Abstract
Background
Limb ischemia (LI) is the underlying pathology of peripheral artery disease (PAD). Macrophages play a critical role in inflammation and can contribute to the exacerbation or reduction of inflammation. Transplantation of mesenchymal stem cells (MSCs) is an emerging therapeutic strategy for PAD. However, the mechanism by which human placenta-derived mesenchymal stem cells (PMSCs) regulate macrophage differentiation in ischemic tissue remains unclear.
Material/Methods
Placentas were obtained from healthy donors with normal 38- to 40-week gestation, and PMSCs were isolated from the placentas and cultured. A mouse model of hind-limb ischemia was established. Ischemic limbs were injected intramuscularly with about 5×106 PMSCs in the PMSCs group or a placebo solution (phosphate-buffered saline) in the control group at 4 different sites 1 day after the procedure. The blood perfusion of hind-limbs and the histological morphology were observed at day 1, 7, and 14 after the surgical procedure. Macrophages were detected by flow cytometry. The expression of serum tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, and IL-10 were detected by enzyme-linked immunosorbent assay (ELISA). The expression of CD31 and smooth muscle α-actin (α-SMA) in frozen muscle samples were detected by immunofluorescence staining.
Results
In the PMSCs group, blood perfusion was gradually recovered and ischemic injury was markedly alleviated. The percentage of M2-like macrophages was increased dramatically, while the M1/M2 macrophage ratio was reduced. The expression of TNF-α and IL-6 was reduced, while the IL-10 level was elevated. The expression and density of CD31- and α-SMA-positive vessels were both significantly increased.
Conclusions
Transplanted PMSCs alleviated inflammation, promoted neovascularization, and improved hind limb ischemia through regulating macrophage differentiation toward the M2 phenotype and cytokine secretion. Cytokine manipulation of macrophage phenotypes may have potential therapeutic benefits in injured ischemic limbs.
MeSH Keywords: Inflammation; Macrophages; Mesenchymal Stromal Cells; Neovascularization, Pathologic; Peripheral Arterial Disease
Background
Peripheral arterial disease (PAD) affects approximately 8 million people in the United States, and the prevalence of PAD is likely to be underestimated due to poor awareness and recognition in the population [1]. The pathology of PAD is characterized by limb ischemia, which contributes to reduced blood flow to the lower extremities. The critical limb ischemia is associated with a high risk of amputation, along with high morbidity and mortality [2]. Beyond surgical bypass and endovascular intervention, which show limited efficacy and do not substantially reduce the high risk of eventual amputation, transplantation of mesenchymal stem cells (MSCs) is an emerging therapeutic strategy for PAD [3–5]. Several previous studies showed that MSCs have angiogenic effects and simultaneously interacted with macrophages, which secrete cytokines and growth factors that promote angiogenesis [6,7]. Macrophages are hypothesized to play a critical role in inflammation by contributing to the exacerbation or reduction of inflammation. Macrophages can differentiate into M1- or M2-like macrophages, which are classically activated macrophages or alternatively activated macrophages [8] that have polarized phenotypes: pro-inflammatory (M1) or anti-inflammatory (M2). M1 macrophages initiate an inflammatory response and promote T helper cell type 1 (Th1) immunity, which is characterized by the release of proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-12, IL-6, and arginase (ARG)-1 [9]. Conversely, M2 macrophages are associated with tissue repair and fibrosis driven by activation of Th2 responses, which produce an abundance of angiogenic cytokines, including IL-10, IL-13, macrophage-derived chemokine (MDC)/chemokine C-C motif ligand (CCL) 22, and IL-4. Subsequently, IL-10, IL-13, and IL-4, which are implicated in inflammation resolution, stimulate alternatively activated M2 macrophages [10–12].
Thus, we hypothesized that MSCs can ameliorate inflammation and improve limb ischemia through decreasing the M1/M2 macrophage ratio.
Material and Methods
Ethics approval
All experimental procedures were approved by the Ethics Committee of the Tongji Hospital, Tongji University. All mouse-related protocols were approved by the Animal Care and Use Ethics Committee of Tongji Hospital, Tongji University. All animal procedures were in accordance with the guidelines published by the Shanghai Experimental Animal Center of the Chinese Academy of Sciences.
