and 50 μM, respectively), 20.77%, 23.02%, 76.36%, and 92.47% of OHCs were tdT positive, and 14.90%, 44.50%, 89.59%, and 96.13% of IHCs were tdT positive, respectively, per cochlea (Figures 2A–2E). In the control group injected with +36 GFP-Cre, there were no tdT+ OHCs and IHCs (Figure 2D). Further, with higher concentrations, more IHCs were tdT positive than OHCs, and more HCs became tdT positive in the base turn than in other turns. At 22.5 μM, the proportion of tdT+ IHCs and OHCs of the basal turns was 95.08 ± 0.75% and 87.64 ± 3.32%, which indicates that it works well at medium concentrations in neonatal mice via cochleostomy (Figure 2E).
Figure 2.
In Vivo Protein Delivery of Cre Recombinase into Mouse Neonatal Cochleae via Cochleostomy
(A–E) Representative immunofluorescence images of HCs and SCs in cochlea sections injected with aurein 1.2–+36 GFP-Cre or +36 GFP-Cre at 5 days postinjection via cochleostomy. The scala media of P1-2 floxP-tdTomato mice were injected with 0.2 μL of 1 μM (C), 5 μM (B), 22.5 μM (E), and 50 μM (A) aurein 1.2–+36 GFP-Cre or +36 GFP-Cre (D). Myo7a labels HCs, and Sox2 labels SCs. Green, Myo7a; white, Sox2; red, tdTomato; and blue, DAPI. Scale bar, 10 μm. (F–H) The transfection efficiencies of targeting HCs and SCs with different doses of aurein 1.2–+36 GFP in IHCs (F), OHCs (G), and SCs (H). Results were obtained from three animals and are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01; N.S., no significance; unpaired Student’s t test.
When the working concentrations was increased from 1 μM to 5 μM, the transfection efficiency of aurein 1.2–+36 GFP-Cre in IHCs of the whole cochlea increased significantly. However, the percentage of tdT+ OHCs did not increase significantly in the apical and middle turns of the cochlea. It is possible that in the case of the lower concentration, aurein 1.2–+36 GFP-Cre preferentially transduced IHCs, and as the concentration increased more, OHCs could be transduced. The quantitative data of targeting IHCs and OHCs with different doses of aurein 1.2–+36 GFP were shown in Figures 2F and 2G.
Targeting Supporting Cells of Neonatal Mice via Cochleostomy
In addition to HCs, functional aurein 1.2–+36 GFP-Cre was delivered into supporting cells. With an increase in concentrations (5 μM, 22.5 μM, and 50 μM) of aurein 1.2–+36 GFP, the average number of supporting cells (SCs) that became tdT positive increased significantly, from 24.26% and 37.20% to 49.13%, respectively (Figures 2A–2E). The transfection efficiency of aurein 1.2–+36 GFP-Cre via cochleostomy in SCs was lower than that in HCs. We again observed a difference in the number of tdT+ SCs at different cochlear regions. After injection at 50 μM, from the basal, middle, to apical turns, there were 54.80 ± 2.78%, 48.60 ± 2.87%, and 44.0 ± 2.13% tdT+ SCs, respectively. The quantitative data of targeting SCs with different doses of aurein 1.2–+36 GFP were shown in Figure 2H.
Comparison of the Transfection Efficiencies via the RWM Injection
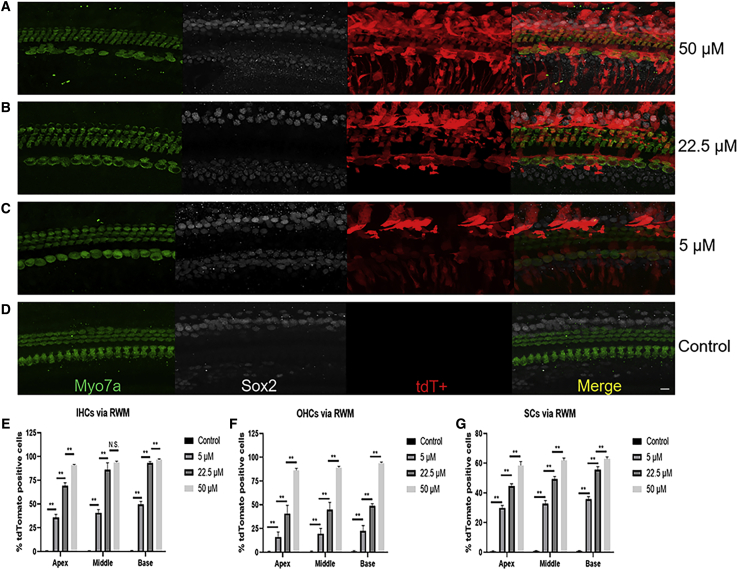
Unlike the tonotopic gradient via cochleostomy, the transfection efficiency of aurein 1.2–+36 GFP-Cre in HCs and SCs was high along the base-to-apex axis via RWM injection. When the working concentration was increased from 5 μM to 50 μM, the transfection efficiency of aurein 1.2–+36 GFP-Cre in HCs and SCs of the whole cochlea increased significantly. The tdT expression was observed in OHCs (19.41%, 44.75%, and 89.63%, respectively), IHCs (41.86%, 82.83%, and 93.70%, respectively), and SCs (32.80%, 49.93%, and 61.01%, respectively) of the whole cochlea with an increasing aurein 1.2–+36 GFP concentration of 5 μM, 22.5 μM, and 50 μM, respectively. In the control group, no obvious tdT+ labeled IHCs and OHCs were observed (Figures 3A–3D). The quantitative data of targeting HCs and SCs with different doses of aurein 1.2–+36 GFP in IHCs, OHCs, and SCs were shown in Figures 3E–3G.
