Abstract
Infection prevention is a high priority for home healthcare (HHC), but tools are lacking to identify patients at highest risk of developing infections. The purpose of this study was to develop and test a predictive risk model to identify HHC patients at risk of an infection-related hospitalization or emergency department visit. A nonexperimental study using secondary data was conducted. The Outcome and Assessment Information Set linked with relevant clinical data from 112,788 HHC admissions in 2014 was used for model development (70% of data) and testing (30%). A total of 1,908 patients (1.69%) were hospitalized or received emergency care associated with infection. Stepwise logistic regression models discriminated between individuals with and without infections. Our final model, when classified by highest risk of infection, identified a high portion of those who were hospitalized or received emergent care for an infection while also correctly categorizing 90.5% of patients without infection. The risk model can be used by clinicians to inform care planning. This is the first study to develop a tool for predicting infection risk that can be used to inform how to direct additional infection control intervention resources on high-risk patients, potentially reducing infection-related hospitalizations, emergency department visits, and costs.
Keywords: infection, risk modeling, home healthcare, OASIS
Introduction
Home healthcare (HHC) has become a leading source of postacute care in the United States., providing services to the expanding aging population of acutely ill patients moving between hospitals and community.1 In 2016, over 3.4 million Medicare beneficiaries received HHC services nationwide. Medicare expenditures for this population totaled more than $18.1 billion, or 4.6 percent of all fee-for-service spending.2 A substantial proportion of HHC episodes results in rehospitalization, placing patients at increased risk of suffering adverse events and incurring costs, which may be as high as $17 billion per year.3
One leading cause of hospitalization among HHC patients is infection. An analysis of national data revealed that approximately 18% of unplanned hospitalizations among HHC patients were associated with four types of infections4, which are costly and often preventable.5 National efforts, guided by the Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections (HAIs) ,6 were associated with a 58% reduction in bloodstream infections in intensive care units between 2001 and 2009.7 However, comparable efforts to reduce infection in HHC settings have been hampered by a lack of data and tools for identifying HHC patients who have high risk of infection-related hospitalization.8 Several organizations have highlighted this gap and listed infection control and prevention as a top priority for HHC.9 An important step in this direction is to assist HHC agencies with identifying patients at high risk of developing an infection and resultant hospitalization or emergency care. Although previous research has attempted to identify risk factors for infection in HHC, a systematic review found that these studies were often limited by methodological flaws and failed to measure a comprehensive set of risk factors associated with the development of infections.10
Decision making regarding infection prevention and control is a complex process, which involves integration of comprehensive information along with clinical knowledge and experience. Identifying which patients have a high risk of infection can be especially challenging in the HHC setting because of variations in clinical expertise, availability of resources and supplies, and issues in the patient’s home environment. Predictive risk modeling (PRM), a promising method to assist clinicians to identify high-risk individuals,11 has been used for decades in other industries12 and more recently by healthcare researchers to predict adverse health outcomes such as rehospitalization and high-cost procedures.11,13–15 By establishing a statistical relationship between a set of predictor variables and the occurrence of an adverse outcome such as infection, this technique can be used to forecast the likelihood that any given patient will have an adverse outcome.
The purpose of this study was to (1) develop and test a PRM to identify patients’ risk of infection-related hospitalization or emergency department use using secondary data gathered through the Outcome and Assessment Information Set (OASIS) start of care (SOC) and follow-up assessments linked with important clinical data from one large home care agency and (2) identify important risk factors associated with infection-related outcomes.
Methods
Study Design, Setting, and Research Questions
This was a nonexperimental study using secondary data. The study was conducted in a large not-for-profit home and community-based home care agency, which provides over 1.3 million home care visits to more than 130,000 patients in New York City and the surrounding areas. The study addressed the following research questions:
What factors from the OASIS and clinical data are associated with an increased risk of infection-related hospitalization or emergency care outcomes among HHC patients?
Can a PRM, based on factors associated with infection risk, be used to identify HHC patients at higher risk of infection-related outcomes?
Study Sample and Data sets
The study sample included all patients served by the agency’s adult HHC program who were admitted from January 1, 2014, to December 31, 2014. Retrospective data were extracted from the agency’s electronic patient record and administration systems. Data included the OASIS assessment at start and end of care (EOC), as well as additional clinical and administrative data held in the agency’s records.
