Abstract
Smoke inhalation injury (SII) affects more than 50,000 people annually causing carbon monoxide (CO) poisoning. Although the increased blood level of carboxyhemoglobin (CO-Hb) is frequently used to confirm the diagnosis of SII, knowledge of its elimination in the acute phase is still limited. The aim of this study is to determine CO-Hb elimination rates and their differences in arterial (aCO-Hb) and mixed-venous (vCO-Hb) blood following severe SII in a clinically relevant ovine model. Forty-three chronically instrumented female sheep were subjected to SII (12 breaths, 4 sets) through tracheostomy tube under anesthesia and analgesia. After the SII, sheep were awakened and placed on a mechanical ventilator (FiO2=1.0, tidal volume 12 mL/kg, and PEEP=5cmH2O) and monitored. Arterial and mixed-venous blood samples were withdrawn simultaneously for blood gas analysis at various time points to determine CO-HB half-lifetime and an elimination curve. The mean of highest aCO-Hb level during SII was 70.8±13.9%. The aCO-Hb elimination curve showed an approximated exponential decay during the first 60 minutes. Per mixed linear regression model analysis, aCO-Hb significantly (p<0.001) declined (4.3%/minute) with a decay constant lambda of 0.044. With this lambda, mean lifetime and half-lifetime of aCO-Hb were 22.7 and 15.7 minutes, respectively. The aCO-Hb was significantly lower compared to vCO-Hb at all-time points (0–180 minutes). To our knowledge, this is the first report describing CO-Hb elimination curve in the acute phase after severe SII in the clinically relevant ovine model. Our data shows that CO-Hb is decreasing in linear manner with supportive mechanical ventilation (0–60 minutes). The results may help to understand CO-Hb elimination curve in the acute phase and improvement of pre-hospital and initial clinical care in patients with CO poisoning.
Keywords: Carbon monoxide poisoning, Smoke inhalation injury, Carboxyhemoglobin, Half-life, Elimination curve, Acute lung injury
Introduction
According to the Burn Incidence Fact Sheet of the American Burn Association (ABA), a total of 486,000 burn patients received medical attention in the United States (US) [1]. There are approximately 50,000 emergency department visits per year in the US, as a result of smoke inhalation injury (SII). Carbon monoxide (CO) poisoning is one of the main causes of death by patients with smoke inhalation. An estimated 1,000 – 2,000 accidental deaths are due to CO poisoning each year [2]. The ABA data [1], suggest the presence of SII can lead to acute lung injury (ALI) and increases the mortality rate in burn patients [1, 3]. In addition, recent publications reveal that CO poisoning affects not only the lungs, but other organ systems, such as heart [4–6], kidney [7, 8], liver [9], and also more importantly the brain [10, 11]. The brain involvement causes a syndrome of delayed neurologic sequelae in up to 40% of patients with high blood carboxyhemoglobin (CO-Hb) levels [12, 13].
SII is diagnosed by the clinical triad: clinical symptoms, history of smoke exposure, and elevated blood CO-Hb levels. Elevated CO-Hb levels in the blood should serve as a solid confirmation of the diagnosis of CO poisoning [14], but there are no universal diagnosis criteria for CO poisoning. One of the reasons is that CO-Hb elimination manner, especially in an acute phase, has not been well studied clinically [14, 15].
Because CO has a high affinity to blood hemoglobin, the gold standard therapy for CO poisoning in the acute phase is oxygen therapy to achieve rapid elimination of CO-Hb, alleviate tissue hypoxia, and prevent organ damage [16]. The elimination half-life of CO-Hb with normobaric oxygen therapy is about 74 minutes [16, 17]. Although several publications suggest poor correlations between the blood CO-Hb level and the severity of CO poisoning [18–20], it is well established clinical practice to eliminate CO-Hb as expediently as possible [21–23]. Thus one effective treatment method for a high level of CO poisoning or patients with symptoms of end-organ ischemia is the use of hyperbaric oxygen therapy (HBO) [24 – 26]; however, a limitation of this treatment modality is the limited availability of the HBO chambers. An additional concern that has been under discussion is that hyperbaric oxygen may also induce reactive oxygen species stress [27, 28].
Therefore, the aim of this study was to establish the blood CO-Hb elimination curve and determine elimination rates, their differences in arterial carboxyhemoglobin (aCO-Hb) and mixed-venous carboxyhemoglobin (vCO-Hb), and the effects of mechanical ventilation on these changes.