Figure 3.
In Vivo Protein Delivery of Cre Recombinase into Mouse Neonatal Cochleae via RWM Injection
(A–D) Representative immunofluorescence images of HCs and SCs in cochlea sections injected with aurein 1.2–+36 GFP-Cre or +36 GFP-Cre at 5 days postinjection via RWM injection. The P1-2 floxP-tdTomato mice were injected with 0.5 μL of 50 μM (A), 22.5 μM (B), and 5 μM (C) aurein 1.2–+36 GFP-Cre or +36 GFP-Cre (D). Myo7a labels HCs, and Sox2 labels SCs. Green, Myo7a; white, Sox2; red, tdTomato; and blue, DAPI. Scale bar, 10 μm. (E–G) The transfection efficiencies of targeting HCs and SCs with different doses of aurein 1.2–+36 GFP in IHCs (E), OHCs (F), and SCs (G). Results were obtained from three animals and are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01; N.S., no significance; unpaired Student’s t test.
The Transfection Efficiencies of Targeting Inner-Ear Cells of Adult Mice
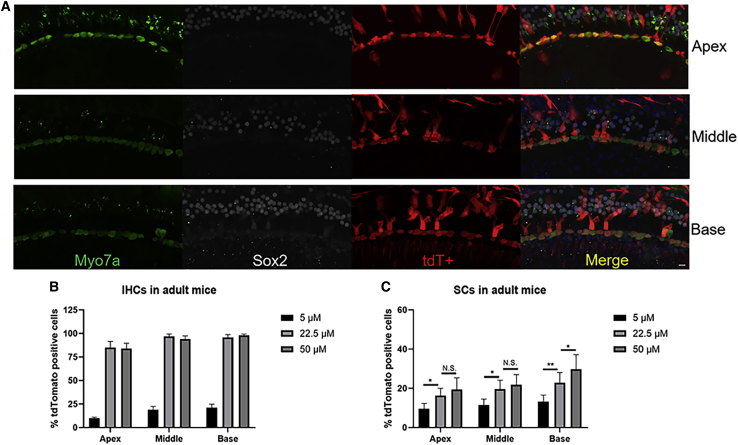
Comparing injection into neonatal mice, the transfection efficiencies of aurein 1.2–+36 GFP-Cre in IHCs were also concentration dependent in adults. As the concentration increased from 5 μM to 22.5 μM, the transfection efficiency of aurein 1.2–+36 GFP-Cre in IHCs in the basal turn of the cochlea increased statistically significantly. However, the difference in transfection efficiencies in IHCs between 22.5 μM and 50 μM was not statistically significant. The proportion of tdT+ labeled cells in IHCs of the basal, middle, and apical turns was 98.10 ± 1.33%, 94.36 ± 3.47%, and 84.67 ± 5.60%, respectively, at 50 μM. The tdT expression was observed in 11.41%, 19.67%, and 23.83%, respectively, in SCs of the whole cochlea with the aurein 1.2–+36 GFP concentration of 5 μM, 22.5 μM, and 50 μM, respectively (Figure 4).
Figure 4.