The OASIS is a standardized patient assessment required for all Medicare-certified HHC agencies nationwide by the Centers for Medicare & Medicaid Services (CMS). Originally developed in 1999, the OASIS has been through several revisions; the OASIS-C was used in 2014. For each HHC patient, there are at least two matching OASIS assessments, including one administered at the SOC and a corresponding assessment at the EOC or recertification conducted after 60 days of care. The OASIS measures several domains of HHC patient characteristics including sociodemographic, medical history, health status, environmental, support system, functional status, and health service utilization. Researchers have found moderate to excellent reliability for most OASIS items,16 including high concordance between expert-derived answers and OASIS items for Activities of Daily Living (ADLs), Instrumental ADL (IADLs), clinical items, and behavioral assessment, but not for depressive symptoms.16,17
Additional data were gathered from the agency’s patient administrative and electronic health records, including variables for primary language spoken at home, medication therapeutic class, and vital signs at admission. A final analytic data set was constructed by merging data sources using the patient’s medical record number and episode sequence numbers. The data for the study sample were randomly divided into two groups with 70% of the data used for model development (training data) and the remaining 30% for model validation (validating data). This study was approved by the institutional review boards of our institution and the agency from which patient data were obtained.
Study Variables
The outcome measure was hospitalization or emergency department use occurring up to 60 days after HHC admission due to four types of infection (respiratory, wound, urinary tract, and intravenous catheter-related), identified by OASIS items M2310 (reasons for emergency care) and M2430 (reasons for hospitalization). Each item lists 19 reasons including these four types of infections. In keeping with the Centers for Disease Control and Prevention’s National Healthcare Safety Network definition for HAIs and HHC guidelines,18 outcomes that occur on or after the third calendar day of admission to HHC were selected, where the day of admission represents the first calendar day.
Selected variables from the SOC OASIS were included in the models as well as the vital signs and medication regimens from the agency’s electronic databases (see Appendix, Supplemental Digital Content 1, http://links.lww.com/JHQ/A87). Disease classifications specifically developed for HHC patients were used.19 This schema of disease classifications included 18 diagnoses such as acute myocardial infarction and cancer. We compared it with other commonly used comorbidity indices and found this disease classifications out-performed them in predicting hospitalization. Temperature was categorized as high (≥100.4 °F) or normal (<100.4 °F).
Data Analysis
Using the training data, first, the distributions of study variables were examined. Categorical variables with one or more cells containing less than 20 observations were collapsed by combining nearby categories. Exceptions were made for variables that have a strong association with infection supported by previous research. Second, bivariate associations between all independent variables and the infection outcome were assessed. Using a criterion of p < .2, variables that were significantly related to the infection outcome were selected for entry into an initial “maximal” multivariate logistic regression model. The initial model was reduced using the stepwise variable selection technique. A logistic regression model that defines the association of p potential predictors Xp, on the probability that a patient will develop an infection outcome with βp regression parameters, was estimated.
To examine model performance, the risk model developed on the training data set was implemented in the validating data to compare estimated probabilities from the final model to the actual observed events. The model was assessed by standard statistical summaries of a confusion matrix for binary classifiers, the area underneath the receiver operating characteristic curve (AUC), sparsity, and face validity among research team members including a HHC clinician.
Results
Table 1 summarizes the characteristics of the study sample (n = 112,788) and a comparison of patient cases with and without infection outcomes; 1,908 patients (1.69%) were hospitalized or received emergency care associated with the four types of infections. The average patient age was 70.8 years (SD = 16.2). Most patients were women (60.9%), insured through Medicare (fee-for-service [42.5%] or a health maintenance organization [22.8%]), primarily spoke English (79.1%), lived with others (60.0%), had a short-stay acute hospital stay within 14 days of HHC admission (62.5%), and were assessed on admission as being in a stable (11.9%) or likely to return to a stable (74.3%) condition. Compared with patients without an infection outcome, patients who were transferred to a hospital or received emergency care due to infections were slightly older, more likely to be male, white, eligible for Medicare and Medicaid and living with others, as well as more likely to have had an acute care hospital stay 14 days before the HHC admission, and to be assessed as in fragile health or having serious progressive conditions.
Table 1.
Descriptive Statistics and Comparison Between Patients With and Without Hospitalization/Emergency Treatment for an Infection
| Total sample (n = 112,788) | Without infection outcome (n = 110,880) | With infection outcome (n = 1,908) | p | |
|---|---|---|---|---|
| Age | ||||
| Age (mean) | 70.8 | 70.78 | 71.97 | .0016 |
| Female, % | 60.9% | 60.9% | 56.4% | .000 |
| Race/Ethnicity | ||||
| non-Hispanic White, % | 42.5% | 42.4% | 48.0% | .000 |
| Hispanic, % | 23.8% | 23.8% | 22.1% | .074 |
| non-Hispanic Black, % | 26.7% | 26.7% | 24.4% | .021 |
| Payer | ||||
| Medicare FFS, % | 42.5% | 42.3% | 52.4% | .000 |
| Medicare HMO, % | 22.8% | 22.9% | 19.8% | .001 |
| Medicaid FFS, % | 4.1% | 4.1% | 3.6% | .222 |
| Medicaid HMO, % | 17.4% | 17.4% | 17.2% | .812 |
| Dual eligible, % | 7.8% | 7.8% | 9.2% | .026 |
| Private insurance HMO, % | 18.5% | 18.5% | 14.3% | .000 |
| Others, % | 3.9% | 3.9% | 3.1% | .083 |
| Language | ||||
| English, % | 79.1% | 79.1% | 79.5% | .686 |
| Spanish, % | 15.9% | 15.9% | 15.6% | .732 |
| Living condition | ||||
| Living alone, % | 37.6% | 37.7% | 34.0% | .001 |
| Living with others, % | 60.0% | 60.0% | 63.9% | .000 |
| Congregate living, % | 2.2% | 2.2% | 2.0% | .524 |
| Inpatient facility stay 14 days before the HHC admission | ||||
| Short-stay acute hospital, % | 62.5% | 62.5% | 65.0% | .024 |
| Long-term care hospital/nursing home/ skilled nursing facility | 9.9% | 9.9% | 11.2% | .055 |
| Rehab/Psych/Other | 6.6% | 6.6% | 6.4% | .699 |
| No inpatient stay | 22.7% | 22.7% | 19.2% | .000 |
| Overall status | .000 | |||
| Stable | 11.9% | 11.9% | 8.6% | |
| Likely to be stable | 74.3% | 74.3% | 71.8% | |
| Fragile | 12.4% | 12.3% | 17.4% | |
| Serious | 0.8% | 0.8% | 1.2% | |
| Situation unknown | 0.6% | 0.6% | 1.1% |
FFS = fee for service; HMO = health maintenance organization; HHC = home healthcare.
