Summary
Background
Lymph node (LN) involvement is an important prognostic indicator for patients with Wilms tumor (WT), and there have been previous reports of utilizing LN density (LND = positive LN/LNs examined) as an advanced metric to risk-stratify patients with WT.
Objective
The purpose of this study was to describe patient characteristics that affect LN yield and assess the effect of LND on the overall survival (OS) in patients with WT, with the expectation that patients with LNDs above a critical cut-point would demonstrate lower OS.
Study design
The Surveillance, Epidemiology, and End Result (SEER) database was queried for all patients diagnosed with unilateral WT from 2004 to 2015. Patient and disease characteristics were collected, and Poisson regression was used to identify characteristics correlated with LN yield. LND was calculated for LN-positive patients, and multivariable survival analysis was performed, including patient demographics and LND as variables.
Results
1489 patients with unilateral WT were identified for analysis, 231 (15.51%) of whom were LN-positive. Median patient age at diagnosis was three years (IQR 1–5). On Poisson regression, the year of diagnosis, patient age, tumor size and laterality, and stage were found to impact LN yield. For patients with positive LNs, five-year OS of patients with LNDs above 0.4 was worse than those below 0.4 (76.1% vs 89.6%, p = 0.041). On multivariable analysis, tumor size and LND remained significant predictors of OS.
Discussion
Administrative databases such as SEER provide an excellent resource for studying conditions where large patient numbers for analysis are difficult to obtain. Unfortunately, the SEER database is unable to account for every factor that could affect LN sampling patterns. Additionally, favorable vs unfavorable histology is not available in SEER, and SEER utilizes its own staging system, which makes comparison to Children’s Oncology Group staging difficult. Despite these limitations, the findings of this study are similar to those previously published using administrative databases analyzing LN sampling patterns and the effect of LND on OS in WT.
Conclusions
Analysis of the SEER database confirms that there are several patient- and disease-specific factors that affect the number of LNs sampled during nephrectomy for WT, and that LND may be a predictor of OS. These findings highlight the need for standardization of LN sampling patterns for pediatric renal tumors and support the investigation of LND in future studies to further risk-stratify WT patients to tailor therapy intensity.
Keywords: Wilms tumor, nephroblastoma, lymph node density, pediatric
Graphical Abstract
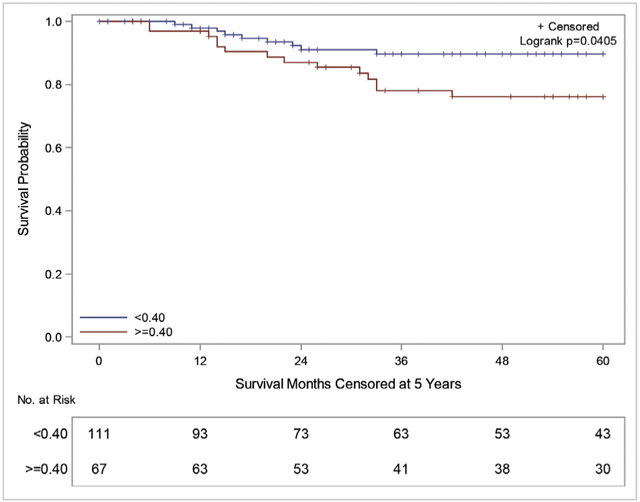
Summary Fig. Kaplan–Meier five-year OS curves for patients with WT with positive LNs stratified by LND of <0.4 (blue) vs ≥ 0.4 (red).
Introduction
Wilms tumor (WT) represents approximately 5% of all pediatric cancers and is the most common primary renal malignancy in children [1]. With an incidence of 7–10 per million children, this results in nearly 600 new cases of WT diagnosed each year in the United States (US) alone [2]. Fortunately, through the work of cooperative groups such as the Children’s Oncology Group (COG), National Wilms Tumor Study Group (NWTS), and the Société Internationale d’Oncologie Pédiatrique (SIOP), the overall survival (OS) rates of WT patients have greatly improved over the last half century [3]. This improvement in OS is in large part due to multimodal therapy, which includes a combination of surgery, chemotherapy, and radiation. More recent efforts have been made to tailor treatment regimens based on disease risk stratification to reduce the burden of therapy required to achieve similar OS and event-free survival (EFS) [4,5].