In Vivo Protein Delivery of Cre Recombinase into Adult Mouse Cochleae
(A) Representative immunofluorescence images of HCs and SCs. 0.4 μL of 50 μM +36 GFP-Cre or aurein 1.2–+36 GFP-Cre was injected into the scala media of 6-week tdTomato mice. 5 days after injection, cochleae were harvested. Myo7a labels HCs, and Sox2 labels SCs. Green, Myo7a; white, Sox2; red, tdTomato; and blue, DAPI. Scale bar, 10 μm. (B and C) The transfection efficiencies of targeting HCs and SCs with different doses of aurein 1.2–+36 GFP in IHCs (B) and SCs (C). Results were obtained from three animals and are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01; N.S., no significance; unpaired Student’s t test.
The Transfection Efficiencies of Targeting the Utricular Cells, Auditory Nerve, and Spiral Ligament
Significantly, aurein 1.2–+36 GFP also extended delivered Cre recombinase activity to other inner-ear cells, such as a majority of utricular cells, auditory nerve, and spiral ligament when injected in neonatal or adult (Figure 5). These results suggest that the aurein 1.2–+36 GFP delivery system may be a promising method for in vivo functional protein delivery into both organ of sensory and nonsensory cells of the inner ear.
Figure 5.
The Transfection Efficiencies of Targeting the Utricular Cells, Auditory Nerve, and Spiral Ligament
(A–C) Representative confocal images of whole-mount fluorescent immunolabeling of the auditory nerve (A), utricular cells (B), and spiral ligament (C). Myo7a labels HCs, and Sox2 labels SCs. Green, Myo7a; white, Sox2; red, tdTomato; and blue, DAPI. Scale bars, 100 μm (A) and 10 μm (B and C).
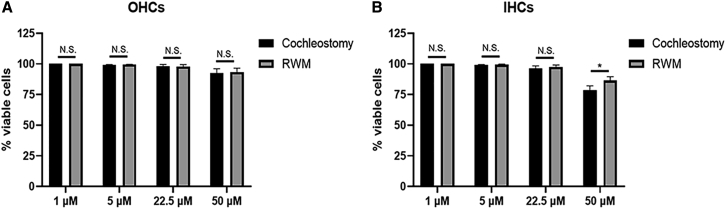
Next, we evaluated the cytotoxicity of aurein 1.2–+36 GFP at a range of concentrations (1 μM to 50 μM) by counting the 4′,6-diamidino-2-phenylindole (DAPI)-stained OHCs and IHCs. We observed no significant cytotoxicity at 22.5 μM, which resulted in 98% viable cells in neonatal cochleae. With the application of 50 μM, aurein 1.2–+36 GFP decreased cell viability to 92.87% and 81.05%, respectively, in OHCs and IHCs. At high concentration, the IHCs were more susceptible to the cytotoxicity. Compared to cochleostomy, injection through the RWM also produced highly efficient tdT+ cells with fewer cell loss. This indicates that the RWM injection may be preferable for intracellular delivery of aurein 1.2–+36 GFP-mediated functional protein (Figure 6). It has a high transfection efficiency for the whole cochlea with low cytotoxicity at medium concentrations.
Figure 6.
The Cytotoxicity of Aurein 1.2–+36 GFP at a Range of Concentrations (5 μM to 50 μM) by Counting the DAPI-Stained OHCs and IHCs
(A and B) The proportion of OHCs (A) and IHCs (B) was measured by counting DAPI-stained cells/100 μm to evaluate cytotoxicity. Results were obtained from three animals and are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01; N.S., no significance; unpaired Student’s t test.
Discussion
The inner ear is a relatively closed cavity in the temporal bone that houses the cochlea and vestibular system, the sensory organs responsible for hearing and balance, respectively. Like other tissue, dysfunction can result from defects in different specialized cell types, which can frequently result in disabling hearing loss or vertigo.20,21 Currently, the medicines in the clinic for inner-ear conditions are primarily antibiotics and glucocorticoids. However, advances in understanding the physiological and biomolecular basis of inner-ear diseases have accelerated the exploration for novel therapeutic reagents, including proteins, polypeptides, gene-editing systems, and small-molecule drugs or nucleotides.22 Compared with the introduction of exogenous DNA into host cells, direct delivery of functional proteins is obviously more advantageous. Many powerful and potentially therapeutic proteins have been discovered or engineered over the past two decades, including antibodies that can neutralize intracellular targets, metabolic complementary enzymes, engineered transcription factors, and enzymes devised for genome editing.23 However, efficient intracellular protein delivery, especially in vivo, has been a persistent challenge in biomedical research and protein therapeutics.