Materials and Methods
Animal
Forty-three adult female Merino sheep (body weight [BW]; 33.1±0.5 kg) were used in this study. The study was approved by the Institutional Animal Care and Use Committee of The University of Texas Medical Branch and conducted in compliance with the guidelines of the National Institutes of Health (NIH), the American Physiological Society for the care and use of laboratory animals, and ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines [29]. Sheep were group-housed during a 14-day quarantine period at the Animal Research Center and placed in individual metabolic cages upon transferring them to the Translational ICU (TICU) for study. Sheep were housed within sight of other sheep in a temperature/humidity-controlled environment with dark/light cycles. Sheep had free access to food (hay and pellets) and water until the day before the start of study. Merino sheep were chosen because of the close resemblance of the pathophysiological changes seen in human respiratory distress, as previously described [3, 30, 31].
Induction of smoke inhalation injury and mechanical ventilation
Fasted animals were anesthetized and multiple vascular catheters were surgically inserted (pulmonary artery and femoral artery catheters), as described previously [30, 32]. After the surgical procedure, animals were awakened and monitored in TICU in a conscious state for 5–7 days with free access to food and water for a recovery. Pre- and post-surgical analgesia were provided with long acting (for 72 hours) Buprenorphine SR (0.1 mg/kg, SR-Veterinary Technologies, Windsor, CO).
After 5–7 days from surgery, baseline (BL) cardiopulmonary function variables were recorded (Table 1). After collecting the baseline data, deeply anesthetized animals were subjected to 48 insufflations (12 breaths x 4 sets) of cooled cotton smoke (<40°C) through a modified bee smoker under deep anesthesia with isoflurane, as we described previously [32]. Immediately after the 4th set breaths, animals were moved to TICU, placed on a mechanical ventilator using the Hamilton G5 ventilator (adaptive pressure ventilation continuous mandatory ventilation mode [APV-cmv], Hamilton Medical AG, Bonaduz, Switzerland) with fraction of inspired oxygen (FiO2) of 1.0, a tidal volume (TV) of 12 mL/kg, a positive end-expiratory pressure (PEEP) of 5 cmH2O, and a respiratory rate (RR) of 20 breaths/minute, and monitored in an awake condition for 3 hours. Notably, sheep require higher TV compared to humans due to a larger anatomical dead space and ratio of dead space to tidal volume compared to humans [33, 34].
Table 1.
Cardiopulmonary hemodynamic and biochemical variables at baseline and 3 hrs after the injury
| Variable | BL | 3h | P | Variable | BL | 3h | P | Variable | BL | 3h | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BT (°C) | 39.0±0.3 | 39.6±0.5 | <0.001 | HR (beats/min) | 88±2 | 109±3 | <0.001 | MAP(mmHg) | 95.7±1.8 | 102.3±1.5 | <0.001 |
| CI (L/min/m2) | 6.1±0.1 | 7.0±0.2 | <0.001 | MPAP (mmHg) | 17.7±0.4 | 21.7±0.5 | <0.001 | PAOP (mmHg) | 9.8±0.3 | 12.8±0.3 | <0.001 |
| LAP (mmHg) | 6.6± 0.4 | 9.2±0.5 | <0.001 | CVP (mmHg) | 4.9±0.4 | 6.9±0.4 | <0.001 | Pc (mmHg) | 13.0±0.3 | 16.4±0.4 | <0.001 |
| LVSWI (gm-m/m2/beat) | 80.8±1.7 | 80.3±3.3 | (NS) | RVSWI (gm-m/m2/beat) | 12.4±0.4 | 13.3±0.7 | (NS) | SVRI (dyne•sec/cm5/m2) | 1232±42 | 1167±59 | 0.02 |
| PVRI (dyn•sec/cm5/m2) | 108±5 | 106±5 | (NS) | P/F ratio (mmHg) | 511±4 | 452±15 | <0.001 | Qt/Qs (%) | 6.6±0.5 | 11.4±0.4 | <0.001 |
| PaCO2 (mmHg) | 35.0±0.4 | 30.9±0.8 | <0.001 | Peak Airway Pressure (cmH2O) | 16.9±0.7 | 17.5±0.8 | (NS) | Plateau Airway Pressure (cmH2O) | 15.6±0.7 | 15.9±0.7 | (NS) |
| C stat (mL/cmH2O) | 43.2±2.0 | 41.0±2.0 | (NS) | Glucose (mg/dL) | 62.5±1.1 | 74.8±1.9 | <0.001 | Lactate (mmol/L) | 0.58±0.05 | 1.20±0.08 | <0.001 |
The animal numbers were n=43. The statistical analysis were performed by Paired t-test or Wilcoxon test, based on data distribution normality test (Shapiro-Wilk test). Data are expressed as mean±SEM.