One unique group of WT patients that may benefit from these efforts is stage III WT patients with lymph node (LN) involvement. Patients with positive LNs have been shown to have worse OS and EFS when compared to patients who qualify for stage III due to alternative criterion [4,6]. In addition, multiple authors have reported on LN density (LND), defined as number of positive LNs/total LN sampled, as an advanced metric to risk-stratify LN-positive patients with various tumors. LND in malignancies involving the esophagus [7] and thyroid [8], as well as urologic cancers such as penile [9], prostate [10], bladder [11], and kidney [12], has been shown to predict OS. The concept of LND as a predictor of OS in WT has also been explored in studies of the National Cancer Database (NCDB) [13] and Surveillance, Epidemiology, and End Result (SEER) database [14].
The aim of this study was twofold: to confirm previously described patient characteristics from the NCDB that may affect LN yield (LNY) during WT surgery and to assess LND as a predictor of OS in WT, regardless of histologic subtype. It was expected that patients with LNDs above a calculated cut-point would have lower OS and cancer-specific survival (CSS).
Materials and methods
Background
The SEER database is a program funded and supported by the Surveillance Research Program within the National Cancer Institute’s Division of Cancer Control and Population Sciences. The stated goal of the database is to help reduce the burden of malignancy on US citizens by providing patient and disease characteristics on all forms of cancer since 1973. This information comes from population-based cancer registries covering approximately 35% of the US population, including over nine million patients [15].
Study population
Data on all patients diagnosed with unilateral WT (Unilateral Adolescents and Young Adults Site Recode = “9.1.1: Wilms Tumor”) from 2004 to 2015 were obtained using SEER*Stat Version 8.3.5 from the SEER 18 Regs Custom Incidence data (with additional treatment fields) 1973–2015 released April 16, 2018. Patients with a histologic diagnosis of malignant cystic nephroma, no or unknown surgery type, and without completed follow-up data were excluded. From these patients, we gathered information on patient demographics, disease characteristics, and OS and CSS. Of note, WT staging in this population was based on the SEER Summary Staging Manual 2000 [16]. This staging system separates kidney tumors into three basic categories: localized, regional, and distant. Localized tumors are limited to the kidney but can demonstrate spread beyond the renal capsule. Regional tumors are those that have spread beyond Gerota’s fascia and can have direct invasion into adjacent organs. Regional tumors can also demonstrate LN involvement if the LNs are confined to local drainage beds (i.e., hilar, aortic, and caval). Distant tumors are those that have hematogenous spread to noncontiguous organs or involve distant LNs.
To verify and expound upon previous work on LND in WT [13,14], a separate survival analysis was performed on only those patients with positive LNs. Patients who did not receive stage-appropriate, standard of care chemoradiation or who had outlier LNYs (LNY = 3 IQR above the 3rd quartile or LNY = 1–2) were excluded from survival analysis.
Data analysis
All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC), and significance was evaluated at a p-value <0.05 with a 95% confidence interval not crossing 1.0. Pertinent patient demographics such as age and year of diagnosis, gender, race, tumor laterality and size, SEER summary stage, LNY, number of positive LNs, and receipt of chemotherapy and radiotherapy were collected. Poisson regression was used to determine the impact of these patient demographics on LNY. The estimated five-year OS was calculated for all patients and LN-positive patients. Kaplan–Meier survival curves and the log-rank test were used to evaluate univariate (UV) effects on five-year OS.
For subanalysis, LN-positive patients were separated into two cohorts based on an optimal LND cut-point of 0.4, which was determined using a log rank test statistic described by Contal and O’Quigley [17]. Using this method, all possible values of LND were tested as cut-point values, and the value that gave the largest separation in outcome (OS) was chosen. OS and CSS were calculated for the whole cohort, those with low LNYs (≤6 LNs), and those with high LNYs (>6 LNs). UV and multivariable (MV) survival analysis was performed. Due to the smaller sample size, p-values <0.10 were considered significant. LND was included as a predictor and evaluated as a continuous and categorical variable. Additional variables included in the MV Cox proportional-hazards model included age, gender, race, tumor size, laterality, and LNY. Proportional-hazards assumption was evaluated using Schoenfeld residuals.
