Summary.
The ArfGAPs are a family of proteins containing an ArfGAP catalytic domain that induces the hydrolysis of GTP bound to the small guanine nucleotide binding-protein ADP-ribosylation factor (Arf). Functional models for Arfs, which are regulators of membrane traffic, are based on the idea that guanine nucleotide-binding proteins function as switches: Arf with GTP bound is active and binds to effector proteins; the conversion of GTP to GDP inactivates Arf. The cellular activities of ArfGAPs have been examined primarily as regulatory proteins that inactivate Arf; however, Arf function in membrane traffic does not strictly adhere to the concept of a simple switch, adding complexity to models explaining the role of ArfGAPs. Here, we review the literature addressing the function Arf and ArfGAP1 in COPI mediated transport, focusing on two critical and integrated functions of membrane traffic, cargo sorting and vesicle coat polymerization. We briefly discuss other ArfGAPs that may have similar function in Arf-dependent membrane traffic outside the ER-Golgi.
Keywords: ArfGAP1, Arf1, Golgi apparatus, Retrograde traffic, Coatomer, COPI
Introduction
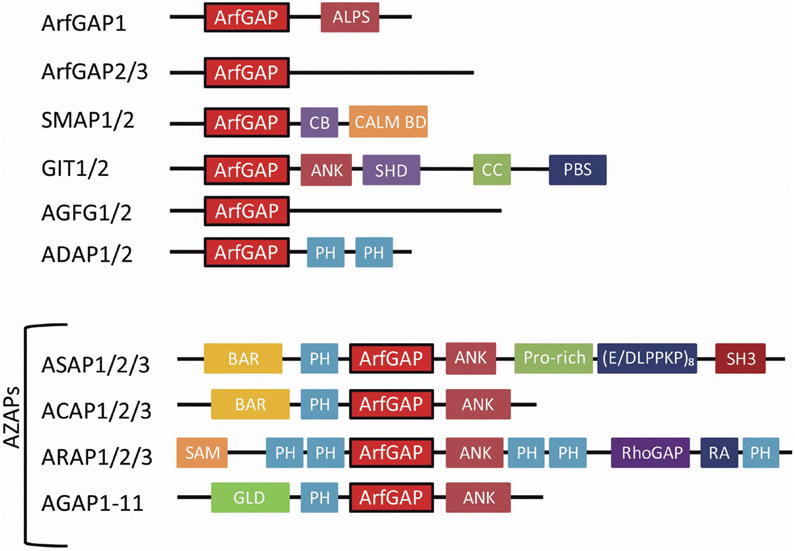
The human genome encodes 31 proteins containing ArfGAP domains, which are comprised of a zinc binding motif conforming to the consensus CX2CX16CX2CX4R (Cukierman el al., 1995; Kahn et al., 2008). The proteins are otherwise structurally diverse. The ArfGAPs have been divided into 10 subfamilies, based on domain structure and on the similarity of the ArfGAP domains (Fig. 1). We will focus on ArfGAP1, which functions with Arf1 to regulate membrane traffic between the Golgi and endoplasmic reticulum.
Fig. 1.
Schematic of domain structure of human ArfGAPs. Not drawn to scale. Abbreviations are; ArfGAP, ArfGAP domain; ALPS, ArfGAP1 lipid-packing sensor; CB, clathrin-box; CALM BD, calm binding domain; ANK, ankyrin repeat; SHD, Spa-homology domain; CC. coiled-coil; PBS, Paxillin binding site; PH, Preckstrin homology; BAR, Bin/Amphiphysin/Rvs; Prorich, Prorin-rich domain; (E/DLPPKP)8, 8 times repeat of E/DLPPKP sequence; SH3, Src homology 3; SAM, sterile α-motif; RhoGAP, Rho GAP domain; RA, Ras association motif; GLD, GTP-binding protein-like domain.
Arf1 is a member of the Arf family of guanine nucleotide binding-proteins, the most ancient group within the Ras superfamily (Kahn et al., 2006). There are 6 known mammalian Arfs, 5 in humans, that fall into three classes based on sequence homology: Arf1,2 and 3 are class I (humans do not have Arf2); Arf4 and Arf5 are class II and Arf6 is class III. Arf1 and Arf6 are the most extensively studied. Arfs regulate actin remodeling and membrane trafficking. Function requires Arf to switch between two forms, Arf•GTP and Arf•GDP1. The conversion between the two forms is catalyzed by regulatory proteins (Jackson and Casanova, 2000; Nie and Randazzo, 2006; Souza-Schorey and Chavrier, 2006; Casanova, 2007; Gillingham and Munro, 2007; Donaldson and Jackson, 2011). Arf•GDP binds with relative low affinity to membranes, and is considered to be soluble (Kahn et al., 1992; Franco et al., 1993; Antonny et al., 1997), i.e. primarily in the cytosol. It is converted to the GTP bound state by guanine nucleotide exchange factors (GEFs) (Jackson and Casanova, 2000; Casanova, 2007). Arf•GTP binds to proteins such as coat proteins or enzymes and binds tightly to lipid membranes (Donaldson et al., 1992; Kahn et al., 1992; Palmer et al., 1993; Randazzo et al., 1995; Zhao et al., 1997; Sun et al., 2007; Donaldson and Jackson, 2000). Arf•GTP is converted to Arf•GDP by hydrolysis of GTP, which is catalyzed by GTPase-activating proteins (GAPs) (Nie and Randazzo, 2006; Souza-Schorey and Chavrier, 2006; Gillingham and Munro, 2007; Inoue and Randazzo, 2007). The role of Arf1 in ER-Golgi membrane traffic is discussed to describe the models into which ArfGAPs were incorporated.
The role of Arf and GTP hydrolysis in membrane traffic
Function of Arf in COPI-dependent traffic – Arf•GTP as a bridge between the membrane and coatomer
The proteins involved in transport from the Golgi apparatus were identified with an in vitro assay (Balch et al., 1984a,b; Rothman et al., 1984; Balch and Rothman, 1985) together with genetic studies in yeast (Stearns et al., 1990; Bednarek et al., 1995). Transport of Golgi enzymes, ER chaperones or other cargos between Golgi stacks or ER and Golgi apparatus is mediated by protein-coated vesicles. The protein coat is called COPI, which is formed from the polymerization of a heteroheptomeric protomer called coatomer (Serafini et al., 1991; Waters et al., 1991; Rabouille and Klumperman, 2005; Hsu et al., 2009). Nonhydrolyzable analogs of GTP were found to be inhibitors of intra-Golgi transport; Arf was identified as the GTP-binding protein that was responsible for the GTPγS dependent inhibition (Melancon et al., 1987; Serafini et al., 1991). Incubating isolated Golgi membranes with GTPγS or [Q71L]Arf1, which is unable to hydrolyze GTP on Arf1, thereby locking Arf1 in the GTP-bound form, was found to cause an accumulation of coated COPI vesicles (Melancon et al., 1987; Tanigawa et al., 1993). The recruitment of coatomer to membranes enriched in the Golgi is increased by Arf•GTP or Arf•GTPγS (Donaldson et al., 1992; Palmer et al., 1993). Arf was a component of COPI vesicles formed in the presence of GTPγS (Serafini et al., 1991).
