Abstract
Tumors evade immune-mediated recognition through multiple mechanisms of immune escape. On chronic tumor antigen exposure, T cells become dysfunctional/exhausted and upregulate various checkpoint inhibitory receptors (IRs) that limit T cells’ survival and function. During the last decade, immunotherapies targeting IRs such as programmed cell death receptor 1 (PD-1) and anticytotoxic T lymphocyte-associated antigen 4 (CTLA-4) have provided ample evidence of clinical benefits in many solid tumors. Beyond CTLA-4 and PD-1, multiple other IRs are also targeted with immune checkpoint blockade in the clinic. Specifically, T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT) is a promising new target for cancer immunotherapy. TIGIT is upregulated by immune cells, including activated T cells, natural killer cells, and regulatory T cells. TIGIT binds to two ligands, CD155 (PVR) and CD112 (PVRL2, nectin-2), that are expressed by tumor cells and antigen-presenting cells in the tumor microenvironment. There is now ample evidence that the TIGIT pathway regulates T cell-mediated and natural killer cell-mediated tumor recognition in vivo and in vitro. Dual PD-1/TIGIT blockade potently increases tumor antigen-specific CD8+ T cell expansion and function in vitro and promotes tumor rejection in mouse tumor models. These findings support development of ongoing clinical trials with dual PD-1/TIGIT blockade in patients with cancer.
Keywords: costimulatory and inhibitory T-cell receptors, immunotherapy, therapies, investigational
Introduction
Ample evidence supports the role of inhibitory receptors (IRs) in regulating innate and adaptive immunity in chronic viral infections and cancer.1 2 On chronic antigen stimulation, T cells become dysfunctional/exhausted and upregulate many IRs, including programmed cell death receptor 1 (PD-1) and T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT). At the same time, IR ligands are expressed by tumor cells and antigen-presenting cells (APCs) in the tumor microenvironment (TME). Targeting IRs with monoclonal antibodies (mAbs) has proven beneficial in mouse tumor models and humans, and immune checkpoint blockade (ICB) with anticytotoxic T lymphocyte-associated antigen 4 (CTLA-4), anti-PD-1, or both mAbs are standard treatments for many solid tumors.3–6 Further, multiple lines of evidence support that TIGIT plays a critical role in limiting adaptive and innate immunity against tumors.7–10 Here, we review results supporting the role of TIGIT in cancer immunology and the potency of TIGIT-based cancer immunotherapy.
TIGIT axis and ligands
TIGIT (also called WUCAM, Vstm3, VSIG9) is a receptor of the Ig superfamily, which plays a critical role in limiting adaptive and innate immunity.5–8 TIGIT participates in a complex regulatory network involving multiple IRs (eg, CD96/TACTILE, CD112R/PVRIG), one competing costimulatory receptor (DNAM-1/CD226), and multiple ligands (eg, CD155 (PVR/NECL-5), CD112 (Nectin-2/PVRL2)8 9 11–13, figure 1). Hence, there is some similarity with the CD28/CTLA-4/CD80/CD86 pathway, for which inhibitory and costimulatory receptors compete for binding to the same ligands. In sharp contrast with CTLA-4−/− mice, TIGIT−/− mice do not develop autoimmunity.10 However, as compared with wild-type mice, Tigit−/− mice develop more severe experimental autoimmune encephalitis when immunized with myelin oligodendrocyte glycoprotein.10 Such an observation supports the role of TIGIT as a negative regulator of T cell functions.
Figure 1.
The TIGIT/CD226/CD96/CD112R axis. TIGIT, CD226, CD96, and CD112R are expressed on activated T cells and NK cells. TIGIT ligands CD115 and CD112 are expressed on APCs or tumor cells. TIGIT binds CD155 and CD112 as well as Fap2, a gut bacterium-derived protein. TIGIT, CD96, CD112R, and CD155 contain ITIM motifs in their cytoplasmic tail that trigger inhibitory signals. TIGIT also contains an ITT-like motif. CD226 associates with LFA-1 and binds CD155 to deliver a positive signal. CD96 binds CD155, and whether this triggers inhibitory or activating signals in human T cells remain to be determined. CD112R binds CD112 to deliver an inhibitory signal through its ITIM. APCs, antigen-presenting cells; ITIM, immunoreceptor tyrosine-based inhibitory motif; ITT, Ig tail-tyrosine; NK cells, natural killer cells; TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domain.