In relation to our first research question, Table 2 lists the variables (together with their odds ratios and 95% confidence intervals [CI]) associated with risk of infection-related outcome from the stepwise logistic regression estimated with the training data (n = 78,951). These predictors were across multiple domains ranging from demographics to medical history and diagnoses, current medical conditions, physical function, care management, medication regimen, and admission vital signs.
Table 2.
Model for Predicting Infection-Related Hospitalization/Emergency Treatment Within the First 60 Days of HHC Admission (n = 78,951)
| Parameter | Stepwise model | ||
|---|---|---|---|
| Odds ratio | Lower 95% CI | Upper 95% CI | |
| Demographics | |||
| Race/Ethnicity (ref: nonwhite) | |||
| non-Hispanic White | 1.19b | 1.05 | 1.34 |
| Payer (ref: all non-Medicare FFS) | |||
| Medicare FFS | 1.45c | 1.28 | 1.63 |
| History and diagnoses | |||
| Diagnoses | |||
| Acute myocardial infarction | 1.25b | 1.08 | 1.45 |
| Peripheral vascular disease | 1.68c | 1.35 | 2.09 |
| Skin ulcer | 1.60c | 1.28 | 2.01 |
| Arthritis | 0.68c | 0.57 | 0.82 |
| No inpatient stay 14 days before HHC | 0.74c | 0.64 | 0.87 |
| Current condition | |||
| Parenteral nutrition treatment | 2.50a | 1.13 | 5.52 |
| Risk of hospitalization | |||
| Multiple hospitalization in past 12 months | 1.37c | 1.22 | 1.55 |
| Frailty (report weight loss or self-reported exhaustion) | 1.15a | 1.02 | 1.29 |
| Risk factors (alcohol dependency) | 1.54a | 1.07 | 2.21 |
| Living condition (living with others) | 1.16a | 1.03 | 1.31 |
| Integumentary status | |||
| Having at least one unhealed pressure ulcer at Stage II (ref: none) | 0.71a | 0.51 | 0.98 |
| Stage of most problematic unhealed (observable) pressure ulcer (ref: none) | |||
| Stage I | 0.81 | 0.53 | 1.25 |
| Stage II | 0.70a | 0.51 | 0.97 |
| Stage III | 1.02 | 0.70 | 1.48 |
| Stage IV | 1.58a | 1.06 | 2.35 |
| Unstageable | 1.55a | 1.04 | 2.32 |
| Status of most problematic (observable) stasis ulcer (ref: none/newly epith/fully/early) | |||
| Not healing | 1.46a | 1.07 | 2.01 |
| Status of most problematic (observable) surgical wound (ref: none) | |||
| Newly epithelialized | 0.78a | 0.61 | 0.99 |
| Fully/Early granulating | 1.23 | 0.90 | 1.69 |
| Not healing | 1.30b | 1.10 | 1.54 |
| Not observable | 0.88 | 0.63 | 1.24 |
| Skin lesion or open wound (yes/no) | 1.21b | 1.05 | 1.38 |
| Respiratory treatments at home (ref: none) | 1.36b | 1.12 | 1.65 |
| Elimination status | |||
| UTI treatment in the past 14 days | 1.30b | 1.07 | 1.57 |
| When urinary incontinence occurred (ref: none/timed voiding/occasional stress incontinence) | |||
| Day and night | 1.21a | 1.04 | 1.42 |
| Night or day only | 1.05 | 0.66 | 1.67 |
| Urinary catheter | 2.02c | 1.61 | 2.53 |
| Neuro/emotional/behavioral status | |||
| Memory deficit | 1.29a | 1.06 | 1.57 |
| ADL/IADLs | |||
| Ambulation/locomotion (ref: totally independent) | |||
| Need one-handed device | 1.07 | 0.77 | 1.49 |
| Need two-handed device | 1.26 | 0.93 | 1.72 |
| Need assistance | 1.38a | 1.00 | 1.91 |
| Chairfast, able to wheel self | 1.82b | 1.22 | 2.71 |
| Chairfast, unable to wheel self | 1.71b | 1.16 | 2.51 |
| Bed rest | 2.10c | 1.36 | 3.25 |
| Previous functioning—transfer (ref: independent) | |||
| Need some help | 1.25b | 1.09 | 1.43 |
| Dependent | 1.32a | 1.04 | 1.67 |
| Injectable medications and injectable medication management | |||
| Previous medication management (ref: independent) | |||
| Need some help | 0.71a | 0.54 | 0.93 |
| Dependent | 0.68b | 0.52 | 0.89 |
| Not applicable | 0.58c | 0.48 | 0.69 |
