Abstract
Objective:
The non-aortic cardiovascular morbidity and mortality in patients with aortic dissection (AD), intramural hematoma (IMH) and penetrating aortic ulcer (PAU) is unknown. This study aimed to define rates of CV events in a cohort of newly diagnosed patients with AD, IMH, and PAU.
Methods:
Retrospective review of all Olmsted County, MN residents diagnosed with AD, IMH, and PAU from 1995–2015. Primary outcome was non-aortic CV death. Secondary outcome was a first-time non-fatal CV event (myocardial infarction (MI), heart failure (HF), or stroke). Outcomes were compared to age- and sex-matched population referents using Cox proportional hazards regression adjusting for comorbidities.
Results:
133 patients (77 AD, 21 IMH, 35 PAU; 57% male) with a mean age of 71.8 years (SD 14.1) were identified. Median follow-up was 10 years. Compared to population referents, AD/IMH/PAU patients had an increased risk of CV death (adjusted HR 2.4, 95% CI 1.4–4.2, p=.003) and an increased risk of any first-time non-fatal CV event (adjusted HR 3.0, 95% CI 1.9–4.8, p<.001), mainly driven by an increased risk of first-time HF (adjusted HR 2.7, 95% CI 1.7–4.3, p<.001). When excluding events within 14 days of diagnosis, AD/IMH/PAU patients remained at increased risk of CV death (adjusted HR 2.6, 95% CI 1.4–4.7, p=.002), any first-time non-fatal CV event (adjusted HR 2.6, 95% CI 1.5–4.4, p<.001), and first-time HF (adjusted HR 2.5, 95% CI 1.5–4.3, p<.001).
Conclusions:
Compared to population referents, AD/IMH/PAU patients have a 2- to 3-fold risk of non-aortic CV death, any first-time non-fatal CV event and first-time HF. These data implicate the need for long-term cardiovascular management in AD/IMH/PAU patients.
Keywords: Aorta, aortic disease, cardiovascular risk, cardiovascular events, cardiovascular death
Table of Contents Summary
In this retrospective population-based study, 133 patients with aortic dissection, intramural hematoma, and penetrating aortic ulcer (PAU) had a 2- to 3-fold increased risk of non-aortic cardiovascular death, any first-time non-fatal cardiovascular event and first-time heart failure when compared to population referents. The study highlights the need for rigorous long-term cardiovascular management in this patient group.
Introduction
Aortic dissection (AD), intramural hematoma (IMH), and penetrating aortic ulcer (PAU) are related aortic pathologies, associated with a high risk of acute and chronic morbidity and mortality.1 While significant improvements in the medical and surgical management of these pathologies and their aortic-related complications have been made over the past decades, we have recently shown that AD, IMH and PAU patients have a significantly higher all-cause mortality in the long-term when compared to the general population.2 However, targets to improve the prognosis in these patients are poorly defined.
The incidence of non-aortic cardiovascular (CV) events in patients with AD, IMH, and PAU is unknown. As >70% of patients with AD and IMH have a history of hypertension3, 4 and atherosclerotic processes are considered fundamental to the pathogenesis of PAU,5, 6 these patients may be at increased risk for CV events. Nevertheless, the rates of CV events and their outcomes have not been well defined in this patient group. Limited studies that report causes of late death in patients with aortic dissection indicate that, apart from aortic-related causes, CV events are the most common cause of death.7,8 However, it is unclear whether the occurrence of AD, IMH, and PAU confers an increased risk of CV events when compared to the general population.
A better understanding of the CV risk associated with AD, IMH, and PAU may help to guide long-term management and improve outcomes in patients with these conditions. The aim of this study was to define the risk of CV death and incident non-fatal CV events (myocardial infarction, heart failure, and stroke) among patients with AD, IMH, and PAU from a population-based approach to assess for the additional CV risk that may be associated with these aortic pathologies.
Methods
This was part of a retrospective, population-based study intended to characterize the incidence of the aortic pathologies AD, IMH, and PAU in Olmsted County, MN.2 The study was conducted utilizing the resources of the Rochester Epidemiology Project (REP), a medical record linkage system that includes virtually all residents and local health care providers in Olmsted County, MN, providing a reliable infrastructure for population-based research.9, 10 As previously described in more detail,2 all adult (≥18 years of age) residents with an incident diagnosis of AD, IMH, and PAU from 1995–2015 were identified from the REP using International Classification of Disease (ICD, 9th and 10th revision) codes and Hospital Adaptation of the International Classification of Diseases (HICDA, 2nd edition) codes. For study inclusion, imaging confirmation of the diagnosis was necessary, or, for immediate decedents, AD/IMH/PAU had to be the confirmed by autopsy or be the primary diagnosis on the death certificate. AD, IMH, and PAU were defined using criteria suggested in current guidelines.11 All identified pathologies meeting the criteria were included regardless of the acuity of presentation. AD was classified using the Stanford and the DeBakey classification, IMH was classified using the Stanford classification and PAU was classified by anatomic location.