The key to the success of functional proteins to play a therapeutic role is to choose safe and efficient vectors and appropriate pathways for protein transfection. However, most functional proteins delivered in existing carriers remain in endosomes and do not reach the cytosol. An ideal vehicle should efficiently bind the protein and protect it against enzymatic degradation, initiate the endocytosis in target cells, trigger the endosomal disruption before protein degradation, and finally, release the bound proteins into cytosols. In addition to maintaining high protein activity during the delivery process, the vehicle should produce minimal toxicity to host cells.24,25 We have previously proven that aurein 1.2 as an AMP that enhances the endosomal escape of a variety of nonendosomal proteins fused to +36 GFP in vitro and shown very preliminary data for hair cell transfection in vivo in neonatal mice.16 Here, we systematically studied functional proteins for the whole regions of the cochlea and vestibular system in vivo at different age stages with different delivery routes. We found that Cre-mediated recombinase was delivered into mouse inner-ear hair cells with much greater potency, and the observed levels have achieved 90% Cre-mediated recombination in hair cells without significant cytotoxicity. The tdT expression was observed in OHCs (20.77%, 23.02%, 76.36%, and 92.47%, respectively) and IHCs (14.90%, 44.50%, 89.59%, and 96.13%, respectively) of the whole cochlea with aurein 1.2–+36 GFP in neonatal mice via cochleostomy at concentrations of 1 μM, 5 μM, 22.5 μM, and 50 μM, respectively. Compared with the reported protein-delivery vectors for the inner ear, such as CPPs, nanocarriers, etc.,26,27 the aurein 1.2–+36 GFP has higher transfection efficiency. The aurein 1.2–+36 GFP is also the method that reported significant recombination levels in both IHCs and OHCs and does not need any virus or other molecules beyond a single polypeptide in vivo.
Because the endogenous regeneration of inner-ear HCs in mammalian is very low, surrounding cells, especially SCs, are ideal candidates for hair cell regeneration by direct transdifferentiation or by renewed proliferation with subsequent differentiation.28 However, the transfection for SCs is a barrier to the study. The fact that aurein 1.2–+36 GFP could transduce SCs efficiently makes it a potential tool in hair cell regeneration studies. In our study, the transfection efficiency of aurein 1.2–+36 GFP-Cre to SCs of the basal, middle, and apical turns was 54.80 ± 2.78%, 48.60 ± 2.87%, and 44.0 ± 2.13%, respectively. It provides bright potential for certain types of deafness, like GJB2-related, induced genetic deafness and supporting cell-based regeneration. Moreover, many cases of sensorineural hearing loss and vestibular dysfunction are caused by auditory nerve, spiral ligament, or utricular cells.29 These are also noteworthy targets for the aurein 1.2–+36 GFP, as it can transfect these cells satisfactorily.
Due to the isolated anatomical position of the mammalian inner ear within the hardest bone, cochlea is superior to other organs in drug delivery. A limited passage through of the blood-labyrinth barrier hampers; however, systemic drug delivery and toxicity are also a concern.30 Local administration via intratympanic and intracochlear routes, bypassing the diffusional barriers that isolate it from the middle ear and the vasculature, has become increasingly important for inner-ear drug delivery. Local delivery routes that have been explored include intratympanic injection, RWM injection, cochleostomy, and posterior semicircular canal canalostomy.31,32 When a drug is administered to the middle ear through intratympanic drug injection, it must remain in the middle ear for long enough and pass the RWM or the annular ligament of oval window (OW) to reach the inner ear. Unfortunately, drugs entering the middle ear are quickly discharged to a Eustachian tube orifice through mucociliary clearance.33 Another challenge with intratympanic administration is the low permeability of the RWM and annular ligament of the OW.34 The latter methods obviously have more advantages in drug-delivery efficiency of the inner ear. Although it exhibits the same concentration gradient trend via between the RWM injection and cochleostomy, the former has a higher expression efficiency at the same concentration, approaching 92% in HCs and 61% in SCs, with better tolerance at 50 μM, which indicates that the RWM injection may be preferable for intracellular delivery of aurein 1.2–+36 GFP-mediated functional protein.
Injection into mouse cochlea via cochleostomy or RWM injection maximizes the efficiency, as it allows aurein 1.2–+36 GFP-Cre to have access to many inner-ear cell types. Previous studies suggested that neonatal mice had a better outcome of cell survival, as OHCs will die due to injection in adult cochlea, causing a barrier for this stage.35 We found that the protein can transduce IHCs and SCs at the adult stage, which is useful. Future study needs to identify a route by which injection can be performed in adults with HCs preservation, like posterior semicircular canal canalostomy.