Isolation of placenta-derived MSCs (PMSCs)
Placentas were obtained from healthy donors with normal 38- to 40-week gestations. PMSCs were isolated from the placentas and cultured according to previously established protocols [13]. Briefly, the placentas were dissected after the umbilical cord blood was drained. After being washed with sterile phosphate-buffered saline (PBS) 4 times, the placental tissues were digested with 0.1% collagenase IV (Sigma-Aldrich, St. Louis, MO, USA) for 60 min at 37°C. The digested placental tissues were filtrated and centrifuged at 350×g for 10 min. Following incubation with red blood cell lysis buffer solution (Sigma-Aldrich) for 5 min at 37 °C, red blood cells were eliminated. The cell suspension was centrifuged at 300×g for 5 min, and resuspended in Dulbecco’s modified Eagle’s medium (Gibco Company, Grand Island, NY, USA), which contained 10% fetal bovine serum (Gibco Company), 2 mM l-glutamine, 100 μg/mL streptomycin, 100 U/mL penicillin, and 1% nonessential amino acids. The medium was subsequently replaced with fresh medium after cells were cultured and maintained at 37°C in 5% CO2 for 4 days. PMSCs were harvested until approximately 75% confluence was obtained.
Established mouse model of hind-limb ischemia
Male 10- to 12-week-old mice were purchased from the Shanghai Experimental Animal Center, the Chinese Academy of Sciences. The mice were randomly divided into a placebo solution (PBS) group (n=5) and a PMSCs group (n=5). Mice were anesthetized by intraperitoneal injection of 50 mg/kg sodium pentobarbital. The left femoral artery, iliac circumflex artery/vein, great saphenous artery, and muscular branch were ligated [14]. Blood flow into ischemic (left) and nonischemic (right) hind limbs was examined by laser Doppler perfusion imaging system (LDPIs, Perimed PSI, Järfälla, Sweden) at day 1, 7, and 14 after the surgery. Blood flow was assessed by the relative blood flow, which was shown as the ratio of ischemic to nonischemic hind-limb blood flow.
Transplantation of PMSCs into the hind-limbs of mice
The ischemic limbs were injected intramuscularly with about 5×106 PMSCs (PMSCs group) or PBS (control group) at 4 different sites 1 day after the procedure. Mice were sacrificed at predetermined time points. The hind limb muscles were harvested and frozen in optimal cutting temperature compound (Bio-Optica, Milan, Italy), and cryosectioned for histological and immunofluorescent assessment. Representative tissue sections were processed for routine hematoxylin and eosin (HE) staining.
Blood perfusion imaging of hind limbs
Given the potential regeneration of ischemic limbs, we assessed the blood perfusion of hind limbs. Repeated measurements of blood perfusion of the hind limbs were obtained by LDPIs at day 1, 7, and 14 after the surgical procedure, respectively. The relative blood flow was expressed as a ratio of blood flow in the ischemic (left) to the nonischemic (right) limb at specific time points.
Histological morphology analysis
To evaluate the inflammation and the fibrosis of the muscle tissue, its morphology was examined microscopically. Following HE staining and Masson’s trichrome staining of sections of tissue extracted at day 7 and 14 after the surgical procedure, at least 10 random fields were assessed. The muscle regeneration was quantified according to muscle fiber diameter based on images of HE-stained sections, using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Interstitial fibrosis was morphometrically evaluated according to the fibrotic area based on images of sections stained with Masson’s trichrome, using ImageJ software.
Fluorescence-activated cell sorting analysis
To assess the M1 or M2 polarization of macrophages, we carried out flow cytometric detection of M1 or M2 macrophages from ischemic lower limb tissue of mice. Cultured macrophages were isolated and harvested at a single-cell level, and surface markers were analyzed. The isolated cells were subsequently incubated with fluorescein isothiocyanate (FITC)-conjugated or phycoerythrin (PE)-conjugated monoclonal antibodies. The anti-F4/80 antibody (Abcam, Cambridge, MA, USA), anti-CD11c antibody (Abcam), and anti-CD206 antibody (Abcam) were conjugated monoclonal antibodies. M1 macrophages were identified on the basis of being F4/80 and CD11c positive, while M2 macrophages were CD206 positive. F4/80 and CD11c double-positive M1 macrophages as well as F4/80 and CD206 double-positive M2 macrophages from ischemic lower limb tissues of mice were detected by flow cytometry (Beckman Coulter, Fullerton, CA, USA) at day 1, 3, 7, and 14 after the surgical procedure, respectively. The data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA). The ratio of M1 to M2 macrophages was observed.