Abbreviations: BT; Body Temperature, HR; Heart Rate, MAP; Mean Arterial Pressure, CI; Cardiac Index, MPAP; Mean Pulmonary Artery Pressure, PAOP; Pulmonary Artery Occlusion Pressure, LAP; Left Atrial Pressure, CVP; Central Venous Pressure, Pc; Pulmonary Capillary Hydrostatic Pressure, LVSWI; Left Ventricular Stroke Work Index, RVSWI; Right Ventricular Stroke Work Index, SVRI; Systemic Vascular Resistance Index, PVRI; Pulmonary Vascular Resistance Index, P/F Ratio; PaO2/FiO2 Ratio, Qt/Qs; Pulmonary Shunt Fraction, C stat; Lung Static Compliance, NS; Not Significant.
Arterial and mixed venous blood carboxyhemoglobin assessment
After each set of smoke inhalation, and until 180 minutes after the 4th set, arterial (femoral artery) and mixed venous (pulmonary artery) blood samples were withdrawn simultaneously for complete blood gas analysis (blood gas analyzer (RAPIDPoint 500; Siemens Healthcare, Erlangen, Germany). The blood gas measurement time points were at the BL, after the 1st, 2nd, 3rd, 4th smoke sets, 1, 2, 3, 4, 5, 7.5, 10, 15, 20, 25, 30, 35, 40, 50, 60, 90, 120, and 180 minutes after the 4th smoke set. During this phase, sheep received 4 mL/kg/hr of maintenance IV fluid with lactated Ringer’s solution (Baxter Healthcare Corporation, Deerfield, IL).
Statistical analysis
The aCO-Hb was initially modeled using a mixed generalized additive model with relation to time following smoke exposure, blocking on sheep and including a continuous AR(1) correlation structure, with a penalized spline allowing for non-linear relation with time. Subsequently, the linear portion of the curve was modeled using a linear mixed regression model, blocking on sheep. Since exponential decay appears linear on the log scale, the slope coefficient from the latter model was used to estimate the decay constant (lambda) and associated parameters mean lifetime and half-life.
The differences between aCO-Hb and vCO-Hb were modeled with relation to time following smoke exposure with a mixed generalized additive model, blocking on sheep and including a continuous AR(1) correlation structure, with a penalized spline accommodating for a non-linear relation with time.
The statistical analyses were performed using R statistical software (R Core Team, 2017, version 3.3.3). The cardiopulmonary hemodynamic statistical analyses were performed using GraphPad Prism (Version 8.1.2, GraphPad Software, La Jolla, CA). In all statistical tests, alpha=0.05. Variables are reported as mean ± standard error of mean (SEM).
Results
Smoke inhalation injury and arterial CO-Hb elimination curve
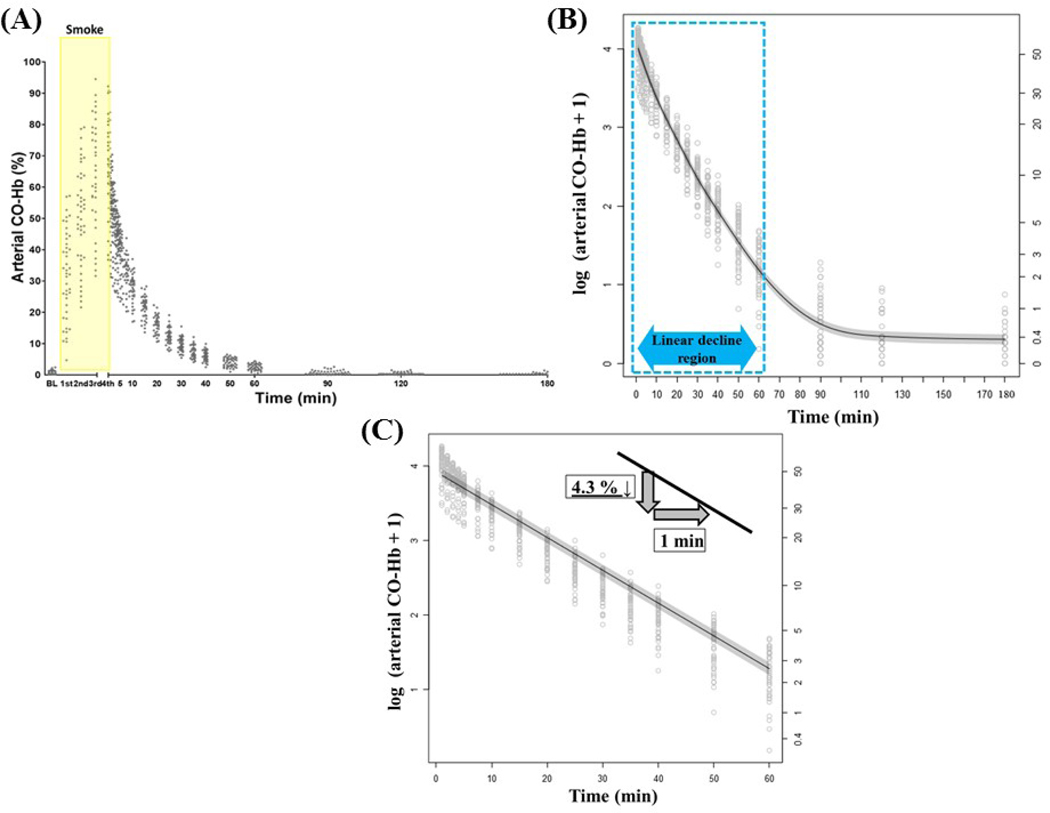
Figure 1A: A scatter plot of aCO-Hb data over the time course reveals an increase in aCO-Hb after four sets of smoke inhalation injury and subsequent return to baseline within 90 minutes. Baseline and each set (1st–4th smoke inhalation) of aCO-Hb were 0.9±0.1, 32.0±2.1, 50.9±2.4, 64.5±2.5, and 70.8±2.1%, respectively.