Three properties of Arf1 relevant to membrane traffic were identified. First, Arf1•GTP binds to membranes much more tightly than does Arf1•GDP (Kahn et al., 1992; Franco et al., 1993; Randazzo et al., 1995). The second property is that Arf1•GTP binds coatomer more tightly than does Arf1•GDP (Sun et al., 2007). Third, Arf1•GTP can bind membranes and coatomer simultaneously (Sun et al., 2007; Beck et al., 2009b). The prevailing paradigm based on these properties is that Arf functions as a component of COPI (Rothman, 2002; Nie and Randazzo, 2006; Beck et al., 2009b). In the model, Arf exchanges nucleotide to form Arf•GTP, which binds tightly to membranes and to coatomer, thereby recruiting coatomer to the Golgi apparatus. Once recruited to the membrane surface, coatomer binds to proteins that are to be transported, called cargos, and polymerizes into COPI to make COPI coated vesicles. In this model, GTP hydrolysis on Arf occurs after the formation of COPI coated vesicles to trigger coatomer uncoating from the vesicles, which is necessary for consumption of the vesicles by the acceptor compartment. Because Arfs have no intrinsic GTPase activity, the model predicts that the function of ArfGAPs is to trigger uncoating. These studies established the role of Arf in recruitment of coatomer on membranes and importance of Arf in vesicular transport, a milestone in membrane trafficking.
Adjustment of model to accommodate function of GTP hydrolysis in cargo sorting
In initial work, the sole role of GTP hydrolysis on Arf was thought to be to trigger uncoating. However, subsequent work supported the idea that GTP hydrolysis regulates cargo sorting. COPI coated vesicles generated in vitro from isolated Golgi in the presence of GTPγS, a slowly hydrolyzable analogue of GTP, or GTP-locked mutant of Arf1, Arf1Q71L, contained lower amounts of cargos than those generated in the presence of GTP, suggesting GTP hydrolysis is required for packaging cargos into vesicles (Nickel et al., 1998; Lanoix et al., 1998, 1999, 2001). In cells, cargos typically transported by COPI are absent from the COPI vesicles induced by microinjection of GTPγS or ectopic expression of Arf1Q71L, while in control cells, 30-60% of the COPI cargos examined colocalized with COPI vesicle marker, β-COP (Pepperkok et al., 2000). These results support the idea that GTP hydrolysis is required for cargo sorting. Since cargo sorting is considered to be coupled with coatomer polymerization which induce vesicle budding, i.e. occurs prior to vesicle budding, GTP hydrolysis should also occur prior to vesicle formation.
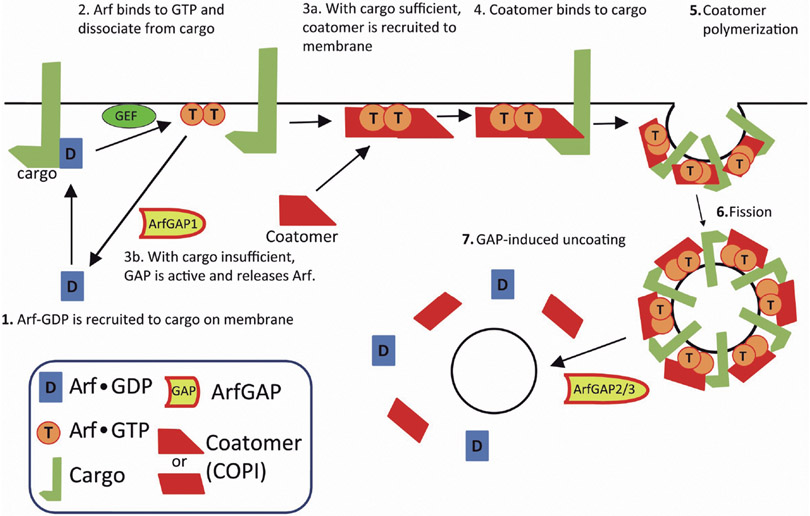
The model for the role of Arf in COPI vesicle formation was modified to explain how GTP hydrolysis could be necessary for sorting and for triggering coat dissociation. In the modified model, GTP hydrolysis is necessary at two sites. First, rapid GTP hydrolysis at sites of low cargo density prevent accumulation of Arf1•GTP and coatomer, ensuring that COPI vesicles do not form. GTP hydrolysis by Arf is slow on a membrane containing cargo. In the presence of cargo, Arf1•GTP recruits coatomer, which reaches sufficient density to drive vesicle formation. Arf1•GTP is subsequently converted to Arf•GDP on transport vesicles to trigger dissociation of COPI (Lanoix et al., 2001; Weiss and Nilsson, 2003; Beck et al., 2009b). This model predicts two distinct sites of GTP hydrolysis by ArfGAPs: (i) on a flat membrane devoid of cargo to cause the dissociation of coatomer and, consequently, prevent the polymerization into COPI when cargo is not present and; (ii) on a vesicle containing cargo to trigger uncoating.
Integration of ArfGAPs into paradigms for Arf- and COPI-dependent traffic
Several mechansims of ArfGAP regulation have been proposed to accommodate the two distinct functions of GTP hydrolysis on Arf in the prevailing model of COPI vesicle dynamics.
Models in which ArfGAPs causes coat dissociation at two sites: (i) prior to vesicle formation to prevent vesicle formation and (ii) after vesicle formation to trigger vesicle uncoating.
ArfGAP1, the first GAP for Arfs identified, was purified from rat liver extracts and the cDNA cloned from a rat library (Makler et al., 1995; Cukierman et al., 1995). The catalytic domain, located at the extreme N-terminus of the protein was found to be comprised of a zinc-finger motif (CX2CX16CX2CX4R), which defines the ArfGAP family (Kahn et al., 2008; Cukierman et al., 1995). ArfGAP1 localizes at the Golgi apparatus (Cukierman et al., 1995) and, in subsequent work, ArfGAP1 was found to bind to the KDEL receptor, a cargo of COPI coated vesicles (Aoe et al., 1997, 1999; Huber et al., 1998). The first published model for the regulation of ArfGAP1 was based on the finding that a peptide from p24, another cargo for COPI, inhibited GAP catalytic activity of ArfGAP1 (Goldberg, 2000; Lanoix et al., 2001). In the model, in the absence of cargo ArfGAP1 rapidly induces the hydrolysis of GTP on Arf1, thereby preventing the formation of an empty vesicle. If cargo is present, GTP hydrolysis is delayed allowing retention of coatomer on the membrane for sufficient time for polymerization and formation of the cargo laden vesicle. Thus, this model accounted for the coupling of cargo selection with coat polymerization.
This model was modified with the identification of ArfGAP2 and ArfGAP3 (Weimer et al., 2008; Beck et al., 2009b). Coatomer bound to and stimulated GAP activity of both ArfGAP2 and ArfGAP3. ArfGAP1 also had been reported to bind to coatomer (Lee et al., 2005; Liu et al., 2005); however, consequences of the interaction in regulation of GAP activity on ArfGAP1 were not clear. At the time, one group reported that coatomer stimulated activity of ArfGAP1 in solution (Goldberg, 2000) and another group found that the association of ArfGAP1 with the Golgi apparatus is in part dependent on coatomer (Liu et al., 2005) but there was also a report that coatomer had little or no effect on the activity of ArfGAP1 in the presence of isolated Golgi (Szafer et al., 2000). The results with ArfGAP2 and ArfGAP3 were clearer with three groups independently reporting that coatomer directly bound and stimulated the catalytic activity (Weimer et al., 2008; Kliouchnikov et al., 2009; Luo et al., 2009). Therefore, the model incorporating a role of ArfGAP2 and ArfGAP3 included two elements; i) the inhibition of GAP activity on ArfGAP1 by cargo and ii) the interaction of coatomer with ArfGAP2/3 to stimulate their GAP activities (Beck et al., 2009b, 2011) ArfGAP1 is active without cargo present, and functions to prevent empty vesicle formation. ArfGAP2 and 3, on the other hand, are incorporated into vesicles where they induce GTP hydrolysis and trigger coatomer uncoating. Indeed, ArfGAP1, 2, and 3 promoted dissociation of coatomer from large unilamellar vesicles in vitro (Bigay et al., 2003; Weimer et al., 2008). This model accounts for sorting and uncoating by having ArfGAP1 and ArfGAP2/3 function at distinct, independent sites (Fig. 2).