Results
A total of 1627 patients diagnosed with unilateral WT (International Classification for Diseases for Oncology histologic code 8960) were identified in the SEER database from 2004 to 2015. Of those patients, 98 were excluded based on further classification as ‘malignant cystic nephroma.’ Of the remaining 1529 patients, 39 had no or an unknown surgical history, and one patient had no survival data. Ultimately, 1489 patients had adequate data for analysis (Supplemental Fig. 1). Baseline patient characteristics of all patients versus LN-positive patients are summarized in Table 1. Among the 1489 patients included in statistical analysis, 231 (15.51%) were recorded as having at least one positive LN. The median number of LNs examined for the total population was 3 (IQR 1–7) compared to 6 (IQR 3–11) for patients with LN-positive disease. The median number of positive LNs among the LN-positive patients was 2 (IQR 1–3). Additionally, it was noted that patients were more likely to have a positive LN as the LNY increased, particularly in those who had LNYs >10 (Supplemental Fig. 2). 314 (21.1%) patients had no or an unknown LNY.
Table 1.
Study patient demographics: all patients vs LN-positive patients.
| All | LN-positive patients |
|
|---|---|---|
| Number of Patients | 1489 | 231 |
| Median Age Years (IQR) Gender | 3.0 (1.0–5.0) | 4.0 (2.0–5.0) |
| Female (%) | 800 (53.7) | 122 (52.8) |
| Male (%) | 689 (46.3) | 109 (47.2) |
| Race/Ethnicity | ||
| White (%) | 738 (49.6) | 111 (48.1) |
| Black (%) | 256 (17.2) | 36 (15.6) |
| Hispanic (%) | 400 (26.9) | 68 (29.4) |
| Other (%) | 84 (5.6) | 14 (6.1) |
| Unknown (%) | 11 (0.7) | 2 (0.9) |
| Laterality | ||
| Right (%) | 739 (49.6) | 88 (38.1) |
| Left (%) | 750 (50.4) | 143 (61.9) |
| Median Tumor Size Centimeters (IQR) | 11 (8.0–13.5) | 12.0 (0.5–13.85) |
| SEER Summary Stage | ||
| Unknown (%) | 18 (1.2) | |
| Localized (%) | 643 (43.2) | |
| Regional (%) | 489 (32.8) | 132 (57.1) |
| Distant | 339 (22.8) | 99 (42.9) |
| Median LNY (IQR) | 3 (1–7) | 6 (3–11) |
| 0 (%) | 246 (16.5) | |
| 1–3 (%) | 483 (32.4) | 76 (32.9) |
| 4–6 (%) | 307 (20.6) | 59 (25.5) |
| 7–9 (%) | 145 (9.7) | 27 (11.7) |
| 10–12 (%) | 85 (5.7) | 23 (10.0) |
| >12 (%) | 155 (10.4) | 46 (19.9) |
| Unknown (%) | 68 (4.6) | |
| Median LNs Positive (IQR) | 0 (0–0) | 2 (1–14) |
| Unknown (%) | 16 (1.1) | |
| 0 (%) | 984 (66.1) | |
| 1 (%) | 114 (7.7) | 113 (48.9) |
| ≥2 (%) | 119 (8.0) | 118 (51.1) |
| LND (IQR) | 0.38 (0.18–0.70) | |
| Radiation (%) | 721 (48.4) | 216 (93.5) |
| Chemotherapy (%) | 1345 (90.3) | 225 (97.4) |
| Estimated five-year OS (95% CI) | 92.2% (90.5–93.7) | 83.5% (77.2–88.2) |
Several factors were identified on Poisson regression to increase LNY and included more recent year of diagnosis, younger patients, white race, larger tumors, left-sided tumors, and higher SEER summary stage (p < 0.001) (Table 2). Five-year OS in all patients was 92.2% (95% CI 90.5–93.7).
Table 2.
Poisson regression predicting number of LNs sampled (n = 1364).