TIGIT is expressed by activated CD8+ T and CD4+ T cells, natural killer (NK) cells, regulatory T cells (Tregs), and follicular T helper cells in humans.7 8 14 15 In sharp contrast with DNAM-1/CD226, TIGIT is weakly expressed by naive T cells. In cancer, TIGIT is coexpressed with PD-1 on tumor antigen-specific CD8+ T cells and CD8+ tumor-infiltrating lymphocytes (TILs) in mice and humans.16 17 It is also coexpressed with other IRs, such as T cell immunoglobulin and mucin domain-containing molecule-3 (TIM-3) and lymphocyte activation gene 3 (LAG-3), on exhausted CD8+ T cell subsets in tumors.16 17 Further, TIGIT is highly expressed by Tregs in peripheral blood mononuclear cells of healthy donors and patients with cancer and further upregulated in the TME.18 19
Increased TIGIT expression is associated with hypomethylation and FOXP3 binding at the TIGIT locus in Tregs, and delineates Tregs from activated effector CD4+ T cells.20 In contrast to mouse splenic NK cells, circulating human NK cells exhibit high TIGIT expression, which regulates their tumor killing activity.21 As compared with TIGIT− NK cells, TIGIT+ NK cells exhibit higher cytotoxic capacity and maturation but paradoxically lower cytotoxicity against CD155+ major histocompatibility complex (MHC) class I-deficient melanoma cells.
In sharp contrast with CD8+ T cells, NK cells present at low frequencies in metastatic tumors are dysfunctional, and downregulate both TIGIT and CD226 expression.22 Membrane-bound CD155 triggers CD226 internalization and degradation, resulting in decreased NK cell-mediated tumor reactivity.22 TIGIT binds two ligands, CD155 and CD112 (figure 1 and table 1), that are expressed on monocytes, dendritic cells (DCs), and many non-hematopoietic cells including tumor cells of different histological types.9 16 23–25 TIGIT binds CD155 with higher affinity than competing receptors CD226 and CD968 9 (table 1). While TIGIT weakly binds CD112, CD112R binds CD112 with higher affinity than CD226.13 Interestingly, CD155 expression increases on reactive oxygen species-dependent activation of the DNA damage response, which regulates interactions of NK cells with T cells and with myeloid-derived suppressive cells (MDSCs).26 27 In addition, the Fap2 protein from Fusobacterium nucleatum, an anaerobic Gram− commensal bacteria associated with colorectal carcinoma, binds directly to TIGIT but not CD226 to inhibit NK-cell and T cell mediated tumor reactivity.28 These findings suggest that the gut microbiome regulates innate immune responses in a TIGIT-mediated fashion.
Table 1.
Ligand binding affinities for TIGIT, CD226, and CD112R
| Ligand/receptor affinity | TIGIT | CD226 | CD96 | CD112R |
| CD155 | 1–3 nM | 114–199 nM | 37.6 nM | – |
| CD112 | Not measurable | 0.31–8.97 µM | – | 88 nM |
Ligand binding affinities for TIGIT, CD226, CD96, and CD112R have been previously reported.8 9 13 61
ITIM, immunoreceptor tyrosine-based inhibitory motif; TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domain.
TIGIT structure and signaling
TIGIT is composed of an extracellular immunoglobulin (Ig) variable domain, a type 1 transmembrane domain, and a cytoplasmic tail with two inhibitory motifs conserved in mouse and human: an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an Ig tail-tyrosine (ITT)-like motif.7–10 The crystal structure of TIGIT bound to CD155 reveals that two TIGIT/CD155 dimers assemble into a heterotetramer with a core TIGIT/TIGIT cis-homodimer, with each TIGIT molecule binding to one CD155 molecule.29 This cis–trans receptor clustering mediates cell adhesion and signaling.
In mice, phosphorylation of either the ITIM (Y227) or ITT-like motif residue (Y233) can trigger TIGIT inhibitory signal. However, in human NK cell line YTS, TIGIT/CD155 engagement initiates major inhibitory signaling through an ITT-like motif, while the ITIM motif mediates a minor inhibitory signal.8 30 On TIGIT/CD155 ligation, the ITT-like motif is phosphorylated at Tyr225 and binds to cytosolic adapter Grb230 and β-arrestin 231 to recruit SH2-containing inositol phosphatase-1 (SHIP-1). SHIP-1 impedes phosphoinositide 3 kinase and mitogen-activated protein kinase signaling.30 SHIP-1 also impairs TRAF6 and NF-κB activation,31 leading to inhibition of interferon (IFN)-γ production by NK cells.
Mechanisms of inhibition