Figure 1: Smoke inhalation injury and arterial CO-Hb elimination curve.
(A) The scatter plot of the arterial CO-Hb data during the smoke inhalation and until 180 minutes after the 4th set of the smoke inhalation.
(B) The log-transformation of the arterial CO-Hb data during the smoke inhalation and until 180 minutes after the 4th set of the smoke inhalation. The elimination curve during 0–60 minutes is (labeled with dotted rectangle) indicating approximately linear decrement. The gray scaled area represents the standard error of mean.
(C) The log-transformation of the arterial CO-Hb data during the first 60 minutes after the smoke inhalation. During this phase, arterial CO-Hb decreased by 4.3% per minute analyzed by the mixed linear regression model analysis. The gray scaled area represents the standard error of mean.
Figure 1B shows log-transformation of the aCO-Hb data over the time course after four sets of smoke inhalation injury and subsequent return to baseline within 90 minutes. The decay curve overlaid on the scatterplot illustrates the mixed generalized additive model relating aCO-Hb to time after the SII. Since the decay curve appeared approximately linear over the first 60 minutes and thereafter approximately constant from 90–180 minutes, the linear portion of the log-transformed aCO-Hb elimination curve during the first 60 minutes was modeled independently, as shown in Figure 1C.
Per Figure 1C, based upon the assumed exponential decay due to linearity on the log scale, the decay constant (lambda) and associated parameters mean lifetime and half-life were estimated. The mixed linear regression model analysis showed a significant decline in aCO-Hb of 4.3% per minute (p<0.001) during the first 60 minutes, with decay constant lambda of 0.044. With this lambda, mean lifetime and half-lifetime of aCO-Hb were 22.7 and 15.7 minutes, respectively.
Difference between arterial and venous blood CO-Hb during the elimination phase
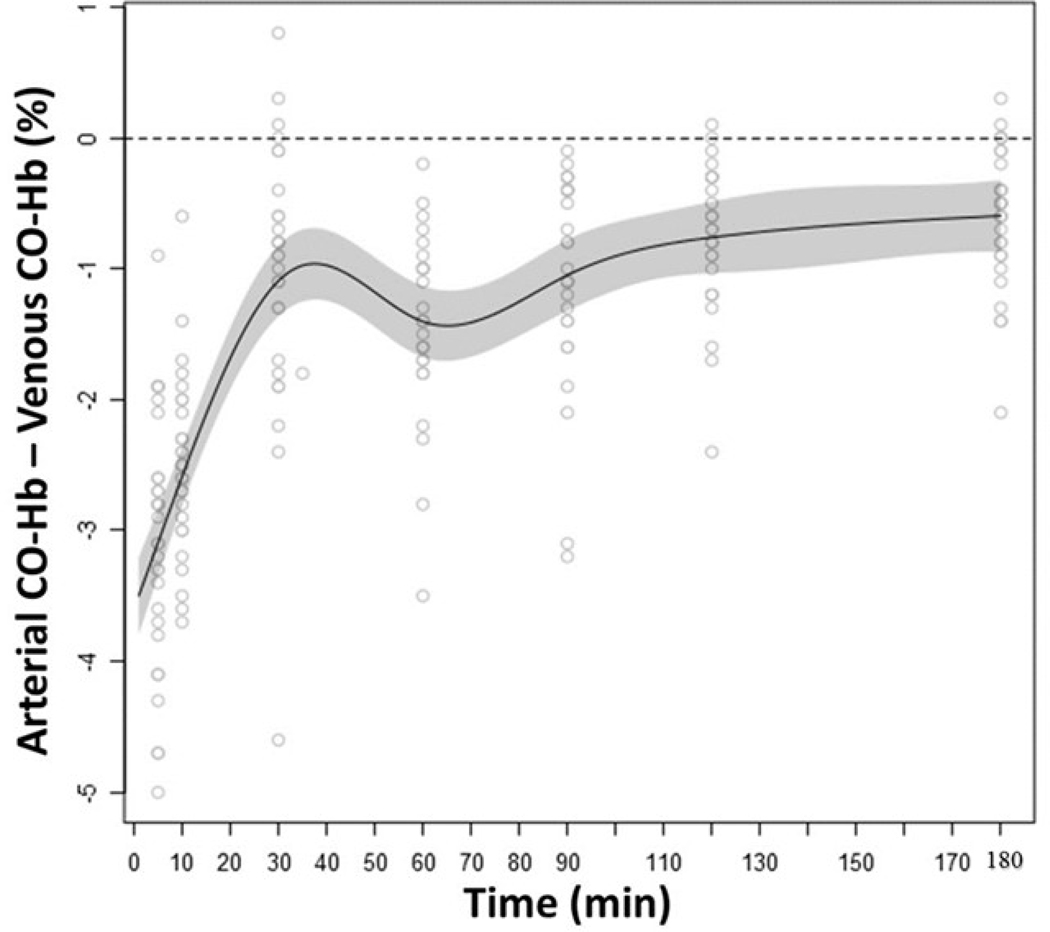
The arterio-venous CO-Hb differences (aCO-Hb - vCO-Hb%) are shown in Figure 2. According to the modeling, illustrated by the model fit line, aCO-Hb was significantly lower compared to vCO-Hb (p<0.05) during all time points until 180 minutes post SII. The largest difference was observed at 5–10 minutes following the SII.
Figure 2: Difference between arterial and venous CO-Hb during the elimination phase.
The difference between arterial CO-Hb and venous CO-Hb during the CO-Hb elimination phase. The arterial CO-Hb was significantly lower compared to venous CO-Hb during all time points until 180 minutes post smoke inhalation (p<0.05). The gray scaled area represents the standard error of mean.