To compare outcomes in this assembled cohort of patients to the general population, a random sample of Olmsted County residents was selected from the REP as a referent cohort, matched to AD/IMH/PAU patients for birth year and sex. Based on survival data for Olmsted County residents and patients with AD, population referents were matched in a 3:1 ratio to detect a minimum hazard ratio (HR) for death of 1.95 with an alpha of 0.05 and power of 0.8. The diagnosis date of the matched AD/IMH/PAU patient was set as the index date for population referents; events and comorbidities known prior to that date were considered preexisting and subsequent events were defined as outcome events.
A review of medical records was performed for AD/IMH/PAU patients and population referents. Comorbidities were assessed using the Charlson Comorbidity Index.12 For the identification of Charlson comorbidities, ICD and HICDA diagnostic codes were used. Assignment of a comorbidity required two occurrences of predefined codes within five years prior to the AD/IMH/PAU diagnosis date (or the index date in population referents), as it has previously been described in a REP study.13 All patients were censored on December 31, 2015.
Assessment of the primary endpoint: CV death
The primary endpoint was CV-related mortality. Vital status of each individual was verified through the REP, which captures information on in state and out of state deaths from multiple sources10 Additionally, an institutionally approved Internet research location service (Accurint, www.accurint.com) was used to confirm vital status of the study subjects if no record of death was found in the REP. Dates and causes of death were obtained from Minnesota State Death Certificates, which are available in the REP for all in state deaths. Out of state death certificates were requested where permissible as per state laws but could not be obtained for three AD/IMH/PAU patients and three population referents known to have died out of state. Cardiovascular death was defined as death related to myocardial infarction, congestive heart failure, other cardiac causes, stroke, or peripheral vascular disease.
Assessment of the secondary endpoint: incident non-fatal CV event (MI, HF, and stroke)
Secondary endpoint was the occurrence of an incident non-fatal cardiovascular event, defined as first ever diagnosis of myocardial infarction (MI), congestive heart failure (HF) or stroke on or after the date of AD/IMH/PAU diagnosis / the index date.
Myocardial infarctions were identified from the REP using ICD-9 codes 410.× and ICD-10 codes I21.× and I22.×. Events with a corresponding code were validated using an algorithm based on biomarker levels, the presence of cardiac pain and Minnesota coding of ECGs. This is an established method that has previously been used to identify and validate MI events within the REP.14 Similarly, HF was detected using ICD-9 codes 428.× and ICD-10 codes I09.81, I11.0, I13.0, I13.2, and I50.×. To verify the diagnosis of HF, Framingham heart failure criteria were abstracted from medical records and heart failure was considered confirmed in the presence of two major criteria or one major criterion accompanied by two minor criteria.15 This approach has previously been used to ascertain incident HF in Olmsted County.16, 17 To identify strokes, ICD-9 codes 430, 431, 432.× – 435.×, 436, V12.54 and 997.02 and ICD-10 codes I60.× – I63.× were used. Medical records and imaging were reviewed to validate the diagnosis of stroke. Stroke was defined as imaging or clinical evidence of focal cerebral injury due to ischemia or non-traumatic hemorrhage. Clinical evidence of stroke was defined as symptoms of neurological dysfunction persisting for 24 hours or longer for which other etiologies had been excluded.18
Only incident diagnoses of MI, HF, and stroke were recorded. Incident CV events that led to death were excluded for analysis of the secondary endpoint as these were captured within the primary endpoint of fatal CV events and censored at death.
Among 133 AD/IMH/PAU patients, 33 (25%) had a CV event prior to AD/IMH/PAU diagnosis. In the 3:1 matched referent cohort, 67 of 399 (17%) had a prior CV event. These individuals were excluded from the corresponding analyses of incident non-fatal CV events, as these would be repeat events. For excluded AD/IMH/PAU patients, matched population referents were excluded likewise to maintain the age/sex-matching. Events were analyzed as a composite endpoint (any incident CV event: MI, HF, or stroke) and by CV event type separately.