| Incidence Rate Ratio |
95% Confidence Interval |
|||
|---|---|---|---|---|
| Lower LImist |
Upper LImist |
P- Value |
||
| Year of Diagnosis | 1.036 | 1.029 | 1.043 | <0.001 |
| Age (Continuous) | 0.986 | 0.982 | 0.991 | <0.001 |
| Race | ||||
| White | 1 | |||
| Black | 0.842 | 0.789 | 0.899 | <0.001 |
| Hispanic | 0.716 | 0.676 | 0.758 | <0.001 |
| Other | 0.974 | 0.879 | 1.079 | 0.615 |
| Unknown | 1.303 | 1.049 | 1.620 | 0.017 |
| Gender | ||||
| Male | 1 | |||
| Female | 0.978 | 0.934 | 1.025 | 0.350 |
| Tumor Size (Continuous) | 1.006 | 1.003 | 1.009 | <0.001 |
| Laterality | ||||
| Left | 1 | |||
| Right | 0.724 | 0.691 | 0.758 | <0.001 |
| Stage | ||||
| Localized | 1 | |||
| Distant | 1.183 | 1.114 | 1.257 | <0.001 |
| Regional | 1.199 | 1.137 | 1.264 | <0.001 |
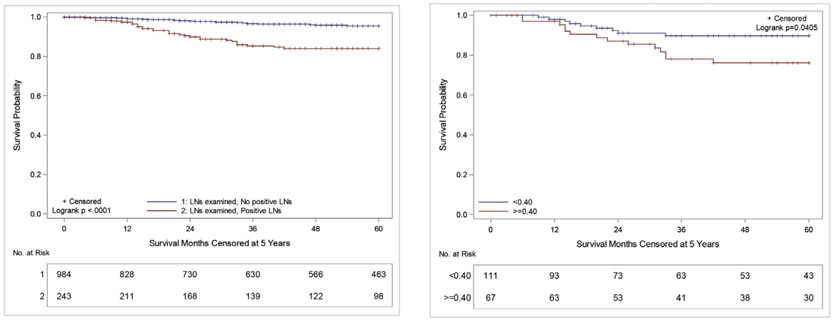
When comparing patients who had undergone LN sampling (LNS) based on LN status (no positive LNs vs positive LNs), there was a significant difference in five-year OS (95.4% vs 84.0%, p < 0.0001) (Fig. 1A). Notably, five-year OS in patients who had no nodes sampled was 89.5%, and the worst OS was seen in those with LNS, but an unknown result (68.6%).
Fig. 1.
A: Kaplan–Meier five-year OS curves of patients with WT who underwent LNS stratified by LN status: No positive LNs (blue) vs positive LNs (red), B: Kaplan–Meier five-year OS curves for patients with WT with positive LNs stratified by LND of <0.4 (blue) vs ≥ 0.4 (red).
On subanalysis of LN-positive patients, the median LND was 0.38, which was similar to the calculated optimal cut-point of 0.4. The average LND was found to be inversely proportional to LNY (Supplemental Fig. 3). These patients were then divided into two cohorts, those with LND <0.4 (n = 111) vs ≥0.4 (n = 67). The estimated five-year OS in patients with LND <0.4 was 89.6% vs 76.1% for those with a LND ≥0.4 (p = 0.041) (Fig. 1B). Likewise, five-year CSS survival in patients with LND <0.4 was 89.6% vs 78.6% in those with LND ≥0.4 (p = 0.107) (Supplemental Fig. 4). Further analysis of patients with LNY ≤6 demonstrated 90.7% vs 76.2% OS (p = 0.1502) when separated by the cut-point LND (Supplemental Fig. 5A). Patients with LNY >6 had an 89.1% vs 76.1% OS (p = 0.2170) when stratified by cut-point LND (Supplemental Fig. 5B). There was no difference in OS in LN-negative patients based on LNY (Supplemental Fig. 6). All survival trends noted in the first five years were stable through 12 years of follow-up. MV survival analysis of LN-positive patients (n = 174) demonstrated that tumor size (p = 0.040) and LND (p = 0.081) alone were associated with OS (Table 3). However, when patients were separated by LNY ≤6 vs > 6, LND was only a significant predictor for those with LNYs ≤6 (p = 0.090) (Supplemental Table 1).
Table 3.
UV and MV survival analysis for LN-positive patients (n = 174).