Enzyme-linked immunosorbent assay
The expression of serum tumor necrosis factor-alpha (TNF-α; Abcam), IL-6 (Abcam), and IL-10 (Abcam) were detected by enzyme-linked immunosorbent assay (ELISA). ELISA was performed according to the manufacturer’s instructions.
Immunofluorescence staining
The expression of CD31 (BD Biosciences, San Diego, CA, USA) and smooth muscle α-actin (α-SMA; BD Biosciences) in frozen muscle samples was detected by immunofluorescence staining. Fluorescence microscopy (Olympus, Japan, BX51) was utilized for observation. At least 10 different random fields were counted. The number of blood vessels and the capillary density were observed at day 7 and 14 after the surgical procedure, respectively.
Statistical analysis
Statistical analysis was performed using Prism Statistical Software (v5.01, GraphPad Software, La Jolla, CA, USA). Quantitative data are expressed as the mean±SEM. The differences between 2 groups were analyzed by either unpaired Student t test or analysis of variance (ANOVA). A value of P<0.05 was considered statistically significant.
Results
Transplanted PMSCs improved blood perfusion in ischemic limbs
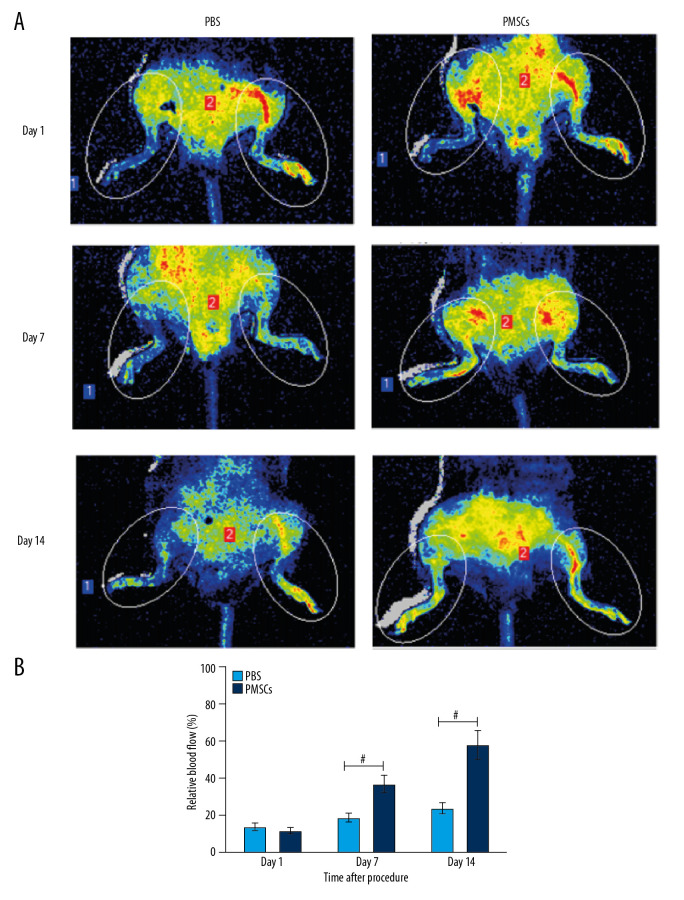
Limb ischemia is the underlying pathology of PAD, and it contributes to reduced blood flow to the lower extremities. In this study, we established a hind-limb ischemia mouse model and assessed blood perfusion of injured hind limbs by LDPIs after the surgical procedure. Minimal perfusion observed at day 1 after the surgery (Figure 1A) demonstrated that the mouse model of limb ischemia was established successfully. Blood perfusion in ischemic hind limbs was gradually recovered in the PMSCs group over 14 days (Figure 1A). Higher blood flow perfusion rates were observed in the injured hind limbs of the PMSCs group compared with the PBS group (Figure 1B). A trend toward improvement in blood perfusion was manifested from day 7, and a significant improvement in blood flow was demonstrated at day 14 (Figure 1B). These findings suggest that transplanted PMSCs promoted the recovery of blood perfusion and facilitated regeneration in the injured hind limbs.