In summary, the present study demonstrates that the aurein 1.2 fused with a supercharged protein (+36 GFP) can effectively deliver functional proteins to the inner ear in vivo at both neonatal and adult stages. The direct protein delivery with aurein dramatically expanded the efficient and wide transfection of mammalian inner-ear cell types in vivo, including HCs, SCs, auditory nerve, and spiral ligament, as well as utricular cells in a single treatment. These findings suggest that the intracellular delivery of functional proteins by aurein 1.2–+36 GFP may greatly expand the scope of research and therapeutic applications of proteins. With a much larger size and operational space, the human inner ear would increase the likelihood of a more accurate delivery, which could facilitate the progression of protein therapy in patients. It can also be developed to be used as protein-based therapy in the mammalian inner ear, such as regeneration and genome editing.
Materials and Methods
Animal Models
Rosa-tdTomatof/f mice (number 007914) were obtained from The Jackson Laboratory. All animal experiments were approved by the Institutional Animal Care and Use Committee of Fudan University. Animal management was performed strictly in accordance with the standards of the Animal Ethics Committee of the Fudan University.
Peptide Synthesis
4 mg of lyophilized aurein 1.2 peptide was obtained from China Peptides (Shanghai, China), with purity >90%. High-performance liquid chromatography (HPLC) and MALDI data were provided. The peptide was dissolved in DMSO (Sigma, St. Louis, MO, USA) to a final concentration of 10 mM.
Sortase-Mediated Peptide Conjugation
To facilitate the conjugation of aurein 1.2 to the N terminus of triglycine (GGG)–+36 GFP, aurein 1.2 was synthesized with an N-terminal histidine (His) tag and a tobacco etch virus (TEV) cleavage site (His6-ENLYFQ) and C-terminal LPETGG. The N-terminal tag prevents sortase reaction with the N-terminal glycine (Gly) on aurein 1.2. 20 μM of GGG–+36 GFP (positively charged GFP with N-terminal Gly-Gly-Gly) was incubated with 400 μM of aurein 1.2 in the presence of 1 μM sortase in 100 mM Tris buffer (pH 7.5) with 5 mM CaCl2 and 1 M NaCl for 2 h at room temperature. The unreacted peptides were removed through Amicon Ultra-0.5 spin filtration (Merck Millipore, St. Louis, MO, USA). 200 μM TEV protease (Sigma, St. Louis, MO, USA) was added to the reaction mixture to remove His tag at 4°C for 16 h. The cleaved peptides were removed, and the reaction mixture was washed twice with 500 μL of buffer concentrated to 50 μL. Conjugation efficiency was examined through liquid chromatography/mass spectrometry (LC/MS) by comparing relative peak intensities.
Protein Expression and Purification
Genes encoding +36 GFP and a (Gly-Gly-Ser)9 (GGS)9 linker were inserted N-terminal of the Cre recombinase gene by uracil-specific excision reagent (USER) cloning, and all overexpression plasmids were constructed on a pETDuet-1 backbone. Plasmids created in this work will be available at Addgene. The E. coli BL21 STAR (DE3) competent cells (Invitrogen, Gaithersburg, MD, USA) were transformed with pETDuet-1 expression plasmids.
The harvested cells were lysed by sonication (1 s pulse-on, 1 s pulse-off for 6 min, twice, at 6 W output), and the soluble lysate was obtained by centrifugation at 10,000 × g for 30 min. The cell lysate was transferred to a His-Ni-NTA (nitrilotriacetic acid [NTA]) column (Invitrogen, Gaithersburg, MD, USA) at 4°C for 45 min to capture His-tagged protein. Protein was eluted in lysis buffer with 500 mM imidazole (I5513; Sigma, St. Louis, MO, USA) and concentrated by Amicon Ultra-30-kDa molecular weight filtration (Merck Millipore, St. Louis, MO, USA) to 50 mg/mL. Protein was eluted with PBS from a 0.1- to 1-M NaCl gradient over five column volumes. The eluted fractions containing protein were concentrated to 50 μM, as quantified by absorbance at 488 nm, and stored in aliquots at −80°C.
In Vivo Microinjection
Microinjection into the Inner Ear of Neonatal Mice
Rosa26-tdTf/f mice of either sex were used for injections. The mice were randomly assigned to the different experimental groups. At least 5 mice were injected in each group. All surgical procedures were done in a clean, dedicated space. Instruments were thoroughly cleaned with 70% ethanol and autoclaved prior to surgery.