| Care management | |||
| Medical procedures/treatments (e.g., changing wound dressing) (ref: no assistance is needed) | |||
| Caregiver provide assistance | 1.46c | 1.24 | 1.72 |
| Caregiver needs training | 1.75c | 1.44 | 2.14 |
| Caregiver unlikely/unclear/no caregiver | 2.16c | 1.80 | 2.60 |
| Medication regimen | |||
| Bronchodilators | 1.31c | 1.13 | 1.52 |
| Penicillins | 1.48c | 1.18 | 1.86 |
| Antibacterials for Urinary Tract Infection | 1.42b | 1.11 | 1.82 |
| Glucocorticoids | 1.22a | 1.02 | 1.44 |
| Lipotropics | 0.81c | 0.72 | 0.91 |
| Fungicides | 1.28a | 1.01 | 1.62 |
| Vital signs | |||
| Pulse | 1.01b | 1.00 | 1.02 |
| High temperature (>100.4) | 1.92a | 1.07 | 3.47 |
| AUC | 0.75 | ||
<0.5.
<0.01.
<0.001.
ADL = Activities of Daily Living; AUC = area underneath the receiver operating characteristic curve; CI = confidence interval; FFS = fee-for-service; HHC = home healthcare; IADL = Instrumental Activities of Daily Living; UTI = urinary tract infection.
To address our second research question, we used AUC statistics to measure the model’s ability to discriminate different levels of infection risk. The model demonstrated reasonably good fit with the patient data (likelihood ratio = 1,108.93, df = 51, p < .0001). Area underneath the receiver operating characteristic curve c-statistics of 0.7517 and 0.7162 was observed for the training and validating data sets, respectively.
Finally, to compare the accuracy of a predicted event in the validating data versus an actual event, we generated a summary of a confusion matrix of binary classification (Table 3), including the true positive rate, true negative rate, positive predictive value, and negative predictive value. The split of the predicted binary classifier in this analysis is varied by deciles of the expected risk of infection. Both positive predictive value and negative predictive value are sensitive to the incidence of an infection outcome in the underlying population. The table also includes a ratio of the false positives to true positives to describe how many false-positive patients would need to be treated to potentially avoid one infection-related outcome in each decile.
Table 3.
Confusion Matrix With Varying Cutoffs (Test Data, n = 33,726)a
| Cutoff percentile | True positive rate, (%) | True negative rate, (%) | Positive predictive rate, (%) | Negative predicted value, (%) | Ratio of false positives to true positives |
|---|---|---|---|---|---|
| 10th | 97.3 | 10.5 | 1.87 | 99.54 | 52.4 |
| 20th | 94.0 | 20.6 | 2.04 | 99.49 | 48.1 |
| 30th | 88.5 | 30.6 | 2.19 | 99.34 | 44.7 |
| 40th | 83.8 | 40.6 | 2.42 | 99.31 | 40.4 |
| 50th | 78.9 | 50.4 | 2.72 | 99.27 | 35.8 |
| 60th | 70.6 | 60.6 | 3.05 | 99.16 | 31.8 |
| 70th | 60.5 | 70.5 | 3.48 | 99.03 | 27.8 |
| 80th | 50.3 | 80.6 | 4.36 | 98.93 | 21.9 |
| 90th | 42.3 | 90.5 | 8.50 | 98.69 | 10.8 |
The true positive rate is the percentage of patients who actually developed an infection outcome, in the group of patients who were predicted to have an infection outcome by the statistical model. The true negative rate is the percentage of patients who did not have an infection outcome, in the group of patients who were predicted not to have an infection outcome by the statistical model. The positive predictive value is the ability of the statistical model to accurately predict whether a patient who is identified as having an infection outcome by the model actually has an infection outcome. The negative predictive value is the ability of the statistical model to accurately predict a patient who does not have an infection outcome.
