ABSTRACT
In late 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged as the cause of a cluster of pneumonia cases in China, and the corresponding disease was designated as Coronavirus Disease 2019 (COVID-19), spreading quickly around the world resulting in a pandemic. COVID-19 is associated with a set of coagulation abnormalities that increase the risk of thromboembolic events, especially in patients with severe/critical disease. We describe a series of five cases of mild COVID-19, treated in an outpatient clinic, which, after an apparent clinical improvement, developed acute pulmonary embolism (APE) between the third and the fourth week after the onset of symptoms, when they are mostly related to acute illness disappearance. Thromboembolic events are also a potential complication of mild COVID-19 and can manifest later in the disease course. This finding raises discussion about the prevention of thromboembolic events in selected group of patients with mild COVID-19.
Keywords: COVID-19, Pulmonary embolism, Thromboembolism, Severe acute respiratory syndrome coronavirus 2, Pneumonia
INTRODUCTION
In late 2019, a novel coronavirus, now designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged as the cause of a cluster of pneumonia cases in Wuhan, a city of the Hubei Province in China1. It spread quickly around the world resulting in a pandemic2. The disease caused by the SARS-CoV-2 was named as coronavirus disease 2019 (COVID-19) by World Health Organization (WHO)3. According to the last WHO report, COVID-19 affected more than 18 million people and around 700,000 people died4.
The clinical spectrum of COVID-19 ranges from mild infection with or without pneumonia, severe pneumonia with respiratory distress and/or hypoxia in ambient air to critical disease characterized by respiratory failure, shock and/or multiorgan dysfunction5. In patients with severe disease, the acute respiratory distress syndrome (ARDS) is the most frequent complication (19.6% - 29%)6,7, resulting in a remarkable hypoxemic respiratory failure. However, several other clinical events could aggravate the course of COVID-19 including thromboembolic events.
Thromboembolic complications seem to be an important issue in patients with severe COVID-19. The risk of venous thromboembolism (VTE), which is already increased in critically ill patients, is probably higher in those with critical COVID-19, even when prophylactic anticoagulation is used8. The SARS-CoV-2 infection is associated with a number of coagulation abnormalities that include increased levels of fibrinogen and d-dimer, modestly prolonged prothrombin time, normal or slightly prolonged activated partial thromboplastin time, normal or slightly decreased platelet counts and markedly hypercoagulable thromboelastometry profiles9,10. This hypercoagulability state is named COVID-19-associated coagulopathy and may correlate with the increased risk of VTE events11.
In a French case series of critical COVID-19 patients, the incidence of acute pulmonary embolism (APE) was 20.6%, a frequency comparatively higher than those observed in patients with Influenza infections and other clinical conditions with similar severity score on admission to the ICU (7.5% and 6.1%, respectively)12. A Dutch study showed a cumulative incidence of thromboembolic events of 31% among ICU patients with proven COVID-19 pneumonia and pulmonary embolism was the most frequent thrombotic complication (81%)13.
Most of the studies reported thromboembolic events in patients with COVID-19 admitted to the hospital. A PubMed search for articles published up to July 31, 2020 using the following search strategy – (“Thrombosis” OR “Embolism” OR “Thromboemb*”) AND “COVID-19” – found ten articles reporting cases of VTE in patients after mild COVID-1914-23. Here we describe a case series of five patients with mild COVID-19 that, after an apparent clinical improvement, developed acute pulmonary embolism more than fourteen days after the onset of symptoms. We also summarized the epidemiological, clinical, laboratory and imaging features of the cases in Tables 1 and 2.