P1-2 Rosa26-tdTf/f mice cochlea was used for aurein 1.2–+36 GFP-Cre and +36 GFP-Cre injection. Aurein 1.2–+36 GFP-Cre was divided into different concentrations: 1 μM 5 μM, 22.5 μM, and 50 μM. Mice were anesthetized by hypothermia on ice, and a skin incision was made behind the right ear of the mouse before 10% povidone iodine-wiping disinfection to expose the tympanic ring and the stapedial artery under the operating microscope (Zeiss, Germany). Glass micropipettes (World Precision Instruments [WPI], Sarasota, FL, USA), held by a Nanoliter 2000 Microinjection System (WPI), were used to deliver the complexes manually into the scala media and tympanic canal through the soft cochlear lateral wall and RWM, which all allows access to inner-ear cells. The total injection volume was 0.2 μL per cochlea for cochleostomy and 0.5 μL for round window injection, and the release rate was 3 nL/s, controlled by a MICRO4 microinjection controller (WPI). The skin was closed with a 6-0 nylon suture (Ethicon, USA).
Microinjection into the Inner Ear of Adult Mice
6-week-old Rosa26-tdTf/f mice were used in this study, with the same grouping methods and concentrations as used in neonatal inner ear. Mice were anesthetized by intraperitoneal injection of a combination of xylazine (10 mg/kg) and ketamine (100 mg/kg). The right postauricular region was exposed by shaving and disinfected by 10% povidone iodine.
A 10-mm postauricular incision was made under the operating microscope (Zeiss), and the sternocleidomastoid muscle was separated to expose the otic bulla and the stapedial artery. We used a Bonn micro probe (Fine Science Tools, Germany) to drill a small hole on the cochlear lateral wall. The scala media and tympanic canal were also injected in the same way as in the neonatal mice. The total injection volume was 0.3 μL for cochleostomy and 1 μL for round window injection, and the release rate was 3 nL/s, controlled by a MICRO4 microinjection controller (WPI). The hole was sealed with tissue adhesive (3M Vetbond), and the skin was closed with a 5-0 nylon suture (Ethicon).
Immunohistochemistry
5 days after injection, the mice were sacrificed by CO2 inhalation, and the cochleae were fixed with 4% (weight/volume) paraformaldehyde (Sigma) at 4°C overnight and then decalcified in 10% EDTA (Sigma) for 3 days if necessary. Tissues were permeabilized with 0.3% Triton X-100 (1% PBS-Tween 20 [PBS-T]) and blocked with 10% donkey serum for 1 h at room temperature. All primary and secondary antibodies were diluted in 1% PBS-T. To observe the tdT expression in IHCs and OHCs of the cochlear and utricle sensory epithelium, we used 1:600 rabbit anti-myosin (Myo)7a (#25-6790; Proteus BioSciences, Ramona, CA, USA) and 1:300 goat anti-Sox2 (sc-17320; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. Appropriate Alexa-conjugated secondary antibodies (A21206 and A11058; Invitrogen, Gaithersburg, MD, USA) were incubated for 1 h after three rinses with PBS rinses, and DAPI was used to label the nuclei (1:1,000 dilution; Sigma).
Cell Counting and Statistics
To quantify the number of tdT-positive cells after aurein 1.2–+36 GFP-Cre and +36 GFP-Cre transfection, we counted the total number of HCs and SCs in a region spanning 100 μm with a laser-scanning confocal microscope (Leica TCS SP8) in the apex, middle, and base turns of the cochlea. The cytotoxicity was evaluated by counting the DAPI-stained OHCs and IHCs. If the cell died, DAPI-stained hair cells were lost. The results are presented as the mean ± SEM, and all statistical analyses were performed with GraphPad Prism 8.0. A difference was considered statistically significant when ∗p < 0.05 and ∗∗p < 0.01. When multiple comparison tests were applied to compare the transfection efficiencies of aurein 1.2–+36 GFP-Cre among different groups in the three regions of the cochlear, p < 0.0167 was considered statistically significant after Bonferroni correction.
Author Contributions
Y.S., B.C., and K.Z. designed the research and analyzed the data. K.Z., X.C., and L.Z. performed experiments in the mouse model and created a draft of the manuscript. Y.S. and B.C. supervised the research. M.H., Y.T., and H.Z. analyzed the data and edited the manuscript. J.M.R., M.Z., and Z.-Y.C. interpreted the data and revised the manuscript critically. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by National Natural Science Foundation of China, China (81822011, 81771013) (Y.S.); Science and Technology Commission of Shanghai Municipality, China (18410712400) (Y.S.); NIH, USA (R01DC006908, R56DC006908) and UG3, USA (UG3TR002636) (Z-Y.C.).
Contributor Information
Bing Chen, Email: bingchen@fudan.edu.cn.
Yilai Shu, Email: yilai_shu@fudan.edu.cn.