| UV |
MV |
|||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio |
95% Lower CL | 95% Upper CL | P-Value | Hazard Ratio |
95% Lower CL | 95% Upper CL | P-Value | |
| Age (Continuous) | 1.047 | 0.974 | 1.126 | 0.215 | 1.040 | 0.952 | 1.136 | 0.383 |
| Gender | ||||||||
| Male | 1.000 | |||||||
| Female | 1.195 | 0.524 | 2.726 | 0.672 | 1.545 | 0.633 | 3.772 | 0.340 |
| Race | ||||||||
| White | 1.000 | |||||||
| Other | 1.593 | 0.698 | 3.634 | 0.268 | 1.656 | 0.706 | 3.886 | 0.246 |
| Tumor Size Laterality | 1.124 | 1.003 | 1.258 | 0.044 | 1.120 | 1.005 | 1.247 | 0.040 |
| Left | 1.000 | |||||||
| Right | 0.655 | 0.258 | 1.661 | 0.373 | 0.680 | 0.264 | 1.752 | 0.424 |
| SEER Summary Stage | ||||||||
| Regional | 1.000 | |||||||
| Distant | 1.491 | 0.658 | 3.380 | 0.339 | 1.304 | 0.558 | 3.048 | 0.540 |
| LNY | ||||||||
| ≤6 LNs | 1.000 | |||||||
| >6 LNs | 0.762 | 0.334 | 1.739 | 0.519 | 1.227 | 0.468 | 3.217 | 0.678 |
| LND (Continuous) | 3.915 | 1.085 | 14.125 | 0.037 | 3.753 | 0.851 | 16.545 | 0.081 |
Discussion
Great improvements have been made in the treatment of WT over the last 50 years. The five-year OS rate for children diagnosed with WT in the US has increased from 70% in the early 1970s to over 90% today [3]. The current SEER data analysis mirrors this survival trend, which supports the validity of these findings. The advancements made by cooperative groups such as COG, NWTS, and SIOP have allowed for greater OS across all disease stages, and in some cases, have allowed a concurrent reduction in therapy intensity. For example, the NWTS demonstrated that abdominal radiation can be avoided in children with stages I and II, favorable histology (FH) WT, and that chemotherapy regimens for these patients can be limited to actinomycin and vincristine for a shorter duration [18,19]. Green et al. also published on the success of nephrectomy alone in children less than two years old with small (<550 gm), favorable-histology WTs (FHWTs) [20].
Treatment reduction without compromising OS is especially pertinent for local stage III WT patients, as they are typically subjected to additional doxorubicin chemotherapy and radiotherapy. There have been reports on late, dose-dependent cardiovascular effects of doxorubicin, which lead to increased cardiac mortality rates [21,22]. COG has determined multiple clinicopathologic features that result in a local stage III designation, including LN involvement, tumor spillage, prior biopsy, and residual disease, of which LN involvement is the most common. These factors have also been independently associated with varying OS rates [4], raising the question of whether the current COG staging system should be refined to account for further stage III subgroup risk stratification.
Unfortunately, LNS omission rates during surgery for WT are reported between 9 and 42% [23,24], and the omission rate for this study was 16.5%. Shamberger et al. analyzed data from the NWTS-4 and found that the absence of LNS resulted in a 2.6 relative risk of local recurrence. That analysis also found that two-year survival after recurrence was only 43% [25]. Decreased OS and EFS in patients who do not undergo LNS are likely the result of under-staging, which affects the intensity of stage-directed therapy [26]. The present study supports this conclusion as patients with no or unknown LNS had significantly lower OS. However, had the LN status in these patients been more clearly defined, it is possible that the predicted survival in the cohorts with a known LN status, positive or negative, would be lower. In addition, reliance on the surgeon to determine the need for LNS at the time of surgery based on intraoperative findings has been shown to be unreliable [27]. This suggests the need for surgeon education and templated LNS at the time of WT nephrectomy.
The current study using the SEER population reinforces the relationship between factors that influence LNY in recently published NCDB data [13]. Although the SEER database could not confirm findings on insurance status and facility volume, it did confirm that age, race, tumor size, laterality, and stage are significant predictors of total LNs sampled (p < 0.0001) (Table 2). Interestingly, individuals recorded as Black or Hispanic were more likely to have lower LNYs than Caucasians. The reasoning behind this race-related trend is unclear, but it does not appear to lead to differences in OS between racial groups with positive LNs (p = 0.246). The correlation demonstrated between LNY and tumor laterality may be due to lymphatic drainage of right-sided tumors into the often under-sampled interaortocaval space [13]. As in this study, tumor size is a known predictor of LNY in colorectal cancer [28], which could be explained by the removal of larger surgical specimens or surgeon bias to obtain more nodal tissue during removal of larger tumors. Ultimately, further studies into which of these factors may be modifiable are warranted.
There are two previous studies that specifically addressed the issue of LND in patients with WT. The first, published by Saltzman et al., used the NCDB to compare OS in LN-positive patients with FHWT based on LND above or below the median [13]. Compared to the current analysis, the NCDB contains more patients and has additional histologic information, which allowed for the investigators to control for anaplasia. Despite the differences in the two databases, LND remained predictive of five-year OS (p = 0.081). The current study expanded the analysis to all WT histologies, included CSS, utilized a different metric to determine the optimal LND cut-point, and investigated the effect of LNY on LND and survival. It is important to note that there is crossover of patients between the NCDB and SEER database.