Statistical analysis
Summary statistics including mean (standard deviation) or median (range), and frequencies (percent) were used to describe baseline characteristics and descriptive outcomes. Univariate associations between AD/IMH/PAU patients and referents at baseline were tested using Student’s t-test for continuous and χ2 test (Fisher’s exact test when appropriate) for categorical variables. Univariate associations between the AD, IMH, and PAU cohorts were tested using ANOVA and x for continuous variables and categorical variables respectively. CV deaths and incident CV events were evaluated as time to event using life tables and Kaplan Meier plots. Cox proportional hazards modeling adjusting for age, sex, and the Charlson Comorbidity Index was used to determine differences in outcome events between AD/IMH/PAU patients and population referents. Analyses for the primary and secondary endpoints were first performed by including all events from the time of diagnosis forward. Due to the high risk of CV events in the acute setting of AD/IMH/PAU, subset analyses excluding any events within 14 days of AD/IMH/PAU diagnosis were performed (analyses starting on day 15). P-values <.05 were considered significant. Statistical analyses were performed using STATA (StataCorp., College Station, TX) and SAS software (SAS Institute Inc., Cary, NC).
The study was approved by the Institutional Review Boards of Mayo Clinic and Olmsted Medical Center, the two major health care providers within the REP. All individuals included in the study had already provided informed consent for the use of their medical records in research as part of the REP.9
Results
A total of 133 patients were identified; 77 had AD, 21 IMH, and 35 PAU. Mean age at diagnosis was 71.8 years (SD 14.1, range 27 – 93) and 57% were male. Median follow-up was 10.2 years for AD/IMH/PAU patients and 10.1 years for matched population referents. Detailed characteristics of the AD/IMH/PAU cohort have been published elsewhere2 and are summarized in Table I. Mean Charlson Comorbidity Index was 2.6 (SD 2.6) in AD/IMH/PAU patients and 1.7 (SD 2.1) in population referents (p<.001).
Table I.
Baseline characteristics of AD/IMH/PAU patients and population referents
| Referents (n=399) | AD/IMH/PAU (n=133) | p* | AD/IMH/PAU Subtypes | p | |||
|---|---|---|---|---|---|---|---|
| AD (n=77) | IMH (n=21) | PAU (n=35) | |||||
| Age (years), mean (SD) | 71.8 (14.1) | 71.8 (14.1) | 1.0 | 68.9 (15.6) | 73.5 (11.5) | 77.1 (10.0) | .015 |
| Male gender | 228 (57.1%) | 76 (57.1%) | 1.0 | 46 (59.7%) | 11 (52.4%) | 19 (54.3%) | .770 |
| Any prior CV event | 67 (16.8%) | 33 (24.8%) | .040 | 18 (23.4%) | 6 (28.6%) | 9 (25.7%) | .878 |
| Prior MI | 21 (5.3%) | 13 (9.8%) | .066 | 6 (7.8%) | 3 (14.3%) | 4 (11.4%) | .556 |
| Prior HF | 29 (7.3%) | 16 (12.0%) | .087 | 9 (11.7%) | 1 (4.8%) | 6 (17.1%) | .458 |
| Prior stroke | 27 (6.8%) | 11 (8.3%) | .560 | 5 (6.5%) | 4 (19.0%) | 2 (5.7%) | .152 |
| Charlson Comorbidity | 1.7 (2.1) | 2.6 (2.6) | <.001 | 2.1 (2.2) | 2.8 (2.6) | 3.7 (3.2) | .006 |
| Index, mean (SD) | |||||||
| Acuity of presentation† | - | <.001 | |||||
| Acute | - | 79 (59.4%) | 52 (67.5%) | 17 (81.0%) | 10 (28.6%) | ||
| Subacute | - | 4 (3.0%) | 2 (2.6%) | 1 (4.8%) | 1 (2.9%) | ||
| Chronic | - | 3 (2.3%) | 2 (2.6%) | 0 (0%) | 1 (2.9%) | ||
| Unknown | - | 47 (35.3) | 21 (27.3%) | 3 (14.3%) | 23 (65.7%) | ||
| Stanford classification | - | <.001 | |||||
| Type A | - | - | 45 (58.4%) | 5 (23.8%) | - | ||
| Type B | - | - | 32 (41.6%) | 16 (76.2%) | - | ||
| De Bakey classification | |||||||
| Type I | - | - | 24 (31.2%) | - | - | ||
| Type II | - | - | 21 (27.3%) | - | - | ||
| Type IIIa | - | - | 8 (10.4%) | - | - | ||
| Type IIIb | - | - | 24 (31.2%) | - | - | ||
| Anatomic localisation | - | - | |||||
| Thoracic | - | - | - | - | 18 (51.4%) | ||
| Abdominal | - | - | - | - | 17 (48.6%) | ||
p-values for comparisons between the AD/IMH/PAU and the referent cohort do not acoount for matching
Acute: ≤ 14 days of symptom onset; Subacute: 15–90 days; Chronic: > 90 days
Primary endpoint: CV death
There were 73 (55%) deaths among the 133 AD/IMH/PAU patients during follow-up. Twenty-one (29%) were due to a non-aortic cardiovascular cause: HF (n=9), MI (n=5), other cardiac cause (n=5, including arrhythmia and not further specified cardiac causes), stroke (n=1) and peripheral vascular disease (n=1). In the 3:1 matched referent cohort, 144 (36%) of 399 individuals died during follow-up and 40 (28%) deaths were due to a cardiovascular cause. Estimated freedom from CV death in AD/IMH/PAU patients versus referents at 5, 10, and 15 years was 91%, 81%, and 61% versus 95%, 86%, and 81%, corresponding to a significantly increased risk of CV death among AD/IMH/PAU patients (adjusted HR 2.4, 95% CI 1.4 – 4.2, p=003, Table II).