Using the statistics from the confusion matrix (Table 3), we sought to discriminate very high and low levels of risk from average risk and consider the costs to deliver potential interventions within each of these strata. We also provided a visual representation of the stratification (Figure 1) that provides frontline clinicians and HHC administrators with a better interpretation of estimated probability of infection. In the figure, the x-axis labels the proposed risk strata, the left y-axis displays the observed incidence of an infection outcome on the validating data, and the right y-axis represents the risk ratio of the observed incidence and 95% CIs for each stratum compared with the moderate-risk group.
Figure 1.
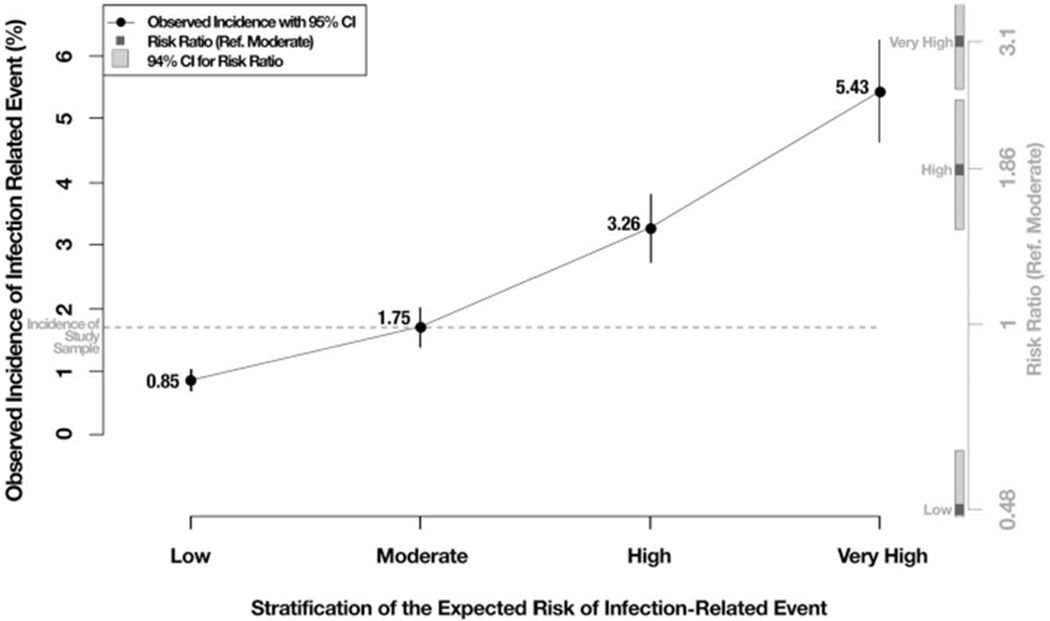
Model’s ability to discriminate risk across strata. CI, confidence interval.
We first grouped the 60–79th percentile of expected risk and defined it as a “moderate” risk group because the observed incidence of infection within this stratum (1.75% shown in Figure 1) is roughly the same as the observed infection in the entire study sample (1.69%). We then defined the entire distribution of risk below the 60th percentile as a “low” risk group because the observed incidence of infection within this stratum (0.85% shown in Figure 1) was roughly half of the “moderate” group, and this group is unlikely to be the focus of additional infection-related interventions. The 80–89th percentile was considered as the high-risk group that identified 50.3% of all infections in the validating data while obtaining an 80% true negative rate. Finally, we defined a very high-risk group as those with predictive probabilities at the 90th percentile or above. This group experienced 42.3% of all infection outcomes, and the model correctly predicted no infection outcome in 90.5% of the cases without events. A potential intervention deployed to this very high-risk group would treat 10.8 patients who will not experience the infection-related hospitalization or emergency care to prevent one infection-related outcome; a significant reduction of the false-positive to true-positive ratio is defined at the 80th percentile. Furthermore, the observed incidence of infection outcomes in the high-and very-high-risk groups (3.26% and 5.43% respectively) is approximately 2 and 3 times as high as that for the moderate-risk group. We recommend that HHC agencies focus interventions on the top two risk strata (i.e. high and very high) as an efficient way of reducing the incidence of infections and adverse outcomes.
Limitations
There were limitations to this study. Despite including comprehensive measures of patient health and functioning, the OASIS is based on nurse observation and reports from patients and family members. Although antibiotic use at SOC is in the models, identifying some patients coming into home care with an infection, there are likely other patients who enter HHC with an infection that is not documented in referral information or picked up by the nurse at the first visit. Therefore, we were unable to include this information in our predictive model. In addition, the infection outcome measure used in our study is based on the clinician’s report on the reasons why a patient was admitted to a hospital or received emergency care. As an alternative, researchers may use claims data to measure infection outcomes. However, obtaining and processing claims data can be time-consuming, especially for agencies that have limited analytic resources.