Fig. 2.
The model of COPI vesicle formation in which GTP hydrolysis occurs in two distinct sites. The image was modified from Beck et al. (2009). GAP is active in two distinct sites; step 3b and 7. For details, see text.
ArfGAP1 as a curvature sensor
In an in vitro experiment, ArfGAP1 was observed to associate preferentially with membranes of low head group packing, which can be achieved by increased membrane curvature (Bigay et al., 2003, 2005; Mesmin et al., 2007). In the model based on this observation, ArfGAP1, through two ArfG AP lipid packing sensor motifs (ALPS), is recruited to the forming vesicle where the lipid head groups are less tightly packed than on the flat lipid surface. The preferential recruitment restricts activity to curved membranes where coatomer has polymerized into COPI and, consequently, deformed the membrane. This model does not explain sorting and is at odds with the previously described model in which ArfGAP1 functions on a flat surface to prevent accumulation of coatomer and its polymerization when cargo is not present.
Reexamination of the in vitro biochemistry of ArfGAPs supports an alternate model for cargo sorting
Recent enzymologic analysis of full length ArfGAP1 and ArfGAP2 with phospholipids revealed that both GAP activities are stimulated by coatomer, as previously described for ArfGAP1 (Goldberg, 1999), and by peptides from cargos (Luo and Randazzo, 2008; Luo et al., 2009). Coatomer decreased the Km of ArfGAP1/2 for the substrate Arf1•GTP and the peptide from cargo tail increased the kcat of the GAP reaction. The previously reported inhibition of ArfGAP1 by a peptide from p24 may have been a nonspecific effect. The concentration of peptide required for the inhibition was greater than 100 μM, and ASAP1, an ArfGAP not involved in COPI vesicles, was also inhibited by p24 with a similar concentration dependence. Furthermore, the inhibition was independent of coatomer (Luo and Randazzo, 2008. In contrast, peptides from p23 and p25, not p24, were found to stimulate ArfGAP1 and ArfGAP2 catalytic activity (Luo et al., 2009). The effect was observed with 1 μM peptide and was dependent on coatomer. The peptides at this concentration had no effect on ASAP1. The combined effects of coatomer and peptide were greater than the effects of curvature on GAP catalytic activity, based on direct comparisons in these studies. These results are consistent with the possibility that GTP hydrolysis stimulated by cargo and coatomer could directly function in cargo sorting prior to vesicle budding. Functions of ArfGAP1 in COPI vesicle formation has been described using mammalian proteins and Golgi membrane isolated from mammalian cells. In an in vitro vesicle formation assay, both ArfGAP1 and GTP hydrolysis were reported to be required for vesicle formation (Yang et al., 2002; Lee et al., 2005; Hsu et al.,2009). Isolated Golgi membranes were incubated with coatomer and GTP. COPI vesicles did not form unless ArfGAP1 was added to the incubation. Formation of vesicles was blocked by the addition of the slowly hydrolyzed GTP analog GTPγS in place of GTP. The vesicles contained ArfGAP1, leading to the proposal that ArfGAP1 is a component of COPI (Yang et al., 2002); however, another group reported that ArfGAP1 is not in the vesicle and, instead, prevented the formation of vesicles (Beck et al., 2009a).
We found that ArfGAP1 and ArfGAP2 contribute to the deformation of large unilamellar vesicles (LUVs), consistent with a role in promoting vesicle formation (Shiba et al., 2011). In these experiments, the effects of Arf1•GTP, coatomer and ArfGAP1 or ArfGAP2 on the shape of LUVs containing a peptide from p25 cargo protein was examined. None of the proteins affected the LUVs by themselves, but a combination of Arf1, coatomer and ArfGAP1 (or ArfGAP2) induced extensive tubulation and vesiculation of the LUVs. The results suggest that ArfGAPs facilitated the polymerization of coatomer. Previously, cargo, Arf1•GTP and coatomer were reported to be sufficient in vesicle formation in vitro (Bremser et al., 1999). However, in those studies, the LUVs contained either high concentrations of negatively charged phospholipids or dioleoyl phospholipids, both favoring bilayer deformation. Further support for the idea that ArfGAPs contribute to coated vesicle formation came from experiments in which soluble coatomer was incubated with p25 cargo peptide and either ArfGAP1 or ArfGAP2. The ArfGAPs facilitated coatomer polymerization into spherical structures (Shiba et al., 2011). These results are consistent with the model of ArfGAP1 promoting vesicle formation by inducing coat polymerization in addition to, or in concert with, its role in cargo sorting. Future studies are required to see if ArfGAP3 behaves as the same way as ArfGAP1/2.
Functional analysis of ArfGAPs in vivo support the idea that ArfGAPs promote Arf-dependent membrane traffic
A number of in vivo studies support the concept that ArfGAPs promote vesicle formation. A genetic screen in yeast identified ArfGAPs as suppressors of Arf insufficiency (Zhang et al., 1998, 2003). To function as suppressors, the ArfGAPs must, in some way, either mediate or promote Arf action. If ArfGAPs function to cause COPI dissociation, it is unlikely that overexpressing ArfGAPs would reverse the effect of Arf insufficiency, which also results in diminished coatomer recruitment to membranes.
The redundant function of ArfGAP1, 2, and 3 in mammalian cells is consistent with the idea that the three ArfGAPs have a common function in vesicle formation. In mammalian cells, the combined knockdown of ArfGAP1, ArfGAP2 and ArfGAP3 was required for a phenotype and expression of any one of these ArfGAPs corrected the phenotype of reduced expression of all three (Saitoh et al., 2009). In the triple knock down cells with reduced expression of ArfGAP1, 2 and 3, the Golgi and ER-Golgi intermediate compartments appear to collapse into a vacuolar structure, in which anterograde and retrograde transport cargos accumulated. The phenotype of ArfGAPs knock down cells is similar to that of coatomer subunit β-COP knock down cells, supporting the idea that the three ArfGAPs regulate COPI mediated transport.
Results in experiments studying yeast also support the idea that the ArfGAPs of this class function redundantly. S. cerevisiae contain two proteins, Gcs1 and Glo3, considered to be orthologs of ArfGAP1 and ArfGAP2/3. Like ArfGAP1, 2 and 3, Gcs1 and Glo3 have overlapping function in COPI mediated transport (Poon et al., 1996, 1999, 2001). Loss of one these genes does not have a detectable effect on cells whereas mutant cells deficient for both Gcs1 and Glo3 cannot proliferate, and are defective in ER-Golgi vesicular transport. Thus, Gcs1 and Glo3 have redundant function (Schindler et al., 2009), consistent with a common function.
Alternate explanation for the effect of ArfGAP1 overexpression
The Golgi disruption in cells overexpressing ArfGAP1 has been considered to be the evidence that ArfGAP1 prevents vesicle formation by hydrolyzing GTP on Arf (Huber et al., 1998). The Golgi disruption is similar in cells overexpressing dominant-negative Arf1(T31N) or BFA treated cells, in which ArfGEF activity is inhibited (Lippincott-Schwartz et al., 1989; Dascher and Balch, 1994). In these cells, coatomer is released from the Golgi apparatus (Donaldson et al., 1990). We re-examined the effect of ArfGAP1 overexpression by electron microscopy (Shiba et al., 2011). We found that in ArfGAP1 overexpressiong cells, the Golgi was disrupted and numerous vesicles had accumulated. The vesicle accumulation was different than tubular-vesicular structures seen in BFA treated cells. This raises the possibility that the Golgi disruption in ArfGAP1 overexpressing cells is caused by the acceleration of vesicle formation, which is not seen in BFA treated cells. Although coatomer is known to be released from Golgi membrane in cells overexpressing ArfGAP1 as well as in cells treated with BFA (Aoe et al., 1997), the release of coatomer in ArfGAP1 overexpressing cells might be caused by the rapid uncoating of COPI vesicles. In cells overexpressing ArfGAP1, anterograde transport is inhibited (Aoe et al., 1997). However, if retrograde transport is accelerated in these cells, the anterograde cargo might be transported back to the ER.