Figure 1.
Transplanted PMSCs rescued blood perfusion in the ischemic hind limb. (A) Representative images from the laser Doppler perfusion imaging system (LDPIs) in the PMSCs group (n=5) and PBS group (n=5) at day 1, 7, and 14 after the surgical procedure, respectively. (B) Quantitative analysis of the relative blood flow in the ischemic (left) to the nonischemic (right) limb at day 1, 7, and 14. Blood perfusion differed significantly between the PMSCs group and PBS group at days 7 and 14 after the surgical procedure. A trend toward improvement was manifested from day 7, and a significant improvement in blood flow was demonstrated at day 14. # P<0.05 for PMSCs group versus PBS group. Data are represented as mean±SEM.
Transplanted PMSCs ameliorated limb ischemic injury
Limb ischemia contributed to reduced blood flow to the lower extremities and the ensuing muscle degeneration as well as typical separated muscle fibers in the ischemic hind limb. HE staining showed that the PMSCs group had less muscle degeneration and smaller reduction in muscle fibers at day 7 and 14 after the procedure (Figure 2A, 2B). Masson’s trichrome staining showed that the fibrotic area was remarkably smaller in the PMSCs group at day 7 and 14 after the procedure (Figure 2C, 2D). These findings indicate that the transplanted PMSCs ameliorated ischemic injury and promoted muscle regeneration in the ischemic hind limbs.
Figure 2.
Transplanted PMSCs ameliorated limb ischemic injury and rescued blood perfusion in the ischemic hind limb. (A) Representative images from the HE staining at day 7 and 14 after the procedure. (B) Quantification of muscle fiber number from the HE staining images at day 7 and 14 after the procedure. (C) Representative images from the Masson’s trichrome staining at day 7 and 14 after the procedure. (D) Quantification of fibrotic area from the Masson’s trichrome staining images at days 7 and 14 after the procedure. # P<0.05 for PMSCs group versus PBS group. Data are represented as mean±SEM.
Transplanted PMSCs modulated macrophage differentiation toward the M2-like phenotype in ischemic tissue
Next, we analyzed the effects of transplanted PMSCs on macrophage polarization. Flow cytometry was used to detect the macrophage phenotype. M1 macrophages were identified based on being positive for F4/80 and CD11c, while M2 macrophages were CD206 positive. F4/80 and CD11c double-positive M2 macrophages as well as F4/80 and CD206 double-positive M2 macrophages in ischemic lower limb tissues of mice were detected at day 1, 3, 7, and, 14, respectively (Figure 3A). Compared with the control group, the PMSCs group demonstrated dramatically increased numbers of M2-like macrophages at day 1, 3, and 7, respectively (Figure 3B). Moreover, the M1/M2 ratio was reduced in the PMSCs group at day 1, 3, 7, and 14, respectively (Figure 3B). Therefore, our results showed that transplanted PMSCs modulated macrophage differentiation toward an M2-like phenotype in ischemic tissue. PMSCs interacting synergistically with macrophages could regulate the immunomodulatory and inflammatory-mediated responses associated with ischemic injury to hind limbs in mice.
Figure 3.
Transplanted PMSCs modulated macrophage differentiation toward an M2-like macrophage phenotype in ischemic tissue. (A) Representative images of flow cytometry. (B) Quantification of macrophage phenotypes. The number of M2-like macrophages increased dramatically in the PMSCs group at day 1, 3, and 7, respectively. Moreover, the M1/M2 ratio was reduced in the PMSCs group at day 1, 3, 7, and 14 after the procedure, respectively. # P<0.05 for PMSCs group versus PBS group. Data are represented as mean±SEM.