Table II.
Risk of CV death in AD/IMH/PAU patients and subtypes versus matched population referents (adjusted for the Charlson Comorbidity Index) Comorbidity Index)
| All deaths | Excluding acute deaths* | |||||||
|---|---|---|---|---|---|---|---|---|
| AD/IMH/PAU No. at risk/events | Referents No. at risk/events | HR (95% CI) | p | AD/IMH/PAU No. at risk/events | Referents No. at risk/events | HR (95% CI) | p | |
| CV death | 133 / 21 | 399 / 40 | 2.4 (1.4 – 4.2) | .003 | 118 / 20 | 354 / 32 | 2.6 (1.4 – 4.7) | .002 |
| AD No. at risk/events | Referents No. at risk/events | HR (95% CI) | p | AD No. at risk/events | Referents No. at risk/events | HR (95% CI) | p | |
| CV death | 77 / 13 | 231 / 25 | 3.2 (1.5 – 6.8) | .002 | 65 / 13 | 195 / 19 | 3.9 (1.8 – 8.6) | <.001 |
| IMH No. at risk/events | Referents No. at risk/events | HR (95% CI) | p | IMH No. at risk/events | Referents No. at risk/events | HR (95% CI) | p | |
| CV death | 21 / 4 | 63 / 5 | 2.8 (0.7 – 11.4) | .145 | 20 / 4 | 60 / 5 | 2.6 (0.6 – 10.6) | .177 |
| PAU No. at risk/events | Referents No. at risk/events | HR (95% CI) | p | PAU No. at risk/events | Referents No. at risk/events | HR (95% CI) | p | |
| CV death | 35 / 4 | 105 / 10 | 1.2 (0.3 – 4.1) | .775 | 33 / 3 | 99 / 8 | 1.1 (0.3 – 4.4) | .917 |
Including deaths >14 days of diagnosis only
Among AD/IMH/PAU patients, 15 (11%) died within two weeks of diagnosis. Of these, 13 were aortic-related deaths, including six immediate deaths with autopsy diagnosis of acute AD Stanford type A. Another patient with AD died within two weeks of diagnosis due to complications of preexisting liver disease and one patient with PAU died due to acute MI (confirmed by autopsy). After exclusion of these acute deaths, AD/IMH/PAU patients remained at increased risk of CV death (adjusted HR 2.6, 95% CI 1.4 – 4.7, p=.002). Overall, 20 (34%) of 58 late deaths among AD/IMH/PAU patients were due to a CV cause, representing the most common cause of late death in these patients. Among subtypes AD, IMH, and PAU separately, only AD was associated with an increased risk of CV death compared to matched referents, both when including and when excluding acute deaths (Table II).
Secondary endpoint: incident non-fatal CV event (MI, HF, and stroke)
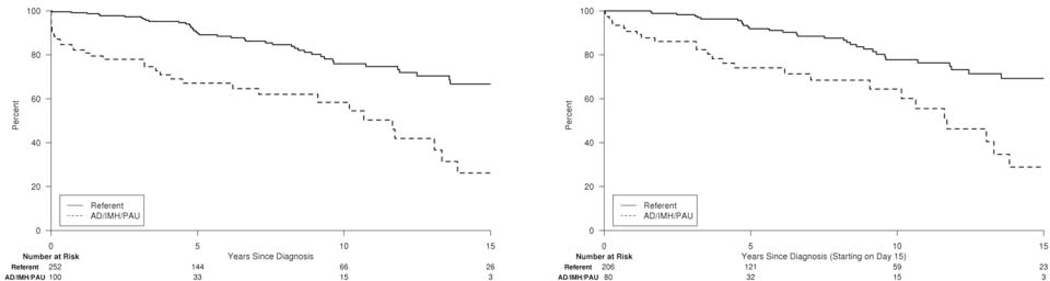
For AD/IMH/PAU patients and population referents with no prior CV event history, estimated freedom from any incident non-fatal CV event at 5, 10, and 15 years was 67%, 58%, and 26% in AD/IMH/PAU patients versus 90%, 76%, and 67% in referents (Figure 1), corresponding to a significantly increased risk of any incident non-fatal CV event in AD/IMH/PAU patients (adjusted HR 3.0, 95% CI 1.9 – 4.8, p<.001). This was mainly due to an increased risk of first-time diagnosis of HF (adjusted HR 2.7, 95% CI 1.7 – 4.3, p<.001), while the risk of first-time MI and stroke alone was not significantly increased among AD/IMH/PAU patients (Table III). Among AD/IMH/PAU patients, three had incident MI, five had incident HF, and four had incident stroke within 14 days of diagnosis. When excluding these acute CV events, AD/IMH/PAU remained similarly associated with an increased risk of any incident CV event (adjusted HR 2.6, 95% CI 1.5 – 4.4, p<.001) and incident HF (adjusted HR 2.5, 95% CI 1.5 – 4.3, p<.001) but not MI and stroke alone (Table III).