Retrograde transport was reexamined using a model cargo, Shiga toxin subunit B fused to KDEL (STxB-KDEL), which depends on coatomer for retrograde transport to the ER (Shiba et al., 2011). Overexpression of either the Arf exchange factor or ArfGAP1 led to fragmentation of the Golgi, but did not disrupt Golgi-to-ER trafficking of STxB-KDEL. Thus, overexpression of ArfGAP1 did not prevent COPI vesicle traffic. An explanation for the fragmented but partially functional Golgi is that the Golgi was not properly positioned because of an imbalance between Golgi formation and retrograde trafficking due to acceleration of the latter.
ArfGAP1 has GAP activity dependent and independent function
The effects of ArfGAP1 mutants lacking GAP activity on STxB-KDEL trafficking were also examined (Shiba et al., 2011). Two mutants were examined. [R50K]ArfGAP1 is still able to bind to Arf•GTP, but does not induce GTP hydrolysis. [CC22,25SS]ArfGAP1, with a poorly folded GAP domain, is unable to bind to Arf•GTP and induce GTP hydrolysis.
Expression of [R50K]ArfGAP1 disrupted the Golgi apparatus, although less efficiently than did wild type ArfGAP1, and supported retrograde traffic of STxB-KDEL. Although there was a report that [R50K] ArfGAP1 did not support the vesicle formation in vitro (Lee et al., 2005), our results indicate that [R50K]ArfGAP1 still supports vesicle formation at least in part, because STxB-KDEL reached the ER. However, some STxB-KDEL remained in disrupted Golgi in cells expressing [R50K]ArfGAP1, raising the possibility that [R50K]ArfGAP1 is able to support the formation of vesicles but did not efficiently sort cargo into the vesicles, consistent with the reported function of GTP hydrolysis in cargo sorting. We predicted an uncoating defect in cells expressing [R50K]ArfGAP1, since GTP hydrolysis is a pre-requisite for uncoating (Melancon et al., 1987; Tanigawa et al., 1993; East and Kahn, 2011) but a fusion defect was not apparent. Although the conclusion that STxB-KDEL reached the ER needs to be confirmed by biochemical analysis (requiring cells that efficiently overexpress [R50K]ArfGAP1), one possibility is that endogenous ArfGAP1 could mediate GTP hydrolysis in these cells. Additional in vitro studies are required to determine if wild type ArfGAP1 has higher affinity for Arf•GTP than does [R50K]ArfGAP1.
In contrast to [R50K]ArfGAP1, [CC22,25SS] ArfGAP1 did not fragment the Golgi, but did block retrograde transport of STxB-KDEL, with which it colocalized at the Golgi (Shiba et al., 2011). The difference in phenotype between [R50K] and [CC22,25SS]ArfGAP1 is consistent with the idea that the GAP domain has roles in addition to GAP activity, such as Arf•TP binding per se or coatomer binding. Given that non-catalytic region of ArfGAP1 has also been reported to bind to KDEL-receptor (Aoe et al., 1999), a plausible explanation for these results is that ArfGAP1 normally delivers KDEL-receptor to the transport vesicle and [CC22,25SS]ArfGAP1, unable to deliver the receptor to a transport vesicle, traps the cargo with KDEL-receptor in the Golgi stack. This idea will be further tested using ArfGAP1 mutants, which are either defective in Arf or coatomer binding.
Yeast ArfGAPs and SNAREs
A role of ArfGAP in yeast COPI vesicle formation has also been proposed. Glo3 binds directly to the ER-Golgi SNAREs Sed5p and Bos1p (Schindler et al., 2009). Binding induces the conformational change in Sed5p and Bos1p that facilitates association with Arf and coatomer (Rein et al., 2002). This result supports the idea that Glo3 mediates recruitment of Arf to SNARE proteins, an initial step in vesicle formation. Glo3 is also reported as a component of COPI coated vesicle, but not Gcs1 (Lewis et al., 2004). The specific mechanism remains to be determined. Questions raised by these results include: (i) why is Glo3 not found in the binding complex of SNARE, Arf and coatomer if Glo3 binds SNARES and; (ii) why are key biochemical properties of Gcs1 and Glo3 different if Gcs1 can replace the biologic function of Glo3 in genetic screens? Note, mammalian ArfGAP1 has been found to interact with ER-Golgi SNARE protein, GS15, Syntaxin5, and Ykt6, which would be a similarity with Glo3, the ArfGAP2/3 homolog (Lee et al., 2005).
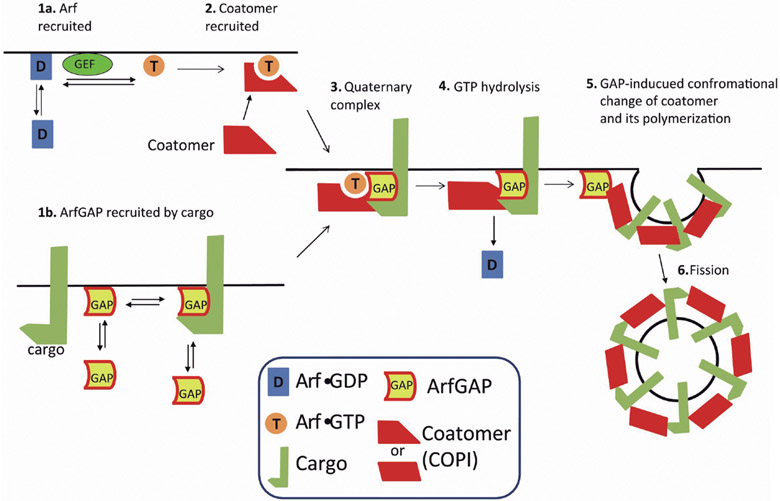
Model with function of ArfGAP1 at single step that integrates cargo sorting with coat polymerization
To accommodate the stimulation of GAP activity by cargo and coat, the role of GTP hydrolysis in cargo sorting and the role of ArfGAP1 in COPI vesicle formation in cells, a proofreading model of ArfGAP1 function in COPI vesicle formation (Nie and Randazzo, 2006) has been proposed. In this model, ArfGAP1 promotes COPI vesicle formation and directly affects cargo sorting. The proofreading model has three important points (Fig. 3). First, two distinct initiation complexes, Arf1-coatomer and ArfGAP1-cargo, combine to form the protein complex that polymerizes into coat. Thus, one function of ArfGAP1 is to deliver cargo proteins to the Arf1-coatomer complex, thereby having a direct role in sorting. Second, efficient incorporation of cargo into coat depends on GTP hydrolysis on Arf1, which is induced by cargo-dependent ArfGAP activity. This is supported by in vitro data that GAP activity of ArfGAP1 and 2 was increased by cargo and coatomer (Luo et al., 2009), and the fact that GTP hydrolysis is required for cargo incorporation into coated vesicles (Lanoix et al., 1998, 1999, 2001; Nickel et al., 1998; Pepperkok et al., 2000). Third, ArfGAP1 contributes to the cargo-dependent conformational changes in coat protein leading to coat polymerization and formation of transport vesicles (Shiba et al., 2011).
Fig. 3.
The model of COPI vesicle formation in which GTP hydrolysis occurs at single site. GAP is active at single site, step 4. For details, see text.