Discussion
A critical component of an effective infection prevention and control program is the identification of those patients at high risk for infection. The risk model developed in this study identified over 30 factors associated with risk of infection-related outcomes among HHC patients. Implementation of this risk model into HHC clinical practice could help HHC agencies focus tailored interventions to patients with the highest levels of infection risk. Consistent with previous research, our results indicate that receiving parenteral nutrition treatment during a home care episode was an important predictor of increased infection risk.10 Although only a small number of patients (0.13%) received parenteral nutrition treatment, their odds of developing an infection outcome was more than 150% higher than patients not receiving this treatment.
Other conditions predictive of infection risk included having a urinary catheter, limited ambulation, and certain skin ulcers. A urinary catheter is a well-known risk factor for urinary tract infection.20 HHC patients with limited mobility or bedridden are prone to developing pressure ulcers and pulmonary congestion that can lead to complications such as wound infection and pneumonia. These findings are particularly significant because CMS is proposing a major shift with higher HHC payments for Medicare patients requiring greater skilled nursing care.21 In the home healthcare-proposed 2020 rules,22 the highest level of reimbursement is for patients with primary diagnoses indicating the presence of a wound.
Our study extends the existing literature on infection in HHC that has focused on risk factors related to underlying medical conditions10 by reporting that the availability of competent caregivers is also a strong predictor of infection outcomes. Unlike the around-the-clock professional care provided in hospitals or nursing homes, HHC clinicians usually visit the patient just 1–2 times a week. The intermittent nature of HHC services, the greater autonomy of patients in their own homes, and limited oversight of informal care limit the HHC clinician’s ability to implement and assess infection prevention and control practices. We found that HHC patients who lacked a caregiver or whose caregiver needed additional training had a significantly higher risk of infection outcome, suggesting their critical role in ensuring patient safety. It has been reported that over 80% of elderly HHC patients receive care from informal caregivers23 with tasks ranging from assistance with basic ADLs/IADLs to more complex medical and nursing procedures.24 For these reasons, a 2009 National Research Council Workshop24 emphasized that informal caregiving is an essential feature of HHC landscape and called for better coordination between professional healthcare providers and informal caregivers.
We also found that HHC patients who were dependent on assistance with medication management had a lower risk of infection-related outcomes. One explanation may be that medication management represents a primary focus of many HHC visits. Any identified gaps in a patient’s ability to manage their medications may trigger educational interventions for patients and caregivers during their HHC visits, thereby improving the surveillance and quality of HHC.
Conclusions
Preventing infections and associated adverse events among HHC patients are a key issue for agencies. HHC agencies currently do not have the ability to identify patients who might be at higher risk of developing infections and, therefore, target preventive interventions accordingly. In this study, patients in the highest risk category (at the 90th centile) accounted for over 40% of all infection-related hospitalizations/emergency care episodes. Hence, HHC agencies can use this model to identify this group and target interventions accordingly, including educating patients and caregivers on adherence to infection prevention and control practices.
Implications
Decision making around infection control can be challenging for HHC nurses, especially for those with less training and education or little experience working autonomously in the patient’s home environment. The risk scores generated by our model can alert HHC nurses to their patients’ risk of infection and serve as special reminders regarding adherence to infection-control protocols.
Our focus on the high-and very-high-risk groups also has important administrative implications, suggesting that risk calculations may be used not only by frontline clinicians but also by clinical operations personnel, compliance and quality departments, and population health managers. Centers for Medicare & Medicaid Services have reduced HHC base episodic payments and nonroutine supply adjustments by 3.5% and 2.82% per year, respectively, from calendar years 2014–2017.25 Risk stratification provides an approach for HHC operations to objectively direct specially tailored interventions and skilled services to the population of patients who have the highest risk of experiencing an infection-related event.
Supplementary Material
CE Objectives and Posttest Questions: A Predictive Risk Model for Infection-Related Hospitalization Among Home Healthcare Patients.
Learning Objectives:
Describe the importance of infection risk and infection control in the home care setting.
State how predictive risk modeling can be used in healthcare decision making to assist home care providers with identifying infection risk.
Describe risk factors associated with infection outcomes in the home care setting.
Questions
-
Healthcare associated infection is defined by which organization?
The Department of Health and Human Services
The Centers for Medicare and Medicaid Services (CMS)
The Office of Disease Prevention and Health Promotion (ODPHP)
The Centers for Disease Control and Prevention (CDC)’s national Healthcare Safety Network
-
What technique did the authors develop in this paper to help homecare clinician in decision making?
Predictive risk modeling
An assessment tool
A staff education package
A patient & caregiver education package
-
Which method does the predictive risk modeling technique use to forecast future adverse outcome?
Clinician’s intuition
Textbook resource
Statistical model
Expert’s opinion
-
Which types of infection did the authors include in the risk modeling?
Surgical site infection, sepsis, respiratory infection, urinary tract infection
Wound infection, sepsis, respiratory infection, urinary tract infection
Wound infection, IV catheter-related, respiratory infection, urinary tract infection
Surgical site infection, IV catheter-related, respiratory infection, urinary tract infection
-
The OASIS is a standardized home care patient assessment required by which organization?