Transplanted PMSCs regulated the local inflammatory response
We subsequently investigated the molecular mechanism of reduced inflammation in ischemic limb injury. Based on our observations, transplanted PMSCs could modulate macrophage differentiation from the M1 to the M2 macrophages. Simultaneously, PMSCs-mediated immune regulation predominantly acted through the secretion of cytokines that were induced or downregulated in response to activated macrophages. The results of ELISA showed that the expression of serum TNF-α and IL-6 was reduced, while IL-10 levels increased in the PMSCs group at day 1, 3, 7, and 14 after the procedure (Figure 4). These findings suggested that transplanted PMSCs suppressed the proinflammatory cytokines and enhanced the anti-inflammatory cytokines, thereby alleviating inflammatory response after limb ischemic injury.
Figure 4.
Transplanted PMSCs regulated the secretion of inflammation-related cytokines. (A–C) The expression of serum TNF-α and IL-6 was reduced, while IL-10 levels increased in the PMSCs group at day 3, 7, and 14 after the procedure, respectively. # P<0.05 for PMSCs group versus PBS group. Data are represented as mean±SEM.
Transplanted PMSCs facilitated neovascularization
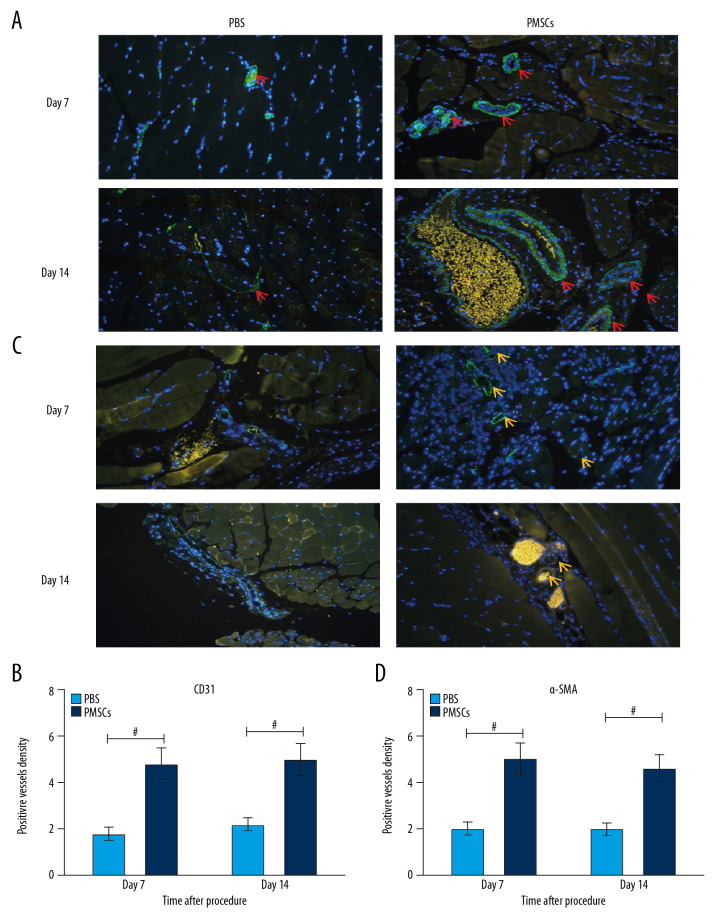
We found that transplanted PMSCs could improve blood perfusion, ameliorate limb ischemic injury, modulate macrophage differentiation toward an M2-like phenotype in ischemic tissue, and regulate the local inflammatory response. We postulated that M2 macrophage differentiation induced by PMSCs may exert angiogenic effects in response to ischemic injury. To evaluate this hypothesis, we assessed the formation of immature blood vessels in ischemic limbs. Immunofluorescent staining showed that the expression of CD31 and α-SMA was significantly elevated in the PMSCs group (Figure 5). Simultaneously, higher densities of CD31 or α-SMA positive vessels were observed in the PMSCs group at day 7 and 14 after the procedure (Figure 5). This outcome indicated that transplanted PMSCs can promote neovascularization in ischemic limbs.
Figure 5.
Transplanted PMSCs promoted the neovascularization in ischemic limbs. (A) Representative images of immunofluorescent staining for CD31 (×200). The red arrows indicate the blood vessel. (B) Quantification of CD31-positive vessel density from the immunofluorescent staining images. The density of CD31-positive vessels was significantly elevated in the PMSCs group at day 7 and 14 after the procedure. (C) Representative images of immunofluorescent staining for α-SMA (×200). The yellow arrows indicate the blood vessel. (D) Quantification of α-SMA-positive vessel density from the immunofluorescent staining images. The density of α-SMA-positive vessels was significantly elevated in the PMSCs group at day 7 and 14 after the procedure. # P<0.05 for PMSCs group versus PBS group. Data are represented as mean±SEM.