ArfGAP1 and AP-1
Results of experiments examining the relationship of ArfGAP1 and AP-1 reveal some mechanistic similarities with the proposed model for ArfGAP1 function with coatomer. In addition, the results support the idea that cargo-induced coat polymerization could be a mechanism by which coatomer persists on membrane independent of Arf. Clathrin/AP-1, which is recruited to membrane by Arf and mediates post-Golgi membrane traffic, polymerizes on artificial membranes in the presence of cargo for AP-1 (Meyer et al., 2005). GAP activity of the catalytic domain of ArfGAP1 is increased by AP-1 and cargo, and AP-1 persists on membrane after addition of ArfGAP1 in the presence of cargo. Cargo has also been reported to increase the affinity of AP-1 for isolated TGN membranes. Two binding sites for AP-1 on the TGN have been described: A high affinity, Arf-dependent site and a low affinity site that is occupied after GTP hydrolysis and subsequent dissociation of Arf from the membrane. One interpretation of these combined results is that cargo affects polymerization of AP-1, which stabilizes AP-1 on membrane after GTP hydrolysis and dissociation of Arf (Zhu et al., 1998, 1999a,b). It is possible that a similar mechanism exist for retaining COPI on membranes after Arf dissociates.
Other ArfGAPs that bind to coat proteins
SMAPs and clathrin coats
Two ArfGAPs, SMAP1 and SMAP2, bind directly to clathrin through a clathrin box motif and affect post Golgi traffic. SMAP1 functions at the plasma membrane. Reduced expression blocks transferrin uptake (Tanabe et al., 2005; Kon et al., 2008). Inhibition was independent of GAP activity. Overexpression of either the wild type or GAP-deficient mutant inhibited endocytosis; however, differences in phenotype led the authors to propose that the wild type and mutant SMAP1 inhibit the endocytosis by different mechanisms. Clathrin binding seems to be critical because a clathrin binding deficient mutant did not block transferrin uptake. SMAP1 is presumed to be an Arf6 GAP because SMAP1 had GAP activity toward Arf6 in vitro; however, SMAP1 did not affect two Arf6 dependent events, endocytosis of CD25 and Arf6-induced actin protrusion formation.
SMAP2 associates with endosomes and the TGN (Natsume et al., 2006; Funaki et al., 2011) and colocalizes with CALM, TGN46/38 and the clathrin adaptor AP-1 and EpsinR but not GGA1. SMAP2 binds to the clathrin assembly protein CALM in addition to clathrin and its overexpression inhibits retrograde transport of TGN38 from endosome to TGN dependent on clathrin binding motif. Preliminary results indicate that Shiga Toxin retrograde transport is upregulated by reduced expression of SMAP2 (Shiba et al., 2010), suggesting that SMAP2 may have inhibitory role in retrograde transport. The anterograde transport of VSV-G to plasma membrane was faster in cells from SMAP2 knock out mice than controls, and SMAP2 overexpression inhibited VSV-G transport dependent on its phospholipid binding motif, suggesting that SMAP2 has also inhibitory role in anterograde transport from the Golgi to PM. These results are compelling support for the hypothesis that SMAP1 and SMAP2 function by directly binding to clathrin to regulate clathrin dependent membrane traffic. The role of SMAP-induced GTP hydrolysis is still not clear. The careful characterization of the effects of SMAPs provides a sound basis for discovering the molecular mechanisms by which Arf and SMAPs control the formation of vesicles and sorting of cargo.
AGAPs
AGAPs are encoded by 11 human genes, which may include pseudogenes (Kahn et al., 2008). AGAP1 and AGAP2 have been the most extensively studied. AGAP1 binds to clathrin adaptor AP-3 (Nie et al., 2003) and AGAP2 binds to AP-1 (Nie et al., 2005). AGAP1 also binds to M5 muscarinic receptors and makes a complex with neuron specific β subunit of AP-3 (Bendor et al., 2010). Reduced expression of AGAP1 or expression of an M5 receptor mutant which is deficient of binding to AGAP1, results in reduced expression of M5 receptor on the cell surface. The elimination of AP-3 or mutant M5 receptor decreased dopamine release potentiation mediated by M5 receptor. These results support the idea that AGAP1 supports AP-3 function. AGAP1 has GAP activity towards Arf1 and Arf5, but a role for the GAP activity remains unexplored.
The role of AGAP2 in endosome function has been studied (Shiba et al., 2010). AGAP2 was found to affect endosome to TGN traffic of Shiga toxin, cholera toxin, cation independent mannose-6-phosphate receptor and TGN46. Shiga toxin traffic, which is AP-1 independent, was examined in more detail. AGAP2 depletion trapped STxB in a very early endosome compartment, colocalizing with clathrin, transferrin and Rab4, but not Rab11 or retromer. STxB trapped in enlarged endosomes with clathrin in AGAP2 knock down cells is consistent with the idea that AGAP2 functions with clathrin. AGAP2 has GAP activity for Arf1 and Arf5. AP-1 binding was found to inhibit GAP activity to a small extent (Nie et al., 2005). The consequences of GAP activity and the role of AP-1 binding for cargo sorting and vesicle formation have not been examined.
ACAPs
ACAP1 and 2 use Arf6 in preference to Arf1 and Arf5 as substrates (Jackson et al., 2000). ACAPs have been proposed to function as a component of clathrin coats that bind to cargos. ACAP1 binds to clathrin (Li et al., 2007), and variety of recycling proteins, such as transferrin receptor, cellubrevin (Dai et al., 2004), β1 integrin (Li et al., 2005) and Glut4 (Li et al., 2007). ACAP1 has been found to affect both constitutive and regulated recycling pathway. Reduction of ACAP1 expression slowed transferrin receptor recycling (Dai et al., 2004). The ACAP1 binding to β1 integrin was found to require stimulation by serum. Reducing ACAP expression slowed β1 integrin recycling to plasma membrane following treatment with serum. Reduced expression of ACAP1 also slowed cell migration (Li et al., 2005). Similarly, Glut4 traffic to the plasma membranes was affected by reducing either ACAP1 or clathrin expression (Li et al., 2007). Arf6 localized on Glut4 enriched compartment with ACAP1 and clathrin, suggesting that these 3 proteins function together to regulate Glut4 recycling. A mutant of ACAP1 that lacked GAP activity failed to restore Glut4 recycling in cells with reduced ACAP1 expression. Thus GAP activity is important for recycling. Together, these results support a model for ACAP1 functioning as a component of clathrin coats in a similar manner as the same authors have proposed that ArfGAP1 functions with coatomer (Li et al., 2005,2007).
Uncoating mechanism independent of GTP hydrolysis on Arf
Mechanisms for uncoating after GTP hydrolysis on Arf have been described for clathrin and, more recently, for COPI. For clathrin/AP-1 vesicles, phosphorylation and dephosphorylation of AP-1, regulated by the fusion machinery, is responsible for dissociation of AP-1 (Ghosh and Kornfeld, 2003). Clathrin uncoating is regulated by Hsc70 (Delucaflaherty et al., 1990; Cheetham et al., 1996; Rothnie et al., 2011). For COPI vesicles, Dsl1p, an ER tethering molecule in yeast, has the potential to be central to one proposed mechanism of uncoating (Munson, 2009; Ren et al., 2009; Tripathi et al., 2009; Zink et al., 2009). Depletion of Dsl1p leads to the massive accumulation of COPI vesicles at ER exit sites. Dsl1p binds to COPI subunits through a site that mediates interaction between COPI subunits. Thus, binding of Dsl1p to COPI subunits would destabilize COPI coat to initiate uncoating (Zink et al., 2009). In this model, uncoating is mediated by the fusion machinery rather than Arf dynamics.