Another recently published study by You et al. looked at OS in WT patients using the SEER database [14]. Survival analysis was performed on patients based on a ‘high’ vs ‘low’ LND defined as above or below 0.22, respectively, which was lower than the LND cut-point for the current study. The former included patients without positive LNs, which likely underestimated the average LND for patients with node-positive disease. Although there is no consensus on the optimal LND cut-point, there are now multiple studies with different cut-points that highlight the utility of LND in WT.
The benefit of using an advanced metric like LND is that it accounts for both disease burden through LN positivity and extent of surgical dissection through LNY. LND may be a way to further risk-stratify LN-positive patients, as LNY alone has not been shown to predict EFS in stages I and II WT [6]. The current study supports these conclusions in LN-positive WT patients, which also showed no correlation between LNY and OS (p = 0.678). Additionally, Kieran et al. have suggested that patients with occult LN involvement and clinical stage II FHWT (but occult pathologically stage III disease) may possibly be successfully treated with two-drug chemotherapy alone [26]. Although seemingly contradictory, it may be that patients with occult LN positivity would be more likely to have lower LND, and therefore, adequately treated with less intense therapy. Collectively, these data suggest that calculating LND may lead to improved risk stratification. It should be noted that the present study was not designed to determine the efficacy of adjuvant therapy based on LND. However, it would be interesting to see if altering the intensity of therapy based on LND could achieve similar outcomes for a subset of stage III WT patients.
Currently, there is great variability in LNS performed for WT, with multiple factors effecting LNY and therefore LND. It is possible that this variability is responsible for the difference in predictive value of LND in high vs low LNYs. It has been reported that identifying patients with positive LNs is maximized by obtaining at least seven LNs [26], with more recent data suggesting 10 LNs are needed to reach a false negative rate of ≤10% [29]. Unfortunately, even with these suggested LNY thresholds, only 25% of surgeries achieve a total LNY of seven or more [26]. Similarly, it has been reported that en-bloc resection of the kidney retrieves fewer LNs than by separate sampling [30], which could skew results. Based on the available data on LN involvement in WT, more precise characterization of true disease stage through a protocolized LNS technique followed by quantification of LND could more accurately and consistently risk-adjust patients. A potential template option familiar to most urologists would be the anatomic boundaries utilized for ipsilateral retroperitoneal LN dissection in testis tumors, which are based on tumor laterality and expected lymphatic drainage patterns, but roughly correspond to where the ureter crosses the iliac inferiorly, the crus of the diaphragm superiorly, and the interaortocaval space medially.
The current study has several limitations worth mentioning. The SEER database, while useful for analyzing large populations with rare diseases, cannot account for every factor that relates to the extent and location of LNS, including institutional volume and individual surgeon preferences. Additionally, the SEER database does not universally track recurrence rates and other outcome measures that could be used to control for situations such as local relapses. The dosing, timing, and choice of chemotherapeutics and radiation therapy are not recorded in SEER, which may compromise adherence to COG protocols and affect clinical outcomes. WT histology is also a missing variable from this database, and there is no centralized pathology review, which limits the ability to control for unfavorable histology.
Lastly, SEER uses a standardized staging system for all renal tumors, regardless of pathology, and it deviates from the more clinically utilized COG staging system for WT in several important ways (Supplemental Table 2). In general, the lower stages of WT are classified by COG according to local tumor extent and completeness of tumor removal. Stage III has the most inductive criteria and is the first stage that accounts for residual disease after surgical extirpation. Positive margins, LN involvement, preoperative biopsy, intraoperative tumor spill, peritoneal involvement, and any inoperable local spread will qualify as local stage III. Stage IV tumors have distant metastasis, and stage V tumors are bilateral on presentation. By comparison, SEER summary stage does not account for residual disease after surgery, preoperative renal biopsy, tumor spill, or bilateral disease. LN involvement has varying effects on the stage depending on location of the positive LNs. The differences in these two staging systems allow for cross-contamination among stages, which makes a direct comparison of stage-based survival impossible.
Despite these limitations, this SEER analysis reinforces the concepts highlighted in prior studies on factors that affect LNS and supports the role of LND in predicting OS. Further characterization of LND in WT is warranted, as this may lead to improved risk stratification of therapeutic regimens in a subset of patients based on LND.