Table 1. Clinical and demographic characteristics of patients with mld COVID-19 and thromboembolic events.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age - year/ Sex | 63/ Male | 28/ Male | 34/ Male | 58/ Male | 55/ Male |
| Clinical characteristics | |||||
| Comorbidity | Type 2 diabetes mellitus and dyslipidemia | No | Obesity, hypertension and gastroesophageal reflux | No | No |
| History of thrombosis | No | No | No | No | No |
| Early symptoms | Fever, arthralgia, abdominal pain, diarrhea, cough and dyspnea | Fever, headache, myalgia, sore throat, abdominal pain, diarrhea, cough. | Fever, chills, myalgia, ocular pain, anosmia and cough | Fever, headache, prostration, myalgia and occasional cough | Fever, myalgia, cough and diarrhea. |
| Epidemiology | Contact with confirmed cases of COVID-19 at work | Household contact with a confirmed case of COVID-19 | Contact with confirmed cases of COVID-19 at work | Household contact with a confirmed case of COVID-19 | Household contact with a confirmed case of COVID-19 |
| Diagnosis | Positive RT-PCR for SARS-COV-2 in respiratory specimens on the 10th day of the disease | Positive RT-PCR for SARS-COV-2 in respiratory specimens on the 6th day of the disease | Positive RT-PCR for SARS-COV-2 in respiratory specimens on the 2nd day of the disease | Positive IgM and IgG anti-SARS-COV-2 on the 21st day of the disease | Positive IgM and IgG anti-SARS-COV-2 on the 15th day of the disease. |
| Imaging features | Chest CT: Bilateral ground-glass opacities with disease extent < 25% of lung parenchyma | Chest X-Ray: normal. | Chest X-Ray: normal. | Not done. | Not done. |
| Disease severity* | Mild | Mild | Mild | Mild | Mild |
| Days from the onset of symptoms to the thromboembolic event | 23 | 16 | 23 | 17 | 17 |
| Thromboembolic event | Acute pulmonary embolism (APE) | APE | APE | APE | APE |
| Symptoms | New onset of fever, left chest pain and hemoptoic sputum | New onset of fever, right chest pain and dyspnea. | Left chest pain and back pain | Right chest pain | New onset of fever, left chest pain, new onset of cough with hemoptoic sputum |
| Imaging features | Filling failures in the arterial branches for the lingula and the lower left lobe | Filling failure in the subsegmental arterial branch to the right lower lobe | Partial failure of filling of the right and left descending pulmonary arteries, as well as its segmental and subsegmental branches | Filling failure of segmental branches of the pulmonary artery located in the right lower lobe, as well as in the left pulmonary artery extending to the upper and lingual lobar artery, and in some segmental branches of the left lower lobe. | Filling failure of left and right lobar arteries, as well as its segmental and subsegmental branches. |
| Treatment | Enoxaparin sodium followed by warfarin | Enoxaparin sodium followed by rivaroxaban | Enoxaparin sodium followed by rivaroxaban | Enoxaparin sodium followed by rivaroxaban | Enoxaparin sodium followed by rivaroxaban |
| Outcome | Alive and well | Alive and well | Alive and well | Alive and well | Alive and well |
*according to the WHO classification of COVID-19 disease severity5.