Figure 1. Freedom from any first-time non-fatal CV event.
Survival free from any first-time CV event (MI, HF, or stroke) for AD/IMH/PAU patients versus population referents; Kaplan-Meier curve including all events from diagnosis forward (left) and events starting on day 15 post AD/IMH/PAU diagnosis (right).
Table III.
First-time non-fatal CV events in AD/IMH/PAU patients versus population referents (adjusted for the Charlson Comorbidity Index). Number at risk includes only subjects without a CV event prior to AD/IMH/PAU diagnosis/the index date.
| All CV events | Excluding acute CV events* | |||||||
|---|---|---|---|---|---|---|---|---|
| AD/IMH/PAU No. at risk/events | Referents No. at risk/events | HR (95% CI) | p | AD/IMH/PAU No. at risk/events | Referents No. at risk/events | HR (95% CI) | p | |
| Any CV event | 100 / 36 | 252 / 43 | 3.0 (1.9 – 4.8) | <.001 | 80 / 27 | 206 / 33 | 2.6 (1.5 – 4.4) | <.001 |
| MI | 120 / 7 | 343 / 14 | 1.6 (0.6 – 4.2) | .342 | 103 / 4 | 295 / 10 | 1.2 (0.3 – 4.0) | .811 |
| HF | 117 / 32 | 328 / 44 | 2.7 (1.7 – 4.3) | <.001 | 98 / 27 | 277 / 34 | 2.5 (1.5 – 4.3) | <.001 |
| Stroke | 122 / 9 | 340 / 18 | 2.3 (0.99 – 5.2) | .051 | 105 / 5 | 294 / 14 | 1.5 (0.5 – 4.3) | .465 |
including deaths >14 days of diagnosis only
Analyses of CV events by subtypes AD, IMH, and PAU showed significantly increased risks of any incident CV event in AD and incident HF in AD and IMH (Supplemental Table). Due to low event rates, these analyses were not adjusted for Charlson comorbidities.
Discussion
This population-based assessment of AD, IMH, and PAU patients in Olmsted County, MN from 1995–2015 is the first to quantify the cardiovascular risk associated with AD, IMH and PAU. When compared to population referents of similar age and sex and after adjustment for comorbidities, patients with these aortic pathologies had a 2.4-fold risk to die from a non-aortic CV cause and a 3-fold risk to suffer a first-time non-fatal CV event (MI, HF, or stroke), predominantly driven by a significantly increased risk of incident HF. The risk of CV death, any first-time CV event, and first-time HF remained similarly increased when excluding acute phase events within 14 days of AD/IMH/PAU diagnosis, demonstrating the long-term CV morbidity and mortality in this patient group. These data highlight potential areas for improvement in the post-acute care of patients with AD/IMH/PAU that has not previously been emphasized. Cardiovascular risk factors among patients with aortic pathology have been previously shown. Although other etiologic factors such as connective tissue disease or the presence of a bicuspid aortic valve are well-established, in particular for AD in younger patients,19 hypertension is the most commonly recognized risk factor for the development of AD and IMH. Registry data have shown that hypertension is present in 74% and 81% of patients with Stanford type A and B dissection respectively3 and in 79% of those with type B intramural hematoma.20 Other cardiovascular risk factors reported for AD and IMH cohorts are diabetes in 3–10%,3, 20, 21 cigarette smoking in 29–55%,21–23 and atherosclerosis in 27–83%.3, 20, 22 PAU on the other hand, is, by itself considered an atherosclerotic lesion of the aortic wall5, 6 and reported risk factors are hypertension in 78–92%, dyslipidemia in 45–51%, and smoking in 36–76%.5, 23, 24 Based on these data, it is evident, that patients with AD, IMH, and PAU exhibit a distinct cardiovascular risk profile. The increased risk of these patients to suffer CV death or a first-time non-fatal CV event may therefore partly be explained by the high prevalence of common underlying risk factors. The association of the aforementioned cardiovascular risk factors with MI, HF, and stroke is widely known. Hypertension specifically, has been established as one of the most important risk factors for heart failure.15, 25 When looking at the subtypes AD, IMH, and PAU separately, AD was the only subtype that was consistently associated with the same outcomes as the entire AD/IMH/PAU cohort, i.e. an increased risk of CV death, any first-time non-fatal CV event and first-time HF. It may be hypothesized that this is due to an even stronger association of AD with cardiovascular risk factors such as hypertension when compared to IMH and PAU. However, other aspects than common risk factors have to be considered.