Discussion
Limb ischemia is the underlying pathology of PAD [2]. In the present study, we successfully established a hind-limb ischemia mouse model and investigated the therapeutic effect of transplanted PMSCs in mice with hind-limb ischemia. The results demonstrated that transplanted PMSCs can improve blood perfusion and ameliorate limb ischemic injury. Simultaneously, we found that PMSCs can modulate macrophage differentiation toward an M2 phenotype, regulate the secretion of inflammation-related cytokines, and facilitate angiogenesis in ischemia-injured hind limbs.
Given the limited efficacy of current therapies, the high risk of amputation, and a lack of medical therapy for critical limb ischemia, novel therapeutic strategies such as cell-based therapy are critically required [2,15]. Mixed results were shown in early clinical trials using therapies based on MSCs [16–18]. Despite some advances in MSCs therapy, some patients still fail to respond to cell-based therapy [15–17]. For example, patients with nondiabetic limb ischemia benefited from transplantation of autologous MSCs, but patients with diabetes seemingly did not [16–18]. Our study provides evidence that blood perfusion in mouse ischemic hind limb was gradually recovered in animals treated with PMSCs over 14 days, suggesting that the transplanted PMSCs could promote the recovery of blood perfusion and facilitate regeneration in the injured hind limbs.
Recently, several studies showed that MSCs interact with macrophages and regulate their function [7–10]. Beyond their transdifferential capacity, MSCs possess the immunomodulatory capacity to modulate macrophage differentiation toward an M2-like phenotype through regulating the secretion of inflammation-related cytokines [8–10]. Macrophages, which compose an important component of innate immunity associated with host defense and inflammation, play essential roles in maintaining tissue homeostasis. It is well known that in response to various signals derived from microbes or injured tissues, classically activated M1 macrophages display pro-inflammatory activities. In contrast to M1 macrophages, the alternatively activated M2 macrophages participate in inflammation resolution [10–12]. Different macrophage populations are implicated in the pathogenesis and regeneration found in a variety of diseases [19,20]. In this study, F4/80 and CD206 were selected as surface markers of M2 macrophages, while F4/80 and CD11c served as markers of M1 macrophages. The results showed that the number of M2-like macrophages increased dramatically in the PMSCs group at day 1, 3, and 7, respectively. Moreover, the M1/M2 ratio was reduced in the PMSCs group at day 1, 3, 7, and 14, respectively. Therefore, it is clear that transplanted PMSCs can modulated macrophage differentiation toward an M2-like phenotype in ischemic tissue.
PMSCs-mediated immune regulation predominantly acted through the secretion of cytokines that were induced or downregulated following interactions with activated macrophages [21]. To investigate the mediators responsible for the interactions with PMSCs, we explored the molecular mechanisms of reduced inflammation in ischemic limb injury. The results of ELISA showed that the expression of secreted TNF-α and IL-6 was reduced, while IL-10 levels increased in the PMSCs group at day 1, 3, 7, and 14 after the procedure, respectively. These findings suggest that transplanted PMSCs suppressed the pro-inflammatory cytokines and enhanced the anti-inflammatory cytokines, and thereby alleviated the inflammatory response after limb ischemic injury.