Table 2. Laboratory tests of patients with mild COVID-19 and thromboembolic events on hospital admission.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Reference Range | |
|---|---|---|---|---|---|---|
| Hemoglobin (g/L) | 115 | 155 | 131 | 147 | 128 | 130 - 180 |
| White-cell count (per mm3) | 10,400 | 9,000 | 10,800 | 14,700 | 9,600 | 4,000 - 10,000 |
| Differential count (per mm3) Total neutrophils Total lymphocytes Total monocytes | 7,488 1,664 1,144 | 6,210 1,890 540 | 7,668 2,700 216 | 12,642 1,323 588 | 5,.952 3,072 384 | 2,160 - 6,200 800 - 3,500 120 - 800 |
| Platelet count (per mm3) | 408,000 | 407,000 | 521,000 | 245,000 | 188,000 | 150,000 – 450,000 |
| Alanine aminotransferase (U/L) | 114 | 50 | 50 | 19 | 59 | 0 –- 42 |
| Aspartate aminotransferase (U/L) | 47 | 26 | 15 | 10 | 21 | 0 - 37 |
| Lactate dehydrogenase (U/L) | 207 | 355 | 161 | 171 | 207 | 100 - 250 |
| Creatine kinase (U/L) | 59 | 92 | 199 | Not done | 107 | 35 - 232 |
| Blood urea nitrogen (mmol/L) | 3.65 | 1.55 | 1.63 | 2.1 | 2.33 | 1.17 - 3.88 |
| Creatinine (μmol/L) | 91.0 | 88.4 | 68.0 | 85.7 | 120.25 | 50 - 110 |
| Sodium (mEq/L) | 134 | 144 | 138 | 132 | 142 | 135 - 145 |
| Potassium (mEq/L) | 4.8 | 3.5 | 3.7 | 4.6 | 5.1 | 3,5 - 5,1 |
| Prothrombine time (sec) | 14.3 | 14.1 | 13.2 | 12.0 | 11.7 | 9 - 13.5 |
| Activated partial-thromboplastin time (sec) | 26.0 | 32.2 | 26.0 | 30.0 | 29.0 | 25.4 - 33.4 |
| Fibrinogen (g/L) | 3.97 | Not Done | 2.16 | 2.26 | 4.46 | 1.5 - 4.5 |
| D-dimer (mg/L) | 3,860 | Not Done | 3,559 | 4,052 | > 10,000 | < 400 |
| High-sensitivity cardiac troponin I (pg/mL) | 1 | 3 | 4 | Not done | 1 | < 26 |
| BNP (pg/mL) | Not Done | Not done | 133 | Not done | Not done | < 100 |
| Serum ferritin (μg/L) | 3,362 | Not done | 455 | 1,066 | 1,310 | 28 – 397 |
| High-sensitivity C-reactive protein (mg/L) | 196.6 | 6 | 36.8 | 80.1 | 87.1 | < 5 |
| C3 (mg/dL) | 175 | Not done | 241 | 129 | Not done | 87 -200 |
| C4 (mg/dL) | 59 | Not done | 35 | 24 | Not done | 19 - 52 |
| Reumatoid factor | Negative | Negative | Negative | Negative | Not done | Negative |
| Antinuclear antibody | Negative | Negative | Negative | Negative | Not Done | Negative |
| VDRL | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive |
| Antiphospholipid antibodies | Presence of Lupus Anticoagulant (AL). Positive anticardiolipin (aCL) IgM and anti-β2-glyprotein 1 (aB2GP1) IgM. Negative aCL IgG and aB2GP1 I IgG. | Presence of AL. Negative aCL IgA, IgM and IgG. | Presence of AL. Positive aCL IgM and negative aB2GP1 IgM. Negative aCL IgG and aB2GP1 IgG. | Presence of AL. Undetermined aCL IgM and negative aB2GP1 IgM. Negative aCL IgG and aB2GP1 IgG. | Presence of AL. Negative aCL IgM and IgG. | Absence of LA. Absence of aCL IgM and IgG. Absence of aB2GP1 IgM and IgG. |
CASE REPORT
Patient 1
A 63-year-old man with a history of type 2 diabetes mellitus and dyslipidemia sought the emergency department on March 26, 2020 with a 10-day clinical course of high fever (axillary temperature of 39.0 ºC), arthralgia, abdominal pain, diarrhea, dry cough and shortness of breath on exertion. On physical examination, vital signs were normal, peripheral oxygen saturation was 97% in ambient air and other parameters were also unremarkable. He had contact with two coworkers diagnosed with COVID-19. He