Conclusions
Ongoing efforts to improve survival in children with WT have been largely successful over the past half century because of the efforts of cooperative groups. Future adjustments in treatment regimens are focused on improved patient risk stratification and subsequent therapy modifications. LN status plays a pivotal role in WT, and this study supports the notion that LND, as an advanced metric of disease burden, predicts OS in WT patients, regardless of the histologic subtype. In addition, patient-specific characteristics such as year of diagnosis, age, race, tumor size and laterality, and SEER stage predict LNY. Future studies assessing the impact of LND on EFS in WT utilizing a standardized approach to LNS may help to confirm the validity of LND as a prognostic indicator.
Supplementary Material
Acknowledgments
Funding
Statistical support was paid for by the Etkin Family Fund of the Apsen Community Foundation and this project was supported by Population Health Shared Resource, University of Colorado Cancer Center P30CA046934.
Footnotes
Ethical approval
Institutional review board-exempt.
Competing interest
None declared.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpurol.2019.10.030.
References
- [1].Birch JM, Breslow N. Epidemiologic features of Wilms tumor. Hematol Oncol Clin North Am 1995;9:1157–78. [PubMed] [Google Scholar]
- [2].Breslow N, Olshan A, Beckwith JB, Green DM. Epidemiology of Wilms tumor. Med Pediatr Oncol 1993;21:172–81. [DOI] [PubMed] [Google Scholar]
- [3].Cotton CA, Peterson S, Norkool PA, Takashima J, Grigoriev Y, Green DM, et al. Early and late mortality after diagnosis of Wilms tumor. J Clin Oncol 2009;27:1304–9. 10.1200/JCO.2008.18.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ehrlich PF, Anderson JR, Ritchey ML, Dome JS, Green DM, Grundy PE, et al. Clinicopathologic findings predictive of relapse in children with stage III favorable-histology Wilms tumor. J Clin Oncol 2013;31:1196–201. 10.1200/JCO.2011.41.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fernandez CV, Perlman EJ, Mullen EA, Chi Y-Y, Hamilton TE, Gow KW, et al. Clinical outcome and biological predictors of relapse after nephrectomy only for very low-risk Wilms tumor: a report from Children’s oncology group AREN0532. Ann Surg 2017;265:835–40. 10.1097/SLA.0000000000001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Graf N, Furtwängler R. Preoperative chemotherapy and local stage III in nephroblastoma. Transl Pediatr 2014;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ooki A, Yamashita K, Kobayashi N, Katada N, Sakuramoto S, Kikuchi S, et al. Lymph node metastasis density and growth pattern as independent prognostic factors in advanced esophageal squamous cell carcinoma. World J Surg 2007;31: 2184–91. 10.1007/s00268-007-9198-9. [DOI] [PubMed] [Google Scholar]
- [8].Amit M, Tam S, Boonsripitayanon M, Cabanillas ME, Busaidy NL, Grubbs EG, et al. Association of lymph node density with survival of patients with papillary thyroid cancer. JAMA Otolaryngol Head Neck Surg 2018;144:108–14. 10.1001/jamaoto.2017.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ball MW, Schwen ZR, Ko JS, Meyer A, Netto GJ, Burnett AL, et al. Lymph node density predicts recurrence and death after inguinal lymph node dissection for penile cancer. Investig Clin Urol 2017;58:20 10.4111/icu.2017.58.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Passoni NM, Abdollah F, Suardi N, Gallina A, Bianchi M, Tutolo M, et al. Head-to-head comparison of lymph node density and number of positive lymph nodes in stratifying the outcome of patients with lymph node-positive prostate cancer submitted to radical prostatectomy and extended lymph node dissection. Urol Oncol: Semin Orig Investig 2014;32:29.e21–8. 10.1016/j.urolonc.2012.10.009. [DOI] [PubMed] [Google Scholar]
- [11].Stein JP, Cai J, Groshen S, Skinner DG. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: the concept of lymph node density. J Urol 2003;170:35–41. 10.1097/01.ju.0000072422.69286.0e. [DOI] [PubMed] [Google Scholar]
- [12].Tilki D, Chandrasekar T, Capitanio U, Ciancio G, Daneshmand S, Gontero P, et al. Impact of lymph node dissection at the time of radical nephrectomy with tumor thrombectomy on oncological outcomes: results from the International Renal Cell Carcinoma-Venous Thrombus Consortium (IRCC-VTC). Urol Oncol: Semin Ori Investig 2018;36: 79.e11–7. 10.1016/j.urolonc.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Saltzman AF, Carrasco A, Amini A, Aldrink JH, Dasgupta R, Gow KW, et al. Patterns of lymph node sampling and the impact of lymph node density in favorable histology Wilms tumor: an analysis of the national cancer database. J Pediatr Urol 2018;14 10.1016/j.jpurol.2017.09.025.161.e1-161161.e8. [DOI] [PubMed] [Google Scholar]
- [14].You H, Yang J, Liu Q, Tang L, Bu Q, Pan Z, et al. The impact of the lymph node density on overall survival in patients with Wilms’ tumor: a SEER analysis. Cancer Manag Res 2018. 10.2147/CMAR.S163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Available at: https://seer.cancer.gov. Accessed 02/26/2019.