Although COPII vesicle use Sar1p instead of Arf as small GTP binding proteins, examination of yeast COPII have provided insights into the role of Arf family GTP-binding proteins in coat dynamics. COPII is comprised of Sec23p/24p and Sec13p/31p complex. Sar1p recruits Sec23p/24p to membrane and polymerization needs Sec13p/31p coat. Sec23p is a Sar1p GAP. Interestingly, COPII coat remains on vesicles after Sar1p falls off. The binding of tethering complex, TRAPPI competes with Sar1p for binding to Sec23p, suggesting TRAPPI binds to Sec23p after Sar1p dissociation. Phosphorylation of Sec23p causes dissociation of TRAPPI from Sec23p before fusion (Lord et al., 2011). The fact that COPII remains on the membrane after GTP hydrolysis of Sar1p is similar to AP-1 and the proposed model for Arf with coatomer. In this model, uncoating occurs near the acceptor Golgi membrane and is not triggered by GTP hydrolysis by Sar1p or by Sar1p dissociation.
Conclusion
The function and regulation of ArfGAP1 is still being discovered and debated (Beck et al., 2011; Hsu, 2011; Kahn, 2011). As more is learned about ArfGAP1, roles beyond negative regulation of Arf are becoming evident. In addition, characterization of other Arf GAPs has led to further understanding of the role of Arf in membrane traffic. We look forward to work that will lead to the description of the molecular mechanisms for cargo sorting and transport vesicle formation.
Acknowledgements:
The authors thank Julie Donaldson for critically reading the manuscript. The work was supported by the Intramural Program of the National Cancer Institute, National Institutes of Health.
Footnotes
Note on use of Ras paradigm for Arf. The signaling proteins Ras and heterotrimeric G-proteins are the prototypes for understanding the action of guanine nucleotide-binding proteins. In these models, the “active” GTP-bound guanine nucleotide-binding proteins bind to an effector, which mediates a response. The role of GTP hydrolysis is to either prevent or terminate a signal. In contrast to Ras, which drives cell proliferation if locked in the GTP bound form, Arf blocks membrane traffic if locked in the GTP bound form. The cycle of GTP binding and hydrolysis is necessary for efficient traffic. Therefore, we will refrain from using the language developed for the Ras paradigm, i.e. “active”, “inactive” and “effector” for this review.
References
- Antonny B, BeraudDufour S, Chardin P and Chabre M (1997). N-terminal hydrophobic residues of the G-protein ADP- ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry 36, 4675–4684. [DOI] [PubMed] [Google Scholar]
- Aoe T, Cukierman E, Lee A, Cassel D, Peters PJ and Hsu VW (1997). The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. EMBO J. 16, 7305–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoe T, Huber I, Vasudevan C, Watkins SC, Romero G, Cassel D and Hsu VW (1999). The KDEL receptor regulates a GTPase-activating protein for ADP-ribosylation factor 1 by interacting with its non-catalytic domain. J. Biol. Chem 274, 20545–20549. [DOI] [PubMed] [Google Scholar]
- Balch WE and Rothman JE (1985). Characterization of protein-transport between successive compartments of the Golgi-apparatus - asymmetric properties of donor and acceptor activities in a cell-free system. Arch. Bioch. Biophys 240, 413–425. [DOI] [PubMed] [Google Scholar]
- Balch WE, Dunphy WG, Braell WA and Rothman JE (1984a). Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-Acetylglucosamine. Cell 39, 405–416. [DOI] [PubMed] [Google Scholar]
- Balch WE, Glick BS and Rothman JE (1984b). Sequential Intermediates in the Pathway of Intercompartmental Transport in A Cell-Free System. Cell 39, 525–536. [DOI] [PubMed] [Google Scholar]
- Beck R, Adolf F, Weimer C, Bruegger B and Wieland FT (2009a). ArfGAP1 activity and COPI vesicle biogenesis. Traffic 10, 307–315. [DOI] [PubMed] [Google Scholar]
- Beck R, Ravet M, Wieland FT, and Cassel D (2009b). The COPI system: Molecular mechanisms and function. FEBS Lett. 583, 2701–2709. [DOI] [PubMed] [Google Scholar]
- Beck R, Brugger B and Wieland F (2011). GAPs in the context of COPI. Cellular Logistics 1, 52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R and Orci L (1995). COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell 83, 1183–1196. [DOI] [PubMed] [Google Scholar]
- Bendor J, Lizardi-Ortiz JE, Westphalen RI, Brandstetter M, Hemmings HC, Sulzer D, Flajolet M and Greengard P (2010). AGAP1/AP-3-dependent endocytic recycling of M-5 muscarinic receptors promotes dopamine release. EMBO J. 29, 2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J, Gounon P, Robineau S and Antonny B (2003). Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature 426, 563–566. [DOI] [PubMed] [Google Scholar]
- Bigay J, Casella JF, Drin G, Mesmin B and Antonny B (2005). ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. EMBO J. 24, 2244–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes CA, Sollner TH, Rothman JE and Wieland FT (1999). Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell 96, 495–506. [DOI] [PubMed] [Google Scholar]
- Casanova JE (2007). Regulation of arf activation: the sec7 family of guanine nucleotide exchange factors. Traffic 8, 1476–1485. [DOI] [PubMed] [Google Scholar]
- Cheetham ME, Anderton BH and Jackson AP (1996). Inhibition of hsc70-catalysed clathrin uncoating by HSJ1 proteins. Biochem. J 319, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Huber I, Rotman M and Cassel D (1995). The ARF1 GTPase-activating protein - Zinc-finger motif and Golgi complex localization. Science 270, 1999–2002. [DOI] [PubMed] [Google Scholar]
- Dai J, Li J, Bos E, Porcionatto M, Premont RT, Bourgoin S, Peters PJ and Hsu VW (2004). ACAP1 promotes endocytic recycling - Short article by recognizing recycling sorting signals. Dev. Cell 7, 771–776. [DOI] [PubMed] [Google Scholar]
- Dascher C and Balch WE (1994). Dominant inhibitory mutants of Arf1 block endoplasmic-reticulum to Golgi transport and trigger disassembly of the Golgi-apparatus. J. Biol. Chem 269, 1437–1448. [PubMed] [Google Scholar]
- Delucaflaherty C, Mckay DB, Parham P and Hill BL (1990). Uncoating protein (Hsc70) binds a conformationally labile domain of clathrin light chain LCA to stimulate ATP hydrolysis. Cell 62, 875–887. [DOI] [PubMed] [Google Scholar]
- Donaldson JG and Jackson CL (2000). Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol 12, 475–482. [DOI] [PubMed] [Google Scholar]
- Donaldson JG and Jackson CL (2011). ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nature Rev. Mol. Cell Biol 12, 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Lippincott-Schwartz J, Bloom GS, Kreis TE and Klausner RD (1990). Dissociation of A 110-Kd Peripheral Membrane-Protein from the Golgi-Apparatus Is An Early Event in Brefeldin-A Action. J. Cell Biol 111, 2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Cassel D, Kahn RA, and Klausner RD (1992). ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-cop to Golgi membranes. Proc. Natl. Acad. Sci. USA 89, 6408–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East MP and Kahn RA (2011). Models for the functions of Arf GAPs. Sem. Cell Dev. Biol 22, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M, Chardin P, Chabre M and Paris S (1993). Myristoylation is not required for GTP-dependent binding of ADP-ribosylation factor- Arf1 to phospholipids. J. Biol. Chem 268, 24531–24534. [PubMed] [Google Scholar]
- Funaki T, Kon S, Ronn RE, Henmi Y, Kobayashi Y, Watanabe T, Nakayama K, Tanabe K and Satake M (2011). Localization of SMAP2 to the TGN and its function in the regulation of TGN protein transport. Cell Struct. Funct 36, 83–95. [DOI] [PubMed] [Google Scholar]