Cardiopulmonary hemodynamic parameters and biochemical variables at the baseline and at 3 hours after smoke inhalation injury
The cardiopulmonary hemodynamic parameters and blood glucose and lactate levels at baseline and at 3 hours are shown in the Table 1. Of the parameters recorded, body temperature (BT), heart rate (HR), mean arterial pressure (MAP), cardiac index (CI), mean pulmonary artery pressure (MPAP), pulmonary artery occlusion pressure (PAOP), left atrial pressure (LAP), central venous pressure (CVP), and pulmonary capillary hydrostatic pressure (Pc), were significantly increased (p<0.001) at 3 hours compared to BL. The systemic vascular resistance index (SVRI) was significantly decreased (p=0.02) after 3 hours compared to the BL values.
In the gas exchange ability and pulmonary mechanics, PaO2/FiO2 ratio, PaCO2, were significantly decreased (p<0.001), and lung shunt fraction (Qt/Qs) was significantly increased (p<0.001) in the values after 3 hours compared to the BL values. There was no difference in left and right ventricular stroke work indexes (LVSWI and RVSWI), pulmonary vascular resistance index (PVRI), peak and plateau airway pressures, and lung static compliance at BL and 3 hours after the SII.
In the biochemical variables, both glucose and lactate were significantly increased (p<0.001) in the values after 3 hours compared to the BL values (Table 1).
Discussions
CO poisoning by SII is one of the common and lethal causes of poisoning death in the US. Our data showed that we had induced severe SII with the high initial CO-Hb level significantly affecting cardiopulmonary hemodynamics, pulmonary oxygenation and mechanics, and biochemical parameters 3 hours after from the induction of SII (Table 1).
One of the gold standard therapies for CO poisoning is eliminating the CO-Hb as fast as possible [21–23]. However, we have only a few options in the initial treatment for CO poisoning in addition to conventional 100% oxygen therapy with face-mask. The mechanism of the elimination of blood CO-Hb with inhaled oxygen methods has two components. One major component is the concentration of inhaled oxygen, and the other minor one is increasing minute ventilation [14, 16, 17]. Regarding to the first component, the HBO therapy (PiO2 of 2100 mmHg at 3 atmospheres) has been increasing its evidence in reducing the SII severity especially in the risk of delayed neurologic sequelae [24–26], however, the limited availability of HBO chambers is an issue. The hyperventilation method relating to the second component, is still under discussion [35].
Given that there are limited therapeutic options in an acute phase of CO poisoning and the few studies conducted in an acute phase of SII with specific focus on the elimination curve of CO-Hb, we chose to conduct this study [17, 36, 37].