underwent a chest computed tomography (CT) that revealed bilateral ground-glass opacities distributed peripherally in the lungs compromising less than 25% of the lung parenchyma. The laboratory tests showed hemoglobin 139 g/L (reference range [RR]: 130 - 180), white blood cells 5,700/mm3 (RR: 4,500 - 10,000), neutrophils 4,446/mm3 (RR: 2,160 - 6,200), lymphocytes 912/mm3 (800 - 3,500) and platelets count 175,000/mm3 (150,000 – 450,000). The lactate dehydrogenase serum level was 253 (RR: 100 - 250 IU/L). High-sensitivity C-reactive protein was 149.9 mg/L (RR: up to 5 mg/L). The nucleic acid amplification test (NAAT) to detect SARS-CoV-2 RNA was performed by RT-qPCR from a nasopharyngeal swab and was positive on the 10th day of the disease. He was given hydroxychloroquine plus azithromycin for 5 days and evolved with improvement of the symptoms.
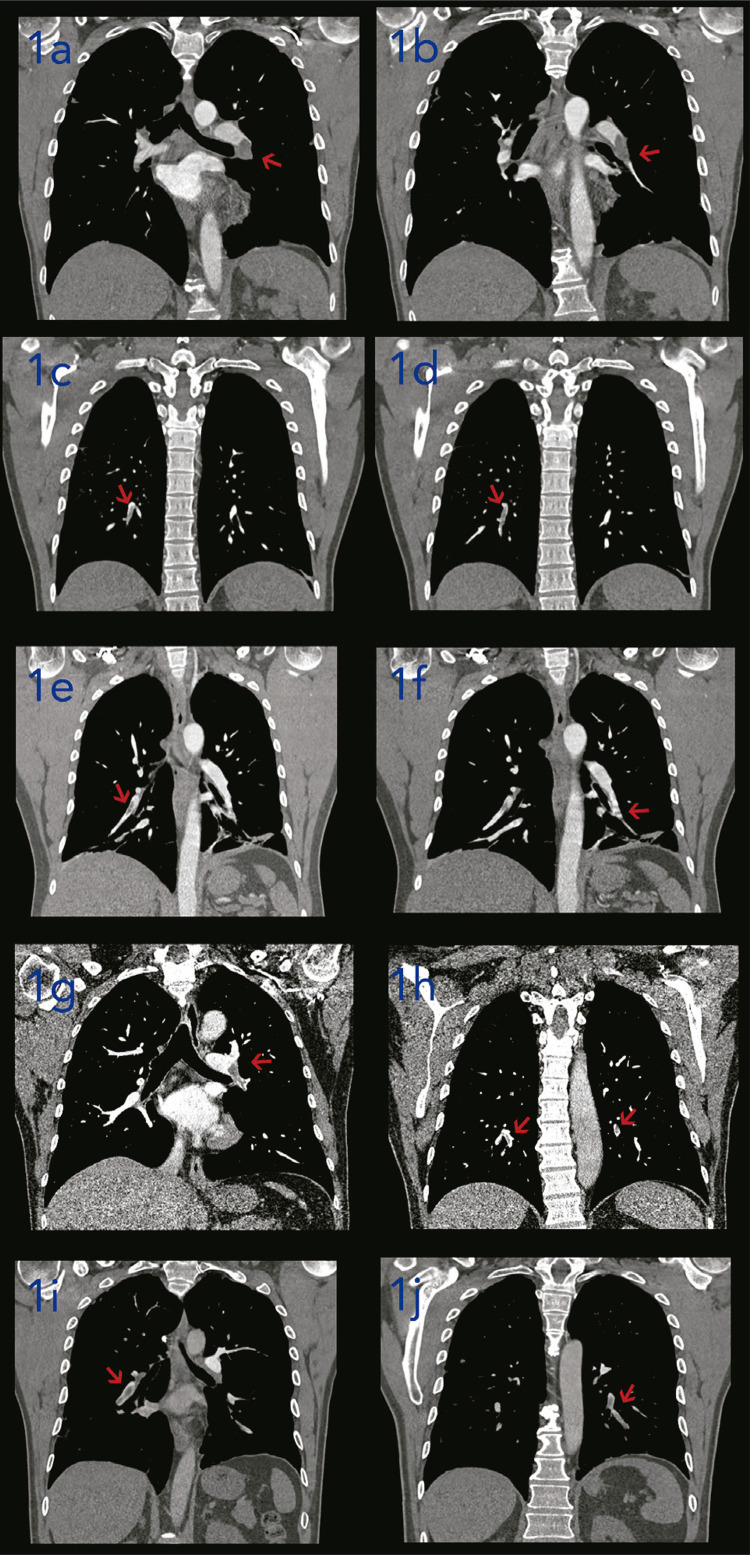
After 8 days, the patient sought again the emergency department with a history of a new onset of fever (axillary temperature of 37.8 ºC), a pleuritic chest pain on the left side associated with hemoptoic sputum. On admission, the vital signs were normal, but fine crackles could be heard on the left side of the chest. The complete blood count showed a mild anemia, a slight leukocytosis with neutrophilia and normal platelet count. The high-sensitivity C-reactive protein level was increased. The d-dimer level was markedly increased. The detailed description of the laboratory tests is shown in the Table 2. He underwent a chest pulmonary CT angiography (Figure 1a and 1b) that showed signs of acute pulmonary embolism (APE) in the lingula and lower left lobe. Moreover, there were bilateral parenchymal consolidations associated with discrete ground-glass opacities distributed peripherally in the lower lobes. The patient was treated with enoxaparin sodium followed by warfarin and discharged from hospital on April 14, 2020 after resolution of the symptoms and reduction in the d-dimer levels. There was no major bleeding. At present, he is alive, asymptomatic and under outpatient follow-up with a hematologist.
Figure 1. CT pulmonary angiography findings of patients with mild COVID-19 complicated with acute pulmonary embolism. In patient 1, filling defects (arrow) are observed in the arterial branches for the lingula and the lower left lobe (1a and 1b) on the CT pulmonary angiography. Filling failure (arrow) in the subsegmental arterial branch to the right lower lobe was revealed by patient 2’s CT pulmonary angiography (1c and 1d). A non-occlusive filling defect (arrow) was observed in the right and left descending pulmonary arteries as well as its segmental and subsegmental branches on the CT pulmonary angiography of patient 3 (1e and 1f). In case of patient 4, the CT pulmonary angiography showed filling failure (arrow) of segmental branches of the pulmonary artery located in the right lower lobe, as well as in the left pulmonary artery extending to the upper and lingual lobar artery, and in some segmental branches of the left lower lobe (1g and 1h). Patient 5’s CT pulmonary angiography (1i and 1j) showed filling failure (arrow) of left and right lobar arteries, as well as its segmental and subsegmental branches.