The Centers for Disease Control and Prevention (CDC)
The Centers for Medicare and Medicaid Services (CMS)
The World Health Organization (WHO)
The Joint Commission
-
Which statistical technique did the authors use to select the variables in the model?
Elastic Net technique
Stepwise variable selection
Forward variable selection
Backward variable selection
-
Based on the study findings, which medical conditions can significantly increase home care patients risk for infection?
Having a urinary catheter, receiving respiratory treatment at home, having a non-healing surgical wound
Having a urinary catheter, receiving respiratory treatment at home, having a newly epithelialized surgical wound
Having urinary incontinence during daytime only, receiving respiratory treatment at home, having a non-healing surgical wound
Having a urinary catheter, receiving respiratory treatment at home, having a stage II pressure ulcer
-
Based on the study findings, which medication regimen is associated with increased risk for infection among home care patients?
Bronchodilators, Fungicides, Lipotropic, Glucocorticoids
Bronchodilators, Penicillin, Lipotropic, Glucocorticoids
Bronchodilators, Fungicides, Lipotropic, Penicillin
Bronchodilators, Penicillin, Fungicides, Glucocorticoids
-
Which of the following issues introduce challenges to infection control and practice in the home care setting? (Check all that apply)
Limited oversight of care provided by informal caregivers
Home care clinicians only visited patients for a limited time
Patients have more friends at home
Patients who receive home care are usually more stable than those who stay in the hospital
-
The majority of elderly home care patients receive care from informal caregivers. The tasks provided by the informal caregivers include:
ADLs/IADLs, coordination of care, giving chemotherapy
ADLs/IADLs, coordination of care, communication with health care providers
ADLs/IADLs, communication with healthcare providers, giving chemotherapy
coordination of care, communication with healthcare providers, giving chemotherapy
Acknowledgments
This study is funded by The Agency for Healthcare Research and Quality (Grant #R01HS024723-03).
Biographies
Author’ Biographies
Jingjing Shang, PhD, RN, is an associate professor at Columbia University, School of Nursing in New York, NY. Her research interests include infection control, home healthcare, nursing workforce, quality of care, patient safety, symptom management, and oncology nursing.
David Russell, PhD, is a research associate in the Center for Home Care Policy & Research at the Visiting Nurse Service of New York and an assistant professor of sociology at Appalachian State University. His research interests include medical sociology, gerontology, home health, and hospice care.
Dawn Dowding, PhD, RN, FAAN, is a professor in clinical decisionmaking, Division of Nursing and Midwifery, School of Health Sciences at the University of Manchester. Her research interests include clinical decisionmaking, clinical decision support techniques, and nursing informatics.
Margaret McDonald, MSW, is an associate director of the Center for Home Care Policy & Research at the Visiting Nurse Service of New York in New York, NY. Her research interests include home healthcare, quality of care, putting evidence into practice, and workforce issues.
Christopher Murtaugh, PhD, is a senior research scientist at the Center for Home Care Policy and Research, Visiting Nurse Service of New York. His research interests include developing the evidence base for home healthcare services and improving the science of home healthcare quality assessment.
Jianfang Liu, PhD, is an assistant professor of quantitative research (in nursing) at Columbia University Medical Center, NYC, NY. She has more than 10 years of professional experience as a statistician/data analyst providing statistical analyses, instruction, and consultation for various research projects and grants. Dr.Liu has expertise in big data processing and analyzing.
Elaine L. Larson, PhD, is a senior associate dean of scholarship and research, Anna C. Maxwell Professor of Nursing Research, Columbia University School of Nursing, and Professor of Epidemiology, Columbia University Mailman School of Public Health.
Sridevi Sridharan, MS, is a senior systems analyst/programmer at the Center for Home Care Policy & Research at the Visiting Nurse Service of New York in New York, NY. Her research interests include home healthcare and quality of care.
Carlin Brickner, DrPH, a biostatistician, is the Director of Analytics at Visiting Nurse Service of New York. His research interests include the development of predictive models, how they can be deployed to support clinical and operational decision making, estimating a transient effect and the onset of an acute event in observational data, and how healthcare policies and payment incentives can be used to impact healthcare delivery.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article at (www.jhqonline.com).
The authors declare no conflicts of interest.
References
- 1.Jarvis WR. Infection control and changing health-care delivery systems. Emerg Infect Dis. 2001;7(2):170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy 2018. 2018. http://www.medpac.gov/docs/default-source/reports/mar18_medpac_entirereport_sec.pdf. Accessed September 10, 2018.
- 3.Rau J Medicare Fines 2,610 Hospitals in Third Round of Readmission Penalties [serial on the Internet], Kaiser Health News, 2014. http://kaiserhealthnews.org/news/medicare-readmissions-penalties-2015/Google Scholar. Accessed September 19, 2018. [Google Scholar]
- 4.Shang J, Larson E, Liu J, Stone P. Infection in home health care: Results from national outcome and assessment information set data. Am J Infect Control. 2015;43(5):454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. 2009. http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed August 22, 2018.