References
- 1.Wilson B.S., Tucci D.L., Merson M.H., O’Donoghue G.M. Global hearing health care: new findings and perspectives. Lancet. 2017;390:2503–2515. doi: 10.1016/S0140-6736(17)31073-5. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham L.L., Tucci D.L. Hearing Loss in Adults. N. Engl. J. Med. 2017;377:2465–2473. doi: 10.1056/NEJMra1616601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller U., Barr-Gillespie P.G. New treatment options for hearing loss. Nat. Rev. Drug Discov. 2015;14:346–365. doi: 10.1038/nrd4533. [DOI] [PubMed] [Google Scholar]
- 4.Wilson B.S. Toward better representations of sound with cochlear implants. Nat. Med. 2013;19:1245–1248. doi: 10.1038/nm.3343. [DOI] [PubMed] [Google Scholar]
- 5.Gu X., Chai R., Guo L., Dong B., Li W., Shu Y., Huang X., Li H. Transduction of Adeno-Associated Virus Vectors Targeting Hair Cells and Supporting Cells in the Neonatal Mouse Cochlea. Front. Cell. Neurosci. 2019;13:8. doi: 10.3389/fncel.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nist-Lund C.A., Pan B., Patterson A., Asai Y., Chen T., Zhou W., Zhu H., Romero S., Resnik J., Polley D.B. Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders. Nat. Commun. 2019;10:236. doi: 10.1038/s41467-018-08264-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuris J.A., Thompson D.B., Shu Y., Guilinger J.P., Bessen J.L., Hu J.H., Maeder M.L., Joung J.K., Chen Z.Y., Liu D.R. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 2015;33:73–80. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z., Shen W., Ling J., Yan Y., Hu J., Cheng Y. The fluorination effect of fluoroamphiphiles in cytosolic protein delivery. Nat. Commun. 2018;9:1377. doi: 10.1038/s41467-018-03779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akishiba M., Takeuchi T., Kawaguchi Y., Sakamoto K., Yu H.H., Nakase I., Takatani-Nakase T., Madani F., Gräslund A., Futaki S. Cytosolic antibody delivery by lipid-sensitive endosomolytic peptide. Nat. Chem. 2017;9:751–761. doi: 10.1038/nchem.2779. [DOI] [PubMed] [Google Scholar]
- 10.Lee K., Rafi M., Wang X., Aran K., Feng X., Lo Sterzo C., Tang R., Lingampalli N., Kim H.J., Murthy N. In vivo delivery of transcription factors with multifunctional oligonucleotides. Nat. Mater. 2015;14:701–706. doi: 10.1038/nmat4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Postupalenko V., Desplancq D., Orlov I., Arntz Y., Spehner D., Mely Y., Klaholz B.P., Schultz P., Weiss E., Zuber G. Protein Delivery System Containing a Nickel-Immobilized Polymer for Multimerization of Affinity-Purified His-Tagged Proteins Enhances Cytosolic Transfer. Angew. Chem. Int. Ed. Engl. 2015;54:10583–10586. doi: 10.1002/anie.201505437. [DOI] [PubMed] [Google Scholar]
- 12.Jenjob R., Phakkeeree T., Crespy D. Core-shell particles for drug-delivery, bioimaging, sensing, and tissue engineering. Biomater. Sci. 2020;8:2756–2770. doi: 10.1039/c9bm01872g. [DOI] [PubMed] [Google Scholar]
- 13.Jain A., Jain A., Gulbake A., Shilpi S., Hurkat P., Jain S.K. Peptide and protein delivery using new drug delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2013;30:293–329. doi: 10.1615/critrevtherdrugcarriersyst.2013006955. [DOI] [PubMed] [Google Scholar]
- 14.Yin L., Yuvienco C., Montclare J.K. Protein based therapeutic delivery agents: Contemporary developments and challenges. Biomaterials. 2017;134:91–116. doi: 10.1016/j.biomaterials.2017.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdurmen W.P.R., Mazlami M., Plückthun A. A quantitative comparison of cytosolic delivery via different protein uptake systems. Sci. Rep. 2017;7:13194. doi: 10.1038/s41598-017-13469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M., Tao Y., Shu Y., LaRochelle J.R., Steinauer A., Thompson D., Schepartz A., Chen Z.Y., Liu D.R. Discovery and characterization of a peptide that enhances endosomal escape of delivered proteins in vitro and in vivo. J. Am. Chem. Soc. 2015;137:14084–14093. doi: 10.1021/jacs.5b05694. [DOI] [PubMed] [Google Scholar]