In the present study, we had demonstrated that the aCO-Hb elimination curve showed a logarithmic decrement in the phase of the first 60 minutes under the mandatory ventilation setting using an established and clinically valid ovine model which closely mimics the response to SII in humans.
The initial 60 minutes period is the most effective phase for the elimination of the CO-Hb and from this log-transformed decay curve showed a significant decline in CO-Hb of 4.3% per minute. The elimination curve analysis showed the CO-Hb mean time and half-lifetime of 22.7 and 15.7 minutes, respectively (Figure 1B, 1C). These elimination times are much faster than those times of humans with conventional face-mask oxygen therapy, reported in previous publications [16, 17]. This discrepancy may be explained by following: 1) Differences in oxygen concentrations delivered by the mechanical ventilator with an FiO2 of 1.0 and PEEP vs. face mask; 2) immediate start of the oxygen support (within the first 60 minutes from SII); and 3) the combined and synergistic effect of the 1) and 2).
Recent publications also show the importance of ventilation support in the acute phase of CO poisoning. Tomruk et al. reported greater effectiveness of the high flow nasal cannula oxygenation in the faster elimination of CO-Hb levels compared to the conventional face mask oxygen therapy [38]. Roth et al. reported a clinical case with similar effectiveness using non-invasive continuous positive airway pressure ventilation in eliminating the blood CO level [39]. In a swine model of CO intoxication, Delvau et al., reported difference in half-life of CO-Hb, when comparing various methods of CO-Hb elimination. They report that pressure support ventilation in a closed system increased CO-Hb elimination, however in their study the FiO2 in that system was the highest at 0.92. Those publications supported our findings in the present study, and the effectiveness of supportive ventilation is maximized in the first 60 minutes following the smoke inhalation injury.
In our model, PaCO2-related hyperventilation, may have resulted in an increased affinity of hemoglobin for oxygen, thus accelerating the CO-Hb elimination. Additionally, CO-Hb is known to increase hemoglobin’s affinity for oxygen [35].
The present study has several limitations. First, we did not compare the elimination curve and mean and half-lifetime of CO-Hb with humans. However, it is impossible to design this kind of experimental study in humans. Previous publications show that sheep have a very close characteristics in genomic [31, 40], anatomical [41], and physiological respiratory system [42, 43] compared to humans. Based on these similarities to humans, we can speculate that a half-lifetime of the CO-Hb in CO poisoning patients may be achieved with immediate mechanical ventilator support much faster than 74 minutes when the patients treated with conventional face-mask oxygen therapy [16]. Effective oxygen delivery (FiO2 of 1.0) can also be delivered with a high-flow system (CPAP or High-flow nasal cannula which do not require intubation). Second, we treated the animal with oxygen therapy with ventilator support (oxygen therapy with mandatory volume-controlled ventilation) started immediately after the SII. We recognize that this could be very difficult to achieve in real clinical pre-hospital situations. Nevertheless, our results indicate necessity for immediate interventions using high flow systems with greater capacity to provide a FiO2 of 1.0 and increase minute ventilation with PEEP support.
To our knowledge, this is the first study demonstrating the CO-Hb elimination curve with the mean lifetime and half-lifetime of the CO-Hb in the acute phase of severe SII associated with elevated CO-Hb and effects of mechanical ventilation in a clinically relevant setting. With our setting (mechanical ventilation with 100% oxygen), CO-Hb was eliminated within the first 60 minutes.
Our finding of the present study emphasize a need for clinical studies comparing non-efficacies of positive pressure ventilation versus conventional face-mask oxygen therapy in the setting of pre-hospital transportation care and initial care in an emergency room in patients with SII.
“Research Highlights”.
Smoke inhalation increases the mortality rate in burn patients.
Carbon monoxide poisoning is one of the causes of death by smoke inhalation injury.
Carboxyhemoglobin elimination manner in the acute phase has not been well studied.
The elimination curve shows logarithmic decrement in the first 60 mins.
Acknowledgements
The authors would like to thank TICU personnel for their work to complete this study.