- Ghosh P and Kornfeld S (2003). AP-1 binding to sorting signals and release from clathrin-coated vesicles is regulated by phosphorylation. J. Cell Biol 160, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK and Munro S (2007). The small G proteins of the Arf family and their regulators. Annu. Rev.Cell Dev. Biol 23, 579–611. [DOI] [PubMed] [Google Scholar]
- Goldberg J (1999). Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell 96, 893–902. [DOI] [PubMed] [Google Scholar]
- Goldberg J (2000). Decoding of sorting signals by coatomer through a GTPase switch in the COPI coat complex. Cell 100, 671–679. [DOI] [PubMed] [Google Scholar]
- Hsu VW (2011). ArfGAP1 in COPI vesicle biogenesis. Cellular Logistics 1, 55–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu VW, Lee SY and Yang JS (2009). The evolving understanding of COPI vesicle formation. Nature Rev. Mol. Cell Biol 10, 360–364. [DOI] [PubMed] [Google Scholar]
- Huber I, Cukierman E, Rotman M, Aoe T, Hsu VW and Cassel D (1998). Requirement for both the amino-terminal catalytic domain and a noncatalytic domain for in vivo activity of ADP-ribosylation factor GTPase-activating protein. J. Biol. Chem 273, 24786–24791. [DOI] [PubMed] [Google Scholar]
- Inoue H and Randazzo PA (2007). Arf GAPs and their interacting proteins. Traffic 8, 1465–1475. [DOI] [PubMed] [Google Scholar]
- Jackson CL and Casanova JE (2000). Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 10, 60–67. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Brown FD, Nie ZZ, Miura K, Foroni L, Sun JL, Hsu VW, Donaldson JG and Randazzo PA (2000). ACAPs are Arf6 GTPase-activating proteins that function in the cell periphery. J. Cell Biol 151, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA (2011). Terminator versus effector function and the role(s) of ArfGAPs in vesicle biogenesis. Cellular Logistics 1, 49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA, Randazzo P, Serafini T, Weiss O, Rulka C, Clark J, Amherdt M, Roller P, Orci L and Rothman JE (1992). The amino terminus of ADP-ribosylation factor (Arf) is a critical determinant of Arf activities and is a potent and specific inhibitor of protein-transport. J. Biol. Chem 267, 13039–13046. [PubMed] [Google Scholar]
- Kahn RA, Cherfils J, Elias M, Lovering RC, Munro S and Schurmann A (2006). Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J. Cell Biol 172, 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA, Bruford E, Inoue H, Logsdon JM, Nie Z, Premont RT, Randazzo PA, Satake M, Theibert AB, Zapp ML and Cassel D (2008). Consensus nomenclature for the human ArfGAP domain containing proteins. J. Cell Biol 182, 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliouchnikov L, Bigay J, Mesmin B, Parnis A, Rawet M, Goldfeder N, Antonny B and Cassel D (2009). Discrete Determinants in ArfGAP2/3 Conferring Golgi Localization and Regulation by the COPI Coat. Mol. Biol. Cell 20, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon S, Tanabe K, Watanabe T, Sabe H and Satake M (2008). Clathrin dependent endocytosis of E-cadherin is regulated by the Arf6GAP isoform SMAP1. Exp. Cell Res 314, 1415–1428. [DOI] [PubMed] [Google Scholar]
- Lanoix J, Ouwendijk J, Stark A, Lin CC, Ostermann J and Nilsson T (1998). GTP gamma S inhibits selective incorporation of resident Golgi enzymes into functional COP-1-dependent vesicular carriers. Mol. Biol. Cell 9, 578. [Google Scholar]
- Lanoix J, Ouwendijk J, Lin CC, Stark A, Love HD, Ostermann J and Nilsson T (1999). GTP hydrolysis by arf-1 mediates sorting and concentration of Golgi resident enzymes into functional COPI vesicles. EMBO J. 18, 4935–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoix J, Ouwendijk J, Stark A, Szafer S, Cassel D, Dejgaard K, Weiss M and Nilsson T (2001). Sorting of Golgi resident proteins into different subpopulations of COPI vesicles: a role for ArfGAP1. J. Cell Biol 155, 1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Yang JS, Hong WJ, Premont RT and Hsu VW (2005). ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J. Cell Biol 168, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SM, Poon PP, Singer RA, Johnston GC and Spang A (2004). The ArfGAP Glo3 is required for the generation of COPI vesicles. Mol. Biol. Cell 15, 4064–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ballif BA, Powelka AM, Dai J, Gygi SP and Hsu VW (2005). Phosphorylation of ACAP1 by Akt regulates the stimulationdependent recycling of integrin beta 1 to control cell migration. Dev. Cell 9, 663–673. [DOI] [PubMed] [Google Scholar]
- Li J, Peters PJ, Bai M, Dai J, Bos E, Kirchhausen T, Kandror KV and Hsu VW (2007). An ACAP1-containing clathrin coat complex for endocytic recycling. J. Cell Biol 178, 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS and Klausner RD (1989). Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin-A - evidence for membrane cycling from Golgi to ER. Cell 56, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Duden R, Phair RD and Lippincott-Schwartz J (2005). ArfGAP1 dynamics and its role in COPI coat assembly on Golgi membranes of living cells. J. Cell Biol 168, 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Bhandari D, Menon S, Ghassemian M, Nycz D, Hay J, Ghosh P and Ferro-Novick S (2011). Sequential interactions with Sec23 control the direction of vesicle traffic. Nature 473, 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo RB and Randazzo PA (2008). Kinetic analysis of Arf GAP1 indicates a regulatory role for coatomer. J. Biol. Chem 283, 21965–21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Ha VL, Hayashi R and Randazzo PA (2009). Arf GAP2 is positively regulated by coatomer and cargo. Cell. Signal 21, 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makler V, Cukierman E, Rotman M, Admon A and Cassel D (1995). ADP-ribosylation factor-directed GTPase-activating protein - purification and partial characterization. J. Biol. Chem 270, 5232–5237. [DOI] [PubMed] [Google Scholar]
- Melancon P, Glick BS, Malhotra V, Weidman PJ, Serafini T, Gleason ML, Orci L and Rothman JE (1987). Involvement of GTP-binding G proteins in transport through the Golgi stack. Cell 51, 1053–1062. [DOI] [PubMed] [Google Scholar]
- Mesmin B, Drin G, Levi S, Rawet M, Cassel D, Bigay J and Antonny B (2007). Two lipid-packing sensor motifs contribute to the sensitivity of ArfGAP1 to membrane curvature. Biochemistry 46, 1779–1790. [DOI] [PubMed] [Google Scholar]
- Meyer DM, Crottet P, Maco B, Degtyar E, Cassel D and Spiess M (2005). Oligomerization and dissociation of AP-1 adaptors are regulated by cargo signals and by ArfGAP1-induced GTP hydrolysis. Mol. Biol. Cell 16, 4745–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson M (2009). Tip20p reaches out to Dsl1p to tether membranes. Nature Struc. Mol. Biol 16, 100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsume W, Tanabe K, Kon S, Yoshida N, Watanabe T, Torii T and Satake M (2006). SMAP2, a novel ARF GTPase-activating protein, interacts with clathrin and clathrin assembly protein and functions on the AP-1-positive early endosome/trans-Golgi network. Mol. Biol. Cell 17, 2592–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Malsam J, Gorgas K, Ravazzola M, Jenne N, Helms JB and Wieland FT (1998). Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTP gamma S in vitro. J. Cell Sci 111, 3081–3090. [DOI] [PubMed] [Google Scholar]
- Nie ZZ and Randazzo PA (2006). Arf GAPs and membrane traffic. J. Cell Sci 119, 1203–1211. [DOI] [PubMed] [Google Scholar]