It is also noteworthy that inflammation, which is typically portrayed in a negative context, might serve as both a negative and a positive participant in neovascularization. The dysregulation of angiogenesis is a phenomenon observed in critical limb ischemia. Therapeutic neovascularization is a pivotal strategy for rescuing tissue from critical limb ischemia. Many studies have provided compelling evidence of therapeutic neovascularization from bone marrow MSCs. Given their immunomodulatory properties, migratory ability, and proliferation, PMSCs appeared comparatively promising, due to having characteristics similar to those of bone marrow MSCs [22,23]. PMSCs regulate the secretion of inflammation-related cytokines, and PMSCs-derived exosomes have been used as a targeted therapy to facilitate neovascularization [23]. Some studies have previously illustrated the promising results of PMSCs in animal models, especially in limb ischemia models [23]. PMSCs mediate augmentation of the number of blood vessels and blood flow and enhance vessel density and perfusion probably through direct differentiation and incorporation or secretion of cytokines in the ischemic tissue [24–26]. Consistent with these reports, the results of our study also demonstrated that transplanted PMSCs can modulate the secretion of inflammation-related cytokines and promote neovascularization in injured ischemic limbs. Our results from immunofluorescent staining showed that the expression of CD31 and α-SMA was significantly elevated in the PMSCs group. Simultaneously, a higher density of CD31− or α-SMA-positive vessels was observed in the PMSCs group at day 7 and 14 after the procedure. The limitation of this experiment is that, although we clarified that PMSCs improve limb ischemia in mice by regulating the ratio of M1 and M2, the mechanism regulating the differentiation of M1 to M2 macrophages was not clear. Future experiments will explore their molecular mechanism.
Conclusions
We hypothesized that transplanted PMSCs alleviate inflammation and promote neovascularization to improve hind limb ischemia through regulating macrophage differentiation toward an M2 phenotype and promoting cytokine secretion. Cytokine manipulation of macrophage phenotypes may have potential therapeutic benefits in injured ischemic limbs.
Footnotes
Conflicts of interest
None.
Source of support: This work was sponsored by the Natural Science Foundation of Shanghai (Grant number: 17ZR1426200) to Ye Song; Health System Leading Talents Project of Shanghai Pudong New Area District (Grant number: PWR12019-06) to Ye Song
References
- 1.Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet. 2013;382(9901):1329–40. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Sinha M, Pandey NN, Chandrashekhara SH. Stem cell therapy in critical limb ischemia: Current scenario and future trends. Indian J Radiol Imaging. 2019;29:397–403. doi: 10.4103/ijri.IJRI_385_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rigato M, Monami M, Fadini GP. Autologous cell therapy for peripheral arterial disease: Systematic review and meta-analysis of randomized, non-randomized, and non-controlled studies. Circ Res. 2017;120:1326–40. doi: 10.1161/CIRCRESAHA.116.309045. [DOI] [PubMed] [Google Scholar]
- 4.Wahid FSA, Ismail NA, Wan Jamaludin WF, et al. Efficacy and safety of autologous cell-based therapy in patients with no-option critical limb ischaemia: A meta-analysis. Curr Stem Cell Res Ther. 2018;13:265–83. doi: 10.2174/1574888X13666180313141416. [DOI] [PubMed] [Google Scholar]
- 5.Xie B, Luo H, Zhang Y, et al. Autologous stem cell therapy in critical limb ischemia: A meta-analysis of randomized controlled trials. Stem Cells Int. 2018;2018 doi: 10.1155/2018/7528464. 7528464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudlik G, Hegyi B, Czibula Á, et al. Mesenchymal stem cells promote macrophage polarization toward M2b-like cells. Exp Cell Res. 2016;348:36–45. doi: 10.1016/j.yexcr.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Jin L, Deng Z, Zhang J, et al. Mesenchymal stem cells promote type 2 macrophage polarization to ameliorate the myocardial injury caused by diabetic cardiomyopathy. J Transl Med. 2019;17:251. doi: 10.1186/s12967-019-1999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Zhao N, Zhang J, et al. Mesenchymal stem cells rejuvenate cardiac muscle through regulating macrophage polarization. Aging (Albany NY) 2019;11:3900–8. doi: 10.18632/aging.102009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Niu L, Tang X, et al. Mesenchymal stem cells prevent podocyte injury in lupus-prone B6.MRL-Faslpr mice via polarizing macrophage into an anti-inflammatory phenotype. Nephrol Dial Transplant. 2019;34:597–605. doi: 10.1093/ndt/gfy195. [DOI] [PubMed] [Google Scholar]