Patient 2
A 28-year-old otherwise healthy man sought the emergency department on April 5, 2020 with a history of fever (axillary temperature of 38.9 ºC) for 6 days associated with sore throat, diffuse myalgia, headache, abdominal pain, diarrhea and dry cough. He denied other respiratory symptoms such as chest pain and shortness of breath. He had a household contact with a proven COVID-19 case on March 20, 2020. His vital signs and physical examination were normal. Chest X-ray was normal. The NAAT for SARS-CoV-2 RNA by RT-qPCR from a nasopharyngeal swab was positive on 6th day of the disease. He was discharged from hospital and treated with supportive measures in an outpatient setting. After 6 days he had no more fever and was completely asymptomatic.
On April 15, 2020 the patient had a sudden onset of low-intensity fever (axillary temperature of 38.0 ºC) associated with chest pain on the right side and shortness of breath. The physical examination was normal, as well the vital signs, except for a sinusal tachycardia (pulse rate of 116 beats/minute). The detailed description of the laboratory tests is shown in Table 2. He underwent a pulmonary CT angiography (Figure 1c and 1d) that showed findings compatible with an APE in the right lower lobe and an area of consolidative opacity located in the posterior basal segment of right lower lobe that might correspond to a pulmonary infarction. There were also ground-glass opacities in the right lower lobe and left upper lobe. He was given enoxaparin sodium followed by rivaroxaban and discharged from hospital on April 22, 2020. No supplemental oxygen was necessary. He had no major bleedings. Nowadays, he is doing well and is under an outpatient follow-up.
Patient 3
A 34-year-old man sought the emergency department on April 10, 2020 with a history of high fever, chills, myalgia, ocular pain, dry cough and anosmia for 2 days. He had contact with proven cases of COVID-19 at work. His medical history was marked by an essential hypertension, obesity and gastroesophageal reflux disease (GERD). The physical examination was normal. He underwent a chest X-ray that was normal. He was discharged from hospital and treated with symptomatic relief medications and supportive measures. The NAAT for SARS-CoV-2 RNA by RT-PCR was positive on the 4th day of the disease. He had a progressive and complete clinical improvement within eight days.
On May 5, 2020 the patient sought again the emergency department with a chest pain on the left side that irradiated to the back. The vital signs and physical examination were normal. The peripheral oxygen saturation was 99% in ambient air. The laboratory tests (Table 2) showed a markedly increased d-dimer level. He was submitted to a pulmonary CT angiography that showed bilateral APE in the lower lobes (Figure 1e and 1f). Moreover, there were subsegmental parenchymal consolidation areas in the lower lingual segment and in the basal segments of the left lower lobe associated with a small pleural effusion, which may correspond to a pulmonary infarction. Anticoagulation was started with enoxaparin sodium and he did not need oxygen supplementation. He was discharged from hospital six days after admission with rivaroxaban. At the present, he is asymptomatic and under an outpatient follow-up with a hematologist and an infectious diseases specialist.
Patient 4
A 58-year-old otherwise healthy man sought the emergency department on May 10, 2020 with a 3-day course of a pleuritic chest pain on the right side that irradiated to the right lateral lumbar region. He denied other symptoms such as cough, hemoptoic sputum and shortness of breath. Seventeen days before, he reported a history of low-intensity fever, headache, asthenia, myalgia and dry cough. The symptoms quickly improved within a week. He had a household contact with a confirmed COVID-19 case. His vital signs were normal, except for a sinusal tachycardia (105 beats/minute). His physical examination was unremarkable. The peripheral oxygen saturation was 99% in ambient air.
Laboratory tests (Table 2) showed leukocytosis and increased levels of high-sensitivity C-reactive protein and d-dimer. The pulmonary CT angiography (Figure 1g and 1h) showed signs of bilateral APE in the right lower lobe and left upper and lower lobes. Two areas of parenchymal consolidation were also observed on the CT, located in the right lower lobe, which might correspond to a pulmonary infarction. On the 21th day of the disease, the anti-SARS-CoV-2 IgM and IgG were positive by immunofluorescence. Anticoagulation was started with enoxaparin sodium and he was discharged from hospital after four days with rivaroxaban. There was need of oxygen supplementation. No major bleeding occurred. Nowadays, he is asymptomatic and under an outpatient follow-up with a hematologist.
Patient 5
A 55-year-old otherwise healthy man reported a sudden onset of medium-intensity fever (axillary temperature of 38.5 ºC), without chills, associated with myalgia, especially in lower limbs and back on May 14, 2020. He also reported dry cough and diarrhea. He had a household contact with a proven COVID-19 case six days before. He was given just supportive measures and symptomatic relief medications. After 13 days, the patient reported no more symptoms. On the 15th day, the anti-SARS-CoV-2 IgM and IgG were positive by immunofluorescence.