- [16].Young JL Jr, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA, editors. SEER summary staging manual – 2000: codes and coding instructions. Bethesda, MD: National Cancer Institute; 2001. NIH Pub. No. 01–4969. [Google Scholar]
- [17].Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal 1999;30:253–70. [Google Scholar]
- [18].D’Angio GJ, Evans A, Breslow N, Beckwith B, Bishop H, Farewell V, et al. The treatment of Wilms’ tumor: results of the second national Wilms’ tumor study. Cancer 1981;47: 2302–11. . [DOI] [PubMed] [Google Scholar]
- [19].D’angio GJ, Breslow N, Beckwith JB, Evans A, Baum E, Delorimier A, et al. Treatment of Wilms’ tumor. Results of the third national Wilms’ tumor study. Cancer 1989;64:349–60. . [DOI] [PubMed] [Google Scholar]
- [20].Green DM, Breslow NE, Beckwith JB, Ritchey ML, Shamberger RC, Haase GM, et al. Treatment with nephrectomy only for small, stage I/favorable histology Wilms’ tumor: a report from the national Wilms’ tumor study group. J Clin Orthod 2001;19:3719–24. 10.1200/JCO.2001.19.17.3719. [DOI] [PubMed] [Google Scholar]
- [21].Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med 1991;324:808–15. 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- [22].Green DM, Hyland A, Chung CS, Zevon MA, Hall BC. Cancer and cardiac mortality among 15-year survivors of cancer diagnosed during childhood or adolescence. J Clin Orthod 1999;17: 3207–15. 10.1200/JCO.1999.17.10.3207. [DOI] [PubMed] [Google Scholar]
- [23].Ehrlich PF, Ritchey ML, Hamilton TE, Haase GM, Ou S, Breslow N, et al. Quality assessment for Wilms’ tumor: a report from the national Wilms’ tumor study-5. J Pediatr Surg 2005;40:208–13. 10.1016/j.jpedsurg.2004.09.044. [DOI] [PubMed] [Google Scholar]
- [24].Raval MV, Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY, et al. Nodal evaluation in Wilms’ tumors: analysis of the national cancer data base. Ann Surg 2010;251:559–65. 10.1097/SLA.0b013e3181cc95d7. [DOI] [PubMed] [Google Scholar]
- [25].Shamberger RC, Guthrie KA, Ritchey ML, Haase GM, Takashima J, Beckwith JB, et al. Surgery-related factors and local recurrence of Wilms tumor in national Wilms tumor study 4. Ann Surg 1999;229:292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kieran K, Anderson JR, Dome JS, Ehrlich PF, Ritchey ML, Shamberger RC, et al. Lymph node involvement in Wilms tumor: results from national Wilms tumor studies 4 and 5. J Pediatr Surg 2012;47:700–6. 10.1016/j.jpedsurg.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Biemann Othersen H, DeLorimer A, Hrabovsky E, Kelalis P, Breslow N, D’Angio GJ. Surgical evaluation of lymph node metastases in Wilms’ tumor. J Pediatr Surg 1990;25:330–1. 10.1016/0022-3468(90)90079-O. [DOI] [PubMed] [Google Scholar]
- [28].Betge J, Harbaum L, Pollheimer MJ, Lindtner RA, Kornprat P, Ebert MP, et al. Lymph node retrieval in colorectal cancer: determining factors and prognostic significance. Int J Colorectal Dis 2017;32:991–8. 10.1007/s00384-017-2778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Saltzman AF, Smith DE, Gao D, Ghosh D, Amini A, Aldrink JH, et al. How many lymph nodes are enough? Assessing the adequacy of lymph node yield for staging in favorable histology Wilms tumor. J Pediatr Surg 2019;54(11):2331–5. 10.1016/j.jpedsurg.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stewart CL, Bruny JL. Maximizing lymph node retrieval during surgical resection of Wilms tumor. Eur J Pediatr Surg 2015;25: 109–12. 10.1055/s-0034-1386637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.