- 10.Wei X, Sun G, Zhao X, et al. Human amnion mesenchymal stem cells attenuate atherosclerosis by modulating macrophage function to reduce immune response. Int J Mol Med. 2019;44:1425–35. doi: 10.3892/ijmm.2019.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heo JS, Choi Y, Kim HO. Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cells Int. 2019;2019 doi: 10.1155/2019/7921760. 7921760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu X, Liu S, Zhang H, et al. Mesenchymal stem cells and extracellular matrix scaffold promote muscle regeneration by synergistically regulating macrophage polarization toward the M2 phenotype. Stem Cell Res Ther. 2018;9(1):88. doi: 10.1186/s13287-018-0821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long C, Lankford L, Kumar P, et al. Isolation and characterization of canine placenta-derived mesenchymal stromal cells for the treatment of neurological disorders in dogs. Cytometry. 2018;93:82–92. doi: 10.1002/cyto.a.23171. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Dardik A. A murine model of hind limb ischemia to study angiogenesis and arteriogenesis. Methods Mol Biol. 2018;17:135–43. doi: 10.1007/978-1-4939-7526-6_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Zhou H, Jin X, et al. Autologous bone marrow mononuclear cells transplant in patients with critical leg ischemia: Preliminary clinical results. Exp Clin Transplant. 2013;11:435–39. doi: 10.6002/ect.2012.0129. [DOI] [PubMed] [Google Scholar]
- 16.Gremmels H, Teraa M, Quax PH, et al. Neovascularization capacity of mesenchymal stromal cells from critical limb ischemia patients is equivalent to healthy controls. Mol Ther. 2014;22(11):1960–70. doi: 10.1038/mt.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benoit E, O’Donnell TF, Iafrati MD, et al. The role of amputation as an outcome measure in cellular therapy for critical limb ischemia: Implications for clinical trial design. J Transl Med. 2011;9:165. doi: 10.1186/1479-5876-9-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris AD, Dalal S, Li H, Brewster LP. Human diabetic mesenchymal stem cells from peripheral arterial disease patients promote angiogenesis through unique secretome signatures. Surgery. 2018;4:870–76. doi: 10.1016/j.surg.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 19.He S, Gleason J, Fik-Rymarkiewicz E, et al. Human placenta-derived mesenchymal stromal-like cells enhance angiogenesis via T cell-dependent reprogramming of macrophage differentiation: PDA-002 enhances angiogenesis via immunomodulation. Stem Cells. 2017;35:1603–13. doi: 10.1002/stem.2598. [DOI] [PubMed] [Google Scholar]
- 20.Ertl J, Pichlsberger M, Tuca AC, et al. Comparative study of regenerative effects of mesenchymal stem cells derived from placental amnion, chorion and umbilical cord on dermal wounds. Placenta. 2018;65:37–46. doi: 10.1016/j.placenta.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Zahavi-Goldstein E, Blumenfeld M, Fuchs-Telem D, et al. Placenta-derived PLX-PAD mesenchymal-like stromal cells are efficacious in rescuing blood flow in hind limb ischemia mouse model by a dose- and site-dependent mechanism of action. Cytotherapy. 2017;19:1438–46. doi: 10.1016/j.jcyt.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Liang T, Zhu L, Gao W, et al. Coculture of endothelial progenitor cells and mesenchymal stem cells enhanced their proliferation and angiogenesis through PDGF and Notch signaling. FEBS Open Bio. 2017;7:1722–36. doi: 10.1002/2211-5463.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew SA, Naik C, Cahill PA, Bhonde RR. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol Life Sci. 2020;77:253–65. doi: 10.1007/s00018-019-03268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CY, Liu SH, Chen CY, et al. Human placenta-derived multipotent mesenchymal stromal cells involved in placental angiogenesis via the PDGF-BB and STAT3 pathways. Biol Reprod. 2015;93(4):103. doi: 10.1095/biolreprod.115.131250. [DOI] [PubMed] [Google Scholar]
- 25.Francki A, Labazzo K, He S, et al. Angiogenic properties of human placenta-derived adherent cells and efficacy in hindlimb ischemia. J Vasc Surg. 2016;64:746–56.e1. doi: 10.1016/j.jvs.2015.04.387. [DOI] [PubMed] [Google Scholar]
- 26.Xie NZ, Li ZH, Adesanya TM, et al. Transplantation of placenta-derived mesenchymal stem cells enhances angiogenesis after ischemic limb injury in mice. J Cell Mol Med. 2016;20:29–37. doi: 10.1111/jcmm.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]