- 17.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 18.Neundorf I., Rennert R., Hoyer J., Schramm F., Löbner K., Kitanovic I., Wölfl S. Fusion of a Short HA2-Derived Peptide Sequence to Cell-Penetrating Peptides Improves Cytosolic Uptake, but Enhances Cytotoxic Activity. Pharmaceuticals (Basel) 2009;2:49–65. doi: 10.3390/ph2020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohner K., Blondelle S.E. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb. Chem. High Throughput Screen. 2005;8:241–256. doi: 10.2174/1386207053764576. [DOI] [PubMed] [Google Scholar]
- 20.Ekdale E.G. Form and function of the mammalian inner ear. J. Anat. 2016;228:324–337. doi: 10.1111/joa.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwan T., White P.M., Segil N. Development and regeneration of the inner ear. Ann. N Y Acad. Sci. 2009;1170:28–33. doi: 10.1111/j.1749-6632.2009.04484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L., Chao T., Brant J., O’Malley B., Jr., Tsourkas A., Li D. Advances in nano-based inner ear delivery systems for the treatment of sensorineural hearing loss. Adv. Drug Deliv. Rev. 2017;108:2–12. doi: 10.1016/j.addr.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadia J.S., Stan R.V., Dowdy S.F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 24.Kintzing J.R., Filsinger Interrante M.V., Cochran J.R. Emerging Strategies for Developing Next-Generation Protein Therapeutics for Cancer Treatment. Trends Pharmacol. Sci. 2016;37:993–1008. doi: 10.1016/j.tips.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugita T., Yoshikawa T., Mukai Y., Yamanada N., Imai S., Nagano K., Yoshida Y., Shibata H., Yoshioka Y., Nakagawa S. Comparative study on transduction and toxicity of protein transduction domains. Br. J. Pharmacol. 2008;153:1143–1152. doi: 10.1038/sj.bjp.0707678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miwa T., Minoda R., Kaitsuka T., Ise M., Tomizawa K., Yumoto E. Protein transduction into the mouse otocyst using arginine-rich cell-penetrating peptides. Neuroreport. 2011;22:994–999. doi: 10.1097/WNR.0b013e32834da8f8. [DOI] [PubMed] [Google Scholar]
- 27.Yoon J.Y., Yang K.-J., Kim D.E., Lee K.-Y., Park S.-N., Kim D.-K., Kim J.-D. Intratympanic delivery of oligoarginine-conjugated nanoparticles as a gene (or drug) carrier to the inner ear. Biomaterials. 2015;73:243–253. doi: 10.1016/j.biomaterials.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 28.White P.M., Doetzlhofer A., Lee Y.S., Groves A.K., Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 29.Kaya S., Hızlı Ö., Kaya F.K., Monsanto R.D., Paparella M.M., Cureoglu S. Peripheral vestibular pathology in Mondini dysplasia. Laryngoscope. 2017;127:206–209. doi: 10.1002/lary.25995. [DOI] [PubMed] [Google Scholar]
- 30.Nyberg S., Abbott N.J., Shi X., Steyger P.S., Dabdoub A. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Sci. Transl. Med. 2019;11:482. doi: 10.1126/scitranslmed.aao0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayoob A.M., Borenstein J.T. The role of intracochlear drug delivery devices in the management of inner ear disease. Expert Opin. Drug Deliv. 2015;12:465–479. doi: 10.1517/17425247.2015.974548. [DOI] [PubMed] [Google Scholar]
- 32.Musazzi U.M., Franzé S., Cilurzo F. Innovative pharmaceutical approaches for the management of inner ear disorders. Drug Deliv. Transl. Res. 2018;8:436–449. doi: 10.1007/s13346-017-0384-5. [DOI] [PubMed] [Google Scholar]
- 33.Hao J., Li S.K. Inner ear drug delivery: Recent advances, challenges, and perspective. Eur. J. Pharm. Sci. 2019;126:82–92. doi: 10.1016/j.ejps.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Glueckert R., Johnson Chacko L., Rask-Andersen H., Liu W., Handschuh S., Schrott-Fischer A. Anatomical basis of drug delivery to the inner ear. Hear. Res. 2018;368:10–27. doi: 10.1016/j.heares.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Shu Y., Tao Y., Wang Z., Tang Y., Li H., Dai P., Gao G., Chen Z.Y. Identification of Adeno-Associated Viral Vectors That Target Neonatal and Adult Mammalian Inner Ear Cell Subtypes. Hum. Gene Ther. 2016;27:687–699. doi: 10.1089/hum.2016.053. [DOI] [PMC free article] [PubMed] [Google Scholar]