On May 31, 2020 the patient reported a new onset of fever (axillary temperature of 38.8 ºC) associated with recurrence of cough and chest pain on the left side. He also reported hemoptoic sputum during the cough bouts. His vital signs and physical examination were normal. The peripheral oxygen saturation was 96% in ambient air. The laboratory tests showed a mild anemia, but the white blood and the platelet count were normal. The high-sensitivity C-reactive protein and d-dimer levels were increased. The detailed description of the laboratory tests is shown in Table 2. He underwent a pulmonary CT angiography (Figure 1i and 1j) that revealed findings compatible with bilateral APE. There were also multiple ground-glass opacities distributed peripherally in the lung parenchyma compromising less than 25% of the lung parenchyma. He was treated with enoxaparin sodium followed by rivaroxaban. He did not need oxygen supplementation. . He was discharged from hospital on June 5, 2020. No major bleeding was observed. At present, he is doing well and asymptomatic.
DISCUSSION
We reported a series of five cases of mild COVID-19 that, after an apparent clinical improvement, developed APE between the third and the fourth week after the onset of symptoms. This is interesting and, so far, still little reported in the literature. Firstly, several studies reported the occurrence of thromboembolic phenomenon in inpatient settings, especially in ICU patients with severe or critical COVID-1912,13,24-27. Secondly, in a PubMed search for papers concerning thromboembolic events after mild COVID-19 cases, we retrieved only ten articles14-23.
According to the last WHO report, patients with mild COVID-19 recover within two weeks28. A few studies reported cases of mild COVID-19 that complicated with venous thromboembolism events still in the active phase of the disease14-19. In contrast, in our cases, the pulmonary embolism occurred in the COVID-19 convalescence phase, when the symptoms related to the acute illness had already disappeared. We found other four cases of late APE after COVID-19 recovery20-23.
Bellieni et al.20 reported a case of a 56-year-old otherwise healthy man with COVID-19 that presented with a 10-day history of fever, cough, asthenia, diarrhea and dyspnea, but without hypoxia. After clinical improvement, on day 20 of the disease, he complicated with APE. The d-dimer levels were increased and the lupus anticoagulant was positive. The prognosis was good. Tveita et al.21 described the occurrence of APE on the 23thday from the COVID-19 onset, after an asymptomatic two-week period, in a previously healthy woman. The patient had cough and upper respiratory tract symptoms. The d-dimer levels were elevated and she presented with clinical improvement.
Vitali et al.22 reported a case of a 70-year-old man with COVID-19 that on March 11, 2020 presented with a history of fever, sore throat, myalgia, weakness and anorexia, which progressively improved within a week. On April 2, 2020 he developed an acute bilateral pulmonary embolism manifesting as dyspnea with tachycardia. The d-dimer level was more than five times the normal values. The prognosis was also good.
Moreira et al.23 described a case of a 52-year-old asthmatic man with a 5-day history of fever, myalgia, headache, abdominal pain, cough and dyspnea. There was no need for oxygen supplementation. The NAAT for SARS-CoV-2 RNA was positive in a nasopharyngeal swab. After two weeks, he complicated with APE on the right lung. The d-dimer levels were increased and the lupus anticoagulant was positive. The patient had clinical and laboratory improvement.
The pathogenesis of COVID-19-related hypercoagulable state is evolving. An intense and uncontrolled inflammatory response seen in some severe COVID-19 cases appears to contribute to thrombosis, especially in the microvasculature due to thromboinflammation11. A subgroup of critical COVID-19 patients exhibits clinical and laboratory features related to a hyperinflammatory syndrome resembling a secondary haemophagocytic lymphohistiocytosis (SHL) such as unremitting fever, hyperferritinemia, hypertriglicerydemia and ARDS29. In these cases, increased levels of proinflammatory cytokines, such as interleukin (IL)-1B, interferon-gamma (IFN-γ), inducible protein 10 (IP10), monocyte chemoattractant protein 1 (MCP1) and tumor necrosis factor-alpha (TNF-α), were observed7. This inflammatory response causes damage to the vascular endothelium, compromising its thrombo-protective state11. Both, inflammation and endothelial injury activate the coagulation cascade, resulting in several of the coagulation abnormalities seen in SARS-CoV-2-infected patients.