First, acute AD can itself cause myocardial infarction when the ascending aorta and/or the coronary arteries are involved, and it can be the cause of stroke when the supra-aortic vessels are involved. AD presenting with heart failure has been described for Stanford type A and type B dissections.26 Although not all AD/IMH/PAU patients in this cohort presented acutely, this increased risk of CV events during the acute phase was accounted for by repeating the analysis including only events that occurred >14 days after AD/IMH/PAU diagnosis. This did not considerably alter the results. The majority of these excess CV deaths and non-fatal incident events in AD/IMH/PAU patients can therefore not be attributed to the acute aortic pathology itself. However, in Stanford type A AD and IMH treated by open surgery using cardiopulmonary bypass, a risk to develop HF that is due to the operation, e.g. due to postoperative atrial fibrillation, may persist after 14 days. Of the 27 patients who developed HF during follow-up (> 14 days after AD/IMH/PAU diagnosis), three patients with Stanford type A AD and IMH treated by open surgery developed HF within a year of surgery. Thus, incident HF may have a potential selection bias. However, the risk of HF in these patients may be an opportunity for improving the quality of postoperative care.
Second, patients with pathologies involving the aortic root (particularly AD), may develop aortic valvulopathy, secondarily resulting in heart failure. This may happen due to degeneration of the native or a prosthetic aortic valve (after valve-sparing surgery or Bentall procedure for initial treatment of Stanford type A AD or IMH).21 In our cohort, 11 (41%) of 27 patients who had incident heart failure > 14 days of AD/IMH/PAU diagnosis were patients with Stanford type A dissection or IMH (9 AD, 2 IMH). Post hoc analysis of these 11 patients showed that at diagnosis of HF, three had no aortic valve abnormality and five were described to have trivial or mild aortic regurgitation (three native, two prosthetic aortic valves). Two had moderate (one native, one prosthetic valve), and one had moderate-severe aortic regurgitation (native valve). Although it is difficult to draw conclusions from these small numbers, aortic valve problems may contribute to the development of heart failure after type A AD/IMH. However overall, those with Stanford type A AD or IMH who developed relevant aortic valve pathologies represent a small proportion that may not explain the increased risk of incident HF or even CV death among AD/IMH/PAU patients alone.
Mortality rates and causes of death in our cohort may be difficult to compare to other reports that include selected patient subgroups only. In a study including surgically treated patients with type A dissections, 5, 10, and 15 year survival rates were 69%, 55% and 48%, respectively.8 For type B dissections, 60%, 35%, and 17% survival has been reported at 5, 10, and 15 years27 and in a cohort of IMH and PAU patients, survival at 5 and 10 years was 58% and 33%.23 As previously reported, we observed similar 5, 10, and 15 year survival rates of 62%, 43%, and 30% in our AD/IMH/PAU cohort. We were able to show an increased long-term risk of all-cause death for AD/IMH/PAU patients when compared to the general population even when excluding acute deaths after AD/IMH/PAU diagnosis (adjusted HR 1.8, 95% CI 1.3 – 2.5, p<.001).2 This emphasizes the need for targets to improve outcomes in these patients. Overall, most patients (32%) in our cohort died from an aortic cause, which is in line with the literature.7,27,28 Non-aortic CV-related causes accounted for 29% of all deaths in our series and have been reported to account for 26–32% of deaths in similar cohorts. 7,27,8 However, when looking at non-acute deaths only (> 14 days after AD/IMH/PAU diagnosis), CV causes were more common than aortic-related causes in our cohort. This translated into a 2.6-fold higher risk of CV death for AD/IMH/PAU patients when compared to population referents. While surgical and medical treatment of AD, IMH, and PAU have significantly been improved over the past decades, medical treatment and follow-up of these patients may still not be rigorous enough to prevent aortic-related and CV deaths and thus, improve overall survival.
Our findings strengthen the recommendation for measures to reduce cardiovascular risk in patients with chronic dissection, which has been included in recent clinical practice guidelines.11 This recommendation has been based on the high percentage of late CV deaths among AD patients. In the present study, we were able to quantify the risk of CV death as well as any first-time non-fatal CV event in AD/IMH/PAU patients in relation to the general population. Our results should prompt more attention to the aforementioned guideline recommendation.