- Nie Z, Boehm M, Boja E, Vass W, Bonifacino J, Fales H and Randazzo PA (2003). Specific regulation of the adaptor protein complex AP-3 by the Arf GAP AGAP1. Dev. Cell 5, 513–521. [DOI] [PubMed] [Google Scholar]
- Nie ZZ, Fei J, Premont RT and Randazzo PA (2005). The Arf GAPs AGAP1 and AGAP2 distinguish between the adaptor protein complexes AP-1 and AP-3. J. Cell Sci 118, 3555–3566. [DOI] [PubMed] [Google Scholar]
- Palmer DJ, Helms JB, Beckers CJM, Orci L and Rothman JE (1993). Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J. Biol. Chem 268, 12083–12089. [PubMed] [Google Scholar]
- Pepperkok R, Whitney JA, Gomez M and Kreis TE (2000). COPI vesicles accumulating in the presence of a GTP restricted arf1 mutant are depleted of anterograde and retrograde cargo. J. Cell Sci 113, 135–144. [DOI] [PubMed] [Google Scholar]
- Poon PP, Wang XM, Rotman M, Huber I, Cukierman E, Cassel D, Singer RA and Johnston GC (1996). Saccharomyces cerevisiae Gcs1 is an ADP-ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. USA 93, 10074–10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon PP, Cassel D, Spang A, Rotman M, Pick E, Singer RA and Johnston GC (1999). Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J. 18, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon PP, Nothwehr SE, Singer RA and Johnston GC (2001). The Gcs1 and Age2 ArfGAP proteins provide overlapping essential function for transport from the yeast trans-Golgi network. J. Cell Biol 155, 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C and Klumperman J (2005). The maturing role of COPI vesicles in intra-Golgi transport. Nature Rev. Mol. Cell Biol 6, 812–817. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Terui T, Sturch S, Fales HM, Ferridge AG and Kahn RA (1995). The myristoylated amino-terminus of ADP-ribosylation factor-1 Is a phospholipid-sensitive and GTP-sensitive switch. J. Biol. Chem 270, 14809–14815. [DOI] [PubMed] [Google Scholar]
- Rein U, Andag U, Duden R, Schmitt HD and Spang A (2002). ARFGAP-mediated interaction between the ER-Golgi v-SNAREs and the COPI coat. J. Cell Biol 157, 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Yip CK, Tripathi A, Huie D, Jeffrey PD, Walz T and Hughson FM (2009). A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1. Cell 139, 1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE (2002). The machinery and principles of vesicle transport in the cell. Nature Med. 8, 1059–1062. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Balch WE, Braell WA, Glick B, Hino Y and Wattenberg B (1984). Reconstitution of protein-transport in the Golgi. J. Cell Biol 99, A230. [Google Scholar]
- Rothnie A, Clarke AR, Kuzmic P, Cameron A and Smith CJ (2011). A sequential mechanism for clathrin cage disassembly by 70-kDa heat-shock cognate protein (Hsc70) and auxilin. Proc. Natl. Acad. Sci. USA 108, 6927–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A, Shin HW, Yamada A, Waguri S and Nakayama K (2009). Three Homologous ArfGAPs Participate in Coat Protein I-mediated Transport. J. Biol. Chem 284, 13948–13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Rodriguez F, Poon PP, Singer RA, Johnston GC and Spang A (2009). The GAP domain and the SNARE, coatomer and cargo interaction region of the ArfGAP2/3 Glo3 are sufficient for Glo3 function. Traffic 10, 1362–1375. [DOI] [PubMed] [Google Scholar]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA and Rothman JE (1991). Adp-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles - A novel role for a Gtp-Binding protein. Cell 67, 239–253. [DOI] [PubMed] [Google Scholar]
- Shiba Y, Romer W, Mardones GA, Burgos PV, Lamaze C and Johannes L (2010). AGAP2 regulates retrograde transport between early endosomes and the TGN. J. Cell Sci 123, 2381–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Luo RB, Hinshaw JE, Szul T, Hayashi R, Sztul E, Nagashima K, Baxa U and Randazzo PA (2011). ArfGAP1 promotes COPI vesicle formation by facilitating coatomer polymerization. Cellular Logistics 1, 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Schorey C and Chavrier P (2006). ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol 7, 347–358. [DOI] [PubMed] [Google Scholar]
- Stearns T, Kahn RA, Botstein D and Hoyt MA (1990). ADP ribosylation factor is an essential protein in saccharomyces-cerevisiae and is encoded by 2 genes. Mol. Cell. Biol 10, 6690–6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Anderl F, Frohlich K, Zhao LY, Hanke S, Brugger B, Wieland F and Bethune J (2007). Multiple and stepwise interactions between coatomer and ADP-ribosylation factor-1 (Arf1)-GTP. Traffic 8, 582–593. [DOI] [PubMed] [Google Scholar]
- Szafer E, Pick E, Rotman M, Zuck S, Huber I and Cassel D (2000). Role of coatomer and phospholipids in GTPase-activating proteindependent hydrolysis of GTP by ADP-ribosylation factor- 1. J. Biol. Chem 275, 23615–23619. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Torii T, Natsume W, Braesch-Andersen S, Watanabe T and Satake M (2005). A novel GTPase-activating protein for ARF6 directly interacts with clathrin and endocytosis. Mol. Biol. Cell 16, 1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms JB and Rothman JE (1993). Hydrolysis of bound GTP by Arf protein triggers uncoating of Golgi-derived COP-coated vesicles. J. Cell Biol 123, 1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Ren Y, Jeffrey PD and Hughson FM (2009). Structural characterization of Tip20p and Dsl1p, subunits of the Dsl1p vesicle tethering complex. Nat. Struct. Mol. Biol 16, 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Serafini T and Rothman JE (1991). Coatomer - A cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature 349, 248–251. [DOI] [PubMed] [Google Scholar]
- Weimer C, Beck R, Eckert P, Reckmann I, Moelleken J, Brugger B and Wieland F (2008). Differential roles of ArfGAP1, ArfGAP2, and ArfGAP3 in COPI trafficking. J. Cell Biol 183, 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M and Nilsson T (2003). A kinetic proof-reading mechanism for protein sorting. Traffic 4, 65–73. [DOI] [PubMed] [Google Scholar]
- Yang JS, Lee SY, Gao MG, Bourgoin S, Randazzo PA, Premont RT and Hsu VW (2002). ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J. Cell Biol 159, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CJ, Cavenagh MM and Kahn RA (1998). A family of Arf effectors defined as suppressors of the loss of Arf function in the yeast Saccharomyces cerevisiae. J. Biol. Chem 273, 19792–19796. [DOI] [PubMed] [Google Scholar]
- Zhang CJ, Bowzard JB, Anido A and Kahn RA (2003). Four ARF GAPs in Saccharomyces cerevisiae have both overlapping and distinct functions. Yeast 20, 315–330. [DOI] [PubMed] [Google Scholar]
- Zhao LY, Helms JB, Brugger B, Harter C, Martoglio B, Graf R, Brunner J and Wieland FT (1997). Direct and GTP-dependent interaction of ADP ribosylation factor 1 with coatomer subunit beta. Proc. Natl. Acad. Sci. USA 94, 4418–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YX, Traub LM and Kornfeld S (1998). ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol. Biol. Cell 9, 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YX, Drake MT and Kornfeld S (1999a). ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes. Proc. Nat’l Acad. Sci. USA 96, 5013–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YX, Traub LM and Kornfeld S (1999b). High-affinity binding of the AP-1 adaptor complex to trans-Golgi network membranes devoid of mannose 6-phosphate receptors. Mol. Biol. Cell 10, 537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink S, Wenzel D, Wurm CA and Schmitt HD (2009). A link between ER tethering and COP-I vesicle uncoating. Dev. Cell 17, 403–416. [DOI] [PubMed] [Google Scholar]