Source of Funding: NIH R01 GM097480
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest in this work.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burn Incidence Fact Sheet. Chicago: American Burn Association (https://ameriburn.org/who-we-are/media/burn-incidence-fact-sheet/). [Google Scholar]
- 2.Hampson NB, Weaver LK. Carbon monoxide poisoning: a new incidence for an old disease. Undersea Hyperb Med. 2007, 34(3), 163–168. [PubMed] [Google Scholar]
- 3.Enkhbaatar P, Pruitt BA, Suman O, et al. Pathophysiology, research challenges, and clinical management of smoke inhalation injury. The Lancet. 2016, 388(10052), 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandini C, Castoldi AF, Candura SM, et al. Carbon monoxide cardiotoxicity. J Toxicol Clin Toxicol. 2001, 39(1), 35–44. [DOI] [PubMed] [Google Scholar]
- 5.Satran D, Henry CR, Adkinson C, et al. Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. J Am Coll Cardiol. 2005, 45(9), 1513–1516. [DOI] [PubMed] [Google Scholar]
- 6.Huang CC, Ho CH, Chen YC, et al. Risk of Myocardial Infarction After Carbon Monoxide Poisoning: A Nationwide Population-Based Cohort Study. Cardiovasc Toxicol. 2019, 19(2), 147–155. [DOI] [PubMed] [Google Scholar]
- 7.Kim YJ, Sohn CH, Seo DW, et al. Analysis of the development and progression of carbon monoxide poisoning–related acute kidney injury according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria. Clin Toxicol (Phila). 2018, 56(8), 759–764. [DOI] [PubMed] [Google Scholar]
- 8.Kim SG, Woo J, Kang GW. A case report on the acute and late complications associated with carbon monoxide poisoning: Acute kidney injury, rhabdomyolysis, and delayed leukoencephalopathy. Medicine (Baltimore). 2019, 98(19), e15551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson A, Williams R. Anoxic hepatic and intestinal injury from carbon monoxide poisoning. Br Med J (Clin Res Ed). 1984, 289(6452), 1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randolph AC, Fukuda S, Ihara K et al. Blood-Brain Barrier Dysfunction After Smoke Inhalation Injury, With and Without Skin Burn. Shock. 2019, 51(5), 634–649. [DOI] [PubMed] [Google Scholar]
- 11.Lo CP, Chen SY, Lee KW, et al. Brain injury after acute carbon monoxide poisoning: early and late complications. AJR Am J Roentgenol. 2007, 189(4), 205–211. [DOI] [PubMed] [Google Scholar]
- 12.Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983, 40(7), 433–435. [DOI] [PubMed] [Google Scholar]
- 13.Thom SR, Taber RL, Mendiguren II, et al. Delayed Neuropsychologic Sequelae After Carbon Monoxide Poisoning: Prevention by Treatment With Hyperbaric Oxygen. Ann Emerg Med. 1995, 25(4), 474–480. [DOI] [PubMed] [Google Scholar]
- 14.Hampson NB, Piantadosi CA, Thom SR, et al. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am J Respir Crit Care Med. 2012, 186(11), 1095–1101. [DOI] [PubMed] [Google Scholar]
- 15.Ozturan IU, Yaka E, Suner S, et al. Determination of carboxyhemoglobin half-life in patients with carbon monoxide toxicity treated with high flow nasal cannula oxygen therapy. Clin Toxicol (Phila). 2019, 57(7), 617–623. [DOI] [PubMed] [Google Scholar]
- 16.Rose JJ, Wang L, Xu Q, et al. Carbon monoxide poisoning: Pathogenesis, management, and future directions of therapy. Am J Respir Crit Care Med. 2017, 195(5), 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver LK, Howe S, Hopkins R, et al. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000, 117(3), 801–808. [DOI] [PubMed] [Google Scholar]
- 18.Hampson NB, Hauff NM. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am J Emerg Med. 2008, 26(6), 665–669. [DOI] [PubMed] [Google Scholar]
- 19.Hampson NB, Dunn SL. Symptoms of carbon monoxide Poisoning do not correlate with the initial carboxyhemoglobin level. Undersea Hyperb Med. 2012, 39(2), 657–665. [PubMed] [Google Scholar]
- 20.Lange M, Cox RA, Traber DL, et al. No correlation between initial arterial carboxyhemoglobin level and degree of lung injury following ovine burn and smoke inhalation. Exp Lung Res. 2014, 40(3), 99–104. [DOI] [PubMed] [Google Scholar]
- 21.Hullin T, Aboab J, Desseaux K, et al. Correlation between clinical severity and different non-invasive measurements of carbon monoxide concentration: A population study. PLoS One, 2017, 12(3), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao SC, Mao YC, Hung YM, et al. Predictive Role of QTc Prolongation in Carbon Monoxide Poisoning-Related Delayed Neuropsychiatric Sequelae. Biomed Res Int. 2018, 2543018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao SC, Mao YC, Yang KJ, et al. Targeting optimal time for hyperbaric oxygen therapy following carbon monoxide poisoning for prevention of delayed neuropsychiatric sequelae: A retrospective study. J Neurol Sci. 2019, 396(September 2018), 187–192. [DOI] [PubMed] [Google Scholar]