Study limitations and strengths
Some aspects have to be considered when interpreting the results of this study. Some of them are inherently associated with the retrospective design of this study as well as with the code-based identification of patients and CV events. However, standardized and consistent methods were used to validate AD/IMH/PAU diagnoses as well as MI, HF and stroke events in patients and referents during the entire study period.
For study inclusion, we used a comprehensive approach, including all identified pathologies meeting the criteria of AD, IMH, and PAU. The majority, but not all cases were acute presentations. Acuity might be difficult to determine in some patients presenting with atypical symptoms. Some patients might be considered asymptomatic but symptoms may have preceded without having led to an emergency consultation or being remembered explicitly. Given our primary aim was to define CV events for patients with the aortic pathologies AD, IMH, and PAU, these patients were not excluded. However, we recognize this approach introduces some heterogeneity into the cohort. Furthermore, AD, IMH and PAU represent a spectrum of diseases considered to have similar risk factors, pathophysiological mechanisms and clinical presentations, but it is apparent that there are important differences between subgroups. As evident in the subtype analyses of the present study, events were mostly associated with AD patients. Only AD was associated with a significantly increased risk of CV death. While IMH and PAU also showed an increased risk of CV death, this was not statistically significant. Similarly, any first-time non-fatal CV event was only significantly associated with AD and first-time HF with AD and IMH. Thus, differences between subtypes have to be considered. However, the subgroups IMH and PAU were relatively small (less than half the size of the AD group) and overall event rates low, which might explain why significance was not reached. The aim of this study was to provide a broad perspective on the CV risk in AD/IMH/PAU patients. Further studies with larger numbers of patients are necessary to identify those subgroups of patients that are more at risk than others.
Due to the retrospective, population-based study design, AD, IMH, and PAU patients were treated by different services and medical management and follow-up were not standardized. It was therefore not feasible to identify specific shortcomings in the medical management of these patients, in particular their long-term blood pressure management. Failure to lower high blood pressure in AD/IMH/PAU patients affects not only aortic-related outcomes but also CV-related endpoints. Further research is needed to determine how medical therapy can be improved in AD, IMH, and PAU patients, with a special focus on blood pressure management.
Our findings are strengthened by several factors. Many reports on late outcomes in AD, IMH, and PAU patients are hospital-based. This may bias results, tending to represent more severe cases referred to specialized centers. Furthermore, patients may be followed elsewhere, resulting in incomplete follow-up. Within the United States, the REP provides unique conditions to conduct population-based research. Because Olmsted County is geographically relatively isolated and all main health care providers in the county are included in the REP, virtually all health care provided to Olmsted County residents is captured. Patients can be followed across providers and death information is registered timely. This may enhance generalizability of our findings to a larger population; although Olmsted County has a predominantly white population, prior REP studies have shown high similarity in age, sex and ethnic characteristics of Olmsted County residents and those of Minnesota and the upper Midwest as well as similar mortality rates for Olmsted County and the United States overall.29
Conclusions
After AD/IMH/PAU diagnosis, patients are at a significantly increased risk of non-aortic CV death and non-fatal incident CV events when compared to the general population. These data highlight the cardiovascular burden in this patient group that should not be underestimated in clinical practice. Our findings emphasize that, due to their increased CV risk, patients should be followed closely after AD, IMH, and PAU diagnosis even in the absence of aortic complications. To improve long-term outcomes for these patients, a focus on rigorous cardiovascular management of all modifiable risk factors is essential.
Supplementary Material
Article Highlights.
Type of research:
Retrospective population-based cohort study
Key findings:
A cohort of 133 patients with aortic dissection, intramural hematoma, and penetrating aortic ulcer (PAU) had a 2- to 3-fold increased risk of non-aortic cardiovascular death, any first-time non-fatal cardiovascular event and first-time heart failure when compared to population referents. (adjusted HR 2.4, 3.0, 2.7, respectively).
Take home message:
Patients with aortic dissection, intramural hematoma, and PAU need for rigorous long-term cardiovascular management.
Acknowledgments
Sources of funding: This study was supported by the American Heart Association (16SDG27250043). It was conducted using the resources of the Rochester Epidemiology Project, which is supported by the National Institutes of Health National Institute on Aging under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Data storage was performed with REDCap (UL1TR002377).