None of our patients exhibited clinical and laboratory features compatible with a SHL. However, we cannot rule out the role of damage to the endothelium caused by inflammation in the pathogenesis of APE in these cases. All patients, except patient 2, had increased levels of serum ferritin. Proinflammatory cytokines, such as IL-1β and TNF-α, increase the ferritin synthesis as part of the hypoferremic response that occurs early in inflammation30.
In addition, the endothelial injury caused by the direct SARS-CoV-2 invasion of the endothelial cells located within the lung parenchyma may be another contributor factor for the development of local thrombotic events. In order to enter the cells, the virus binds to the angiotensin-converting enzyme 2 (ACE) receptor, which is expressed in pneumocytes of the alveolar lining layer and in endothelial cells31. In a post-mortem analysis, there was evidence of the presence of viral elements within endothelial cells and diffuse endothelial inflammation, which can result in endothelial dysfunction and, by consequence, in a procoagulant state32. This endothelitis is also implicated in the lung injury of patients with COVID-1933.
On the admission to the hospital due to APE, all our patients had evidence of lung invasion by SARS-CoV-2 as CT showed multiple and peripheral ground-glass opacifications, the most common abnormality consistent with viral pneumonia in patients with COVID-1934. Interestingly, none of the patients had signs and symptoms related to deep vein thrombosis (DVT), possibly indicating that they had pulmonary thrombosis rather than pulmonary embolism. However, we cannot rule out DVT because they did not undergo the ultrasonography of the lower limbs. On the other hand, bed rest is one of the supportive measures advised during the acute illness, and immobilization causes blood flow stasis increasing the risk of DVT and, by extension, of APE.
All patients were men. The male sex is a risk factor for severe COVID-19. One of the explanations is the possible sex-related differences on the immune response to SARS-CoV-2 infection35. Women produce less inflammatory cytokines after infection, which is associated with a shorter disease duration and higher survival rates36. Male sex is also a risk factor for thrombosis in hospitalized patients with COVID-1924.
Serological tests in some patients were positive for anticardiolipin IgM and anti-β2-glycoprotein I IgM. SARS-CoV-1 has been associated with the presence of anticardiolipin antibodies in patients with post-SARS osteonecrosis and with positive lupus anticoagulant test in children37. These antiphospholipid antibodies were also found in critically ill patients with COVID-19 that developed multiple cerebral infarctions, but lupus anticoagulant was not detected in any of the patients38.
Antiphospholipid antibodies can be non-autoimmune cross-reactive antibodies induced by some exogenous antigens from viral infections39. These antibodies can target endothelial cell membrane phospholipids and promote the endothelial activation and dysfunction, contributing to a procoagulant state40. The lupus anticoagulant was detected in all our patients, except for patient 5. But they were taking rivaroxaban when this test was performed. The use of rivaroxaban affects laboratory coagulation tests and can cause false-positive results to lupus anticoagulant41.
Thrombotic events are associated with worse prognosis in hospitalized patients with COVID-19. In our cases, despite some patients have presented bilateral and extensive APE, there was no need of oxygen supplementation. All patients had a good response to oral anticoagulants. Thus, the prognostic implications of the VTE in mild COVID-19 cases are not necessarily bad.
Therefore, after these cases, we started to discuss and consider thrombosis prophylaxis for up to 14 days after the clinical improvement in patients with a mild form of COVID-19 that present evidence of lung involvement and increased inflammatory markers. Trials addressing the role of thrombosis prophylaxis in the outpatient management of mild COVID-19 cases are necessary.
CONCLUSION
Acute pulmonary embolism is a potential complication of mild COVID-19 cases and may occur late in the course of the disease, when the symptoms related to the acute illness have already disappeared. This raises discussion about the prevention of thromboembolic events in selected group of patients with mild COVID-19.
ACKNOWLEDGMENTS
We would like to thank all the members of the COVID-19 assistance team from Hospital Giselda Trigueiro and Hospital do Coração de Natal who supported us.
REFERENCES
- 1.World Health Organization Novel Coronavirus (2019-nCoV): situation report - 1. Jan 21, 2020. [cited 2020 Aug 12]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4.
- 2.World Health Organization Coronavirus disease 2019 (COVID-19): situation report - 51. [cited 2020 Aug 12]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10.
- 3.World Health Organization Novel Coronavirus (2019-nCoV): situation report - 22. [cited 2020 Aug 12]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=fb6d49b1_2.
- 4.World Health Organization Coronavirus disease (COVID-19): situation report - 197. [cited 2020 Aug 12]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200804-covid-19-sitrep-197.pdf?sfvrsn=94f7a01d_2.
- 5.World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. [cited 2020 Aug 12]. https://apps.who.int/iris/bitstream/handle/10665/331446/WHO-2019-nCoV-clinical-2020.4-eng.pdf?sequence=1&isAllowed=y.