- 6.U.S. Department of Health & Human Services. HHS action plan to prevent healthcare-associated infections. http://www.hhs.gov/ash/initiatives/hai/actionplan/index.html. Accessed September 1,2018.
- 7.Center for Disease control and Prevention. Vital Signs: Central Line-Associated Blood Stream Infections: United States, 2001, 2008, and 2009. 2011. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6008a4.htm. Accessed September 1, 2018.
- 8.Castellucci M Home Healthcare Providers’Infection Prevention Efforts Hampered by Dearth of Data, Tools. Modern Healthcare, 2018. http://www.modernhealthcare.com/article/20180421/NEWS/180429996. Accessed September 10, 2018.
- 9.The Joint Commission. 2019. Home Care National Patient Safety Goals. The Joint Commission on Accreditation of Healthcare Organizations; 2019 Accessed June 12th, 2019 https://www.jointcommission.org/assets/1/6/2019_OME_NPSGs_final.pdf. [Google Scholar]
- 10.Shang J, Ma C, Poghosyan L, Dowding D, Stone P. The prevalence of infections and patient risk factors in home health care: A systematic review. Am J Infect Control. 2014;42(5): 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billings J, Blunt I, Steventon A, Georghiou T, Lewis G, Bardsley M. Development of a predictive model to identify inpatients at risk of re-admission within 30 days of discharge (PARR-30). BMJ Open. 2012;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang C, Schwien T, Blockberge-Miller S, Erickson A. Predictive Modeling—To Improve Outcomes in Patients and Home Care. Seattle, WA: OCS, Inc; 2008. [Google Scholar]
- 13.Rosati RJ, Huang L. Development and testing of an analytic model to identify home healthcare patients at risk for a hospitalization within the first 60 days of care. Home Health Care Serv Q. 2007;26(4):21–36. [DOI] [PubMed] [Google Scholar]
- 14.Moturu ST, Liu H, Johnson WG. Trust evaluation in health information on the world wide web. Conf IEEE Eng Med Biol Soc. 2008;2008:1525–1528. [DOI] [PubMed] [Google Scholar]
- 15.Paryavi E, Stall A, Gupta R, et al. Predictive model for surgical site infection risk after surgery for high-energy lower-extremity fractures: Development of the risk of infection in orthopedic trauma surgery score. J Trauma Acute Care Surg. 2013;74(6): 1521–1527. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor M, Davitt JK. The outcome and assessment information set (OASIS): A review of validity and reliability. Home Health Care Serv Q. 2012;31(4):267–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madigan EA, Tullai-McGuinness S, Fortinsky RH. Accuracy in the outcomes and assessment information set (OASIS): Results of a video simulation. Res Nurs Health. 2003;26(4): 273–283. [DOI] [PubMed] [Google Scholar]
- 18.Center for Disease control and Prevention. HAI Checklists. 2010. https://www.cdc.gov/nhsn/hai-checklists/. Accessed September 6, 2018.
- 19.Murtaugh C, Peng T, Totten A, Costello B, Moore S, Aykan H. Complexity in geriatric home healthcare. J Healthc Qual. 2009; 31(2):34–43. [DOI] [PubMed] [Google Scholar]
- 20.Wilde MH, Brasch J, Getliffe K, et al. Study on the use of longterm urinary catheters in community-dwelling individuals. J Wound Ostomy Continence Nurs. 2010;37(3):301–310. [DOI] [PubMed] [Google Scholar]
- 21.Rosati RJ, Russell D, Peng T, et al. Medicare home health payment reform may jeopardize access for clinically complex and socially vulnerable patients. Health Aff (Millwood). 2014; 33(6):946–956. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; CY 2019 Home Health Prospective Payment System Rate Update and CY 2020 Case-Mix Adjustment Methodology Refinements; Home Health Value-Based Purchasing Model; Home Health Quality Reporting Requirements; Home Infusion Therapy Requirements; and Training Requirements for Surveyors of National Accrediting Organizations. Fed Regist. 2018;83:56406–56638. [PubMed] [Google Scholar]
- 23.Cho E, Kim EY, Lee NJ. Effects of informal caregivers on function of older adults in home health care. West J Nurs Res. 2013;35(1):57–75. [DOI] [PubMed] [Google Scholar]
- 24.Schulz R, Tompkins CA. Informal caregivers in the United States: Prevalence, caregiver characteristics, and ability to provide care. Paper presented at: The Role of Human Factors in Home Health Care; Workshop Summary 2009. https://www.ncbi.nlm.nih.gov/books/NBK210048/. Accessed August 10, 2018. [Google Scholar]
- 25.Department of the Treasury. Internal revenue service. Fed Regist. 2013;78:72257. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