Presentation: This study was presented as a poster on April 7, 2018 at the American Heart Association QCOR 2018 Scientific Sessions, Arlington, Virginia, April 6–7, 2018.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation. 2005;112(24):3802–13. [DOI] [PubMed] [Google Scholar]
- 2.DeMartino RR, Sen I, Huang Y, Bower TC, Oderich GS, Pochettino A, et al. A Population-Based Assessment of the Incidence of Aortic Dissection, Intramural Hematoma and Penetrating Ulcer, and Its Associated Mortality from 1995 to 2015. Circ Cardiovasc Qual Outcomes. 2018;11:e004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66(4):350–8. [DOI] [PubMed] [Google Scholar]
- 4.Evangelista A, Mukherjee D, Mehta RH, O’Gara PT, Fattori R, Cooper JV, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. 2005;111(8):1063–70. [DOI] [PubMed] [Google Scholar]
- 5.Cho KR, Stanson AW, Potter DD, Cherry KJ, Schaff HV, Sundt TM 3rd. Penetrating atherosclerotic ulcer of the descending thoracic aorta and arch. J Thorac Cardiovasc Surg. 2004;127(5):1393–9. [DOI] [PubMed] [Google Scholar]
- 6.Evangelista A, Czerny M, Nienaber C, Schepens M, Rousseau H, Cao P, et al. Interdisciplinary expert consensus on management of type B intramural haematoma and penetrating aortic ulcer. Eur J Cardiothorac Surg. 2015;47(2):209–17. [DOI] [PubMed] [Google Scholar]
- 7.Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population- based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114(24):2611–8. [DOI] [PubMed] [Google Scholar]
- 8.Stevens LM, Madsen JC, Isselbacher EM, Khairy P, MacGillivray TE, Hilgenberg AD, et al. Surgical management and long-term outcomes for acute ascending aortic dissection. J Thorac Cardiovasc Surg. 2009;138(6):1349–57 el. [DOI] [PubMed] [Google Scholar]
- 9.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riambau V, Bockler D, Brunkwall J, Cao P, Chiesa R, Coppi G, et al. Editor’s Choice - Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53(1):4–52. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain AM, Gersh BJ, Alonso A, Chen LY, Berardi C, Manemann SM, et al. Decade-long trends in atrial fibrillation incidence and survival: a community study. Am J Med. 2015;128(3):260–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roger VL, Killian JM, Weston SA, Jaffe AS, Kors J, Santrach PJ, et al. Redefinition of myocardial infarction: prospective evaluation in the community. Circulation. 2006;114(8):790–7. [DOI] [PubMed] [Google Scholar]
- 15.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–6. [DOI] [PubMed] [Google Scholar]
- 16.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175(6):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roger VL, Weston SA, Redfield MA, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA-J Am Med Assoc. 2004;292(3):344–50. [DOI] [PubMed] [Google Scholar]
- 18.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, et al. Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol. 2004;43(4):665–9. [DOI] [PubMed] [Google Scholar]
- 20.Tolenaar JL, Harris KM, Upchurch GR Jr., Evangelista A, Moll FL, di Eusanio M, et al. The differences and similarities between intramural hematoma of the descending aorta and acute type B dissection. J Vasc Surg. 2013;58(6):1498–504. [DOI] [PubMed] [Google Scholar]
- 21.Yang B, Patel HJ, Sorek C, Hornsby WE, Wu X, Ward S, et al. Sixteen-Year Experience of David and Bentall Procedures in Acute Type A Aortic Dissection. Ann Thorac Surg. 2018;105(3):779–84. [DOI] [PubMed] [Google Scholar]
- 22.Jex RK, Schaff HV, Piehler JM, King RM, Orszulak TA, Danielson GK, et al. Early and late results following repair of dissections of the descending thoracic aorta. J Vasc Surg. 1986;3(2):226–37. [DOI] [PubMed] [Google Scholar]
- 23.Chou AS, Ziganshin BA, Charilaou P, Tranquilli M, Rizzo JA, Elefteriades JA. Longterm behavior of aortic intramural hematomas and penetrating ulcers. J Thorac Cardiovasc Surg. 2016;151(2):361–72, 73 e1. [DOI] [PubMed] [Google Scholar]
- 24.Nathan DP, Boonn W, Lai E, Wang GJ, Desai N, Woo EY, et al. Presentation, complications, and natural history of penetrating atherosclerotic ulcer disease. J Vasc Surg. 2012;55(1):10–5. [DOI] [PubMed] [Google Scholar]
- 25.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996–1002. [DOI] [PubMed] [Google Scholar]
- 26.Januzzi JL, Eagle KA, Cooper JV, Fang J, Sechtem U, Myrmel T, et al. Acute aortic dissection presenting with congestive heart failure: results from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2005;46(4):733–5. [DOI] [PubMed] [Google Scholar]
- 27.Umana JP, Lai DT, Mitchell RS, Moore KA, Rodriguez F, Robbins RC, et al. Is medical therapy still the optimal treatment strategy for patients with acute type B aortic dissections? J Thorac Cardiovasc Surg. 2002;124(5):896–910. [DOI] [PubMed] [Google Scholar]
- 28.Yu HY, Chen YS, Huang SC, Wang SS, Lin FY. Late outcome of patients with aortic dissection: study of a national database. Eur J Cardiothorac Surg. 2004;25(5):683–90. [DOI] [PubMed] [Google Scholar]
- 29.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.