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia inWuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 13.Klok FA, Kruip MJ, van der Meer NJ, Arbous MS, Gommers DA, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jasinowodolinski D, Filisbino MM, Baldi BG. COVID-19 pneumonia: a risk factor for pulmonary thromboembolism? J Bras Pneumol. 2020;46:e20200168. doi: 10.36416/1806-3756/e20200168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khodamoradi Z, Boogar SS, Shirazi FK, Kouhi P. COVID-19 and acute pulmonary embolism in postpartum patient. Emerg Infect Dis. 2020;26:1937–1939. doi: 10.3201/eid2608.201383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akiyama Y, Horiuchi K, Kondo Y, Kabata H, Ishii M, Fukunaga K. A case of non-severe COVID-19 complicated by pulmonary embolism. Respirol Case Rep. 2020;8:e00622. doi: 10.1002/rcr2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boccatonda A, Ianniello E, D’Ardes D, Cocco G, Giostra F, Borghi C, et al. Can lung ultrasound be used to screen for pulmonary embolism in patients with SARS-CoV-2 pneumonia? 001748Eur J Case Rep Intern Med. 2020;7 doi: 10.12890/2020_001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin AI, Rao G. COVID-19: a potential risk factor for acute pulmonary embolism. Methodist Debakey Cardiovasc J. 2020;16:155–157. doi: 10.14797/mdcj-16-2-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nauka PC, Oran E, Chekuri S. Deep venous thrombosis in a non-critically ill patient with novel COVID-19 infection. Thromb Res. 2020;192:27–28. doi: 10.1016/j.thromres.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellieni A, Intini E, Taddei E, Baldi F, Larosa L, Murri R, et al. Infect Dis. Vol. 52. Lond: 2020. Challenges in COVID-19: is pulmonary thromboembolism related to overall severity? pp. 585–589. [DOI] [PubMed] [Google Scholar]
- 21.Tveita A, Hestenes S, Sporastøyl ER, Pettersen SA, Neple BL, Myrstad M, et al. Pulmonary embolism in cases of COVID-19. 0526Tidsskr Nor Laegeforen. 2020;140 doi: 10.4045/tidsskr.20.0366. [DOI] [PubMed] [Google Scholar]
- 22.Vitali C, Minniti A, Caporali R, Del Papa N. Occurrence of pulmonary embolism in a patient with mild clinical expression of COVID-19. Thromb Res. 2020;192:21–22. doi: 10.1016/j.thromres.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira BL, Santana PR, Zanetti G, Marchiori E. COVID-19 and acute pulmonary embolism: what should be considered to indicate a computed tomography pulmonary angiography scan? Rev Soc Bras Med Trop. 2020;53:e20200267. doi: 10.1590/0037-8682-0267-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020 doi: 10.1001/jama.2020.13372. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MC, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahum J, Morichau-Beauchant T, Daviaud F, Echegut P, Fichet J, Maillet JM, et al. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;3:e2010478. doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19. Feb 24, 2020. [cited 2020 Aug 12]. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---24-february-2020.
- 29.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connelly KG, Moss M, Parsons PE, Moore EE, Moore FA, Giclas PC, et al. Serum ferritin as a predictor of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;155:21–25. doi: 10.1164/ajrccm.155.1.9001283. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teuwen L-A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kragholm K, Andersen MP, Gerds TA, Butt JH, Østergaard L, Polcwiartek C, et al. Association between male sex and outcomes of Coronavirus Disease 2019 (Covid-19) – a Danish nationwide, register-based study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa924. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34:339–343. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 37.Sun W, Wang BL, Liu BL, Zhao FC, Shi ZC, Guo WS, et al. Osteonecrosis in patients after severe acute respiratory syndrome (SARS): possible role of anticardiolipin antibodies. J Clin Rheumatol. 2010;16:61–63. doi: 10.1097/RHU.0b013e3181cf3464. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petri M. Pathogenesis and treatment of the antiphospholipid antibody syndrome. Med Clin North Am. 1997;81:151–177. doi: 10.1016/s0025-7125(05)70509-5. [DOI] [PubMed] [Google Scholar]
- 40.Simantov R, LaSala JM, Lo SK, Gharavi AE, Sammaritano LR, Salmon JE, et al. Activation of cultured vascular endothelial cells by antiphospholipid antibodies. J Clin Invest. 1995;96:2211–2219. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost. 2011;105:385–386. doi: 10.1160/TH10-08-0511. [DOI] [PubMed] [Google Scholar]