- 24.Huang CC, Ho CH, Chen YC, et al. Hyperbaric Oxygen Therapy Is Associated With Lower Short- and Long-Term Mortality in Patients With Carbon Monoxide Poisoning. Chest, 2017, 152(5), 943–953. [DOI] [PubMed] [Google Scholar]
- 25.Rose JJ, Nouraie M, Gauthier MC, et al. Clinical Outcomes and Mortality Impact of Hyperbaric Oxygen Therapy in Patients With Carbon Monoxide Poisoning. Crit Care Med. 2018, 46(7), e649–e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CH, Su WH, Chen YC, et al. Treatment with normobaric or hyperbaric oxygen and its effect on neuropsychometric dysfunction after carbon monoxide poisoning. Medicine, 2018, 97(39), e12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heyboer M, Sharma D, Santiago W, et al. Hyperbaric Oxygen Therapy: Side Effects Defined and Quantified. Adv Wound Care (New Rochelle). 2017, 6(6), 210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wingelaar TT, van Ooij PAM, van Hulst RA. Oxygen toxicity and special operations forces diving: Hidden and dangerousngl. Front Psychol. 2017, 8(JUL), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8(6), e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soejima K, Schmalstieg FC, Sakurai H, et al. Pathophysiological analysis of combined burn and smoke inhalation injuries in sheep. Am J Physiol Lung Cell Mol Physiol. 2001, 280(6), L1233–41. [DOI] [PubMed] [Google Scholar]
- 31.Enkhbaatar P, Nelson C, Salsbury JR, et al. Comparison of gene expression by sheep and human blood stimulated with the TLR4 agonists lipopolysaccharide and monophosphoryl lipid A. PLoS One, 2015, 10(12), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez E, Fujiwara O, Lima-Lopez F, et al. Nebulized Epinephrine Limits Pulmonary Vascular Hyperpermeability to Water and Protein in Ovine With Burn and Smoke Inhalation Injury. 2016, Crit Care Med. 44(2), E89–E96. [DOI] [PubMed] [Google Scholar]
- 33.Vidal Melo MF, Harris RS, Layfield D, et al. Changes in regional ventilation after autologous blood clot pulmonary embolism. Anesthesiology, 2002, 97(3), 671–681. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda S, Ihara K, Andersen CR, et al. Modulation of Peroxynitrite Reduces Norepinephrine Requirements in Ovine MRSA Septic Shock. Shock. 2019. November;52(5):e92–e99. [DOI] [PubMed] [Google Scholar]
- 35.Fisher JA1, Rucker J, Sommer LZ, et al. Isocapnic hyperpnea accelerates carbon monoxide elimination. Am J Respir Crit Care Med 1999, 159(4 Pt 1):1289–92. [DOI] [PubMed] [Google Scholar]
- 36.Pace N, Strajman E, Walker EL. Acceleration of carbon monoxide elimination in man by high pressure oxygen. Science. 1950, 111(2894), 652–654. [DOI] [PubMed] [Google Scholar]
- 37.Shimazu T, Ikeuchi H, Sugimoto H, et al. Half-Life of Blood Carboxyhemoglobin after Short-Term and Long-term exposure to carbon monoxide. J Trauma. 2000, 49(July), 126–131. [DOI] [PubMed] [Google Scholar]
- 38.Tomruk O, Karaman K, Erdur B, et al. A new promising treatment strategy for carbon monoxide poisoning: High flow nasal cannula oxygen therapy. Med Sci Monit. 2019, 25, 605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roth D, Mayer J, Schreiber W, et al. Acute carbon monoxide poisoning treatment by non-invasive CPAP-ventilation, and by reservoir face mask: Two simultaneous cases. Am J Emerg Med. 2018, 36(9), 1718.e5–1718.e6. [DOI] [PubMed] [Google Scholar]
- 40.Delvau N, Penaloza A, Liistro G, et al. Effect of Pressure Support Ventilation on Carboxyhemoglobin Toxicokinetic after Acute Carbon Monoxide Intoxication: a Swine Model. J Med Toxicol. 2018, 14(2), 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinnapureddy AR, Stayner C, McEwan J, et al. Large animal models of rare genetic disorders: Sheep as phenotypically relevant models of human genetic disease. Orphanet J Rare Dis. 2015, 10(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheerlinck JPY, Snibson KJ, Bowles VM, et al. Biomedical applications of sheep models: from asthma to vaccines. Trends Biotechnol. 2008, 26(5), 259–266. [DOI] [PubMed] [Google Scholar]
- 43.Enkhbaatar P, Connelly R, Wang J, et al. Inhibition of neuronal nitric oxide synthase in ovine model of acute lung injury. Crit Care Med. 2009, 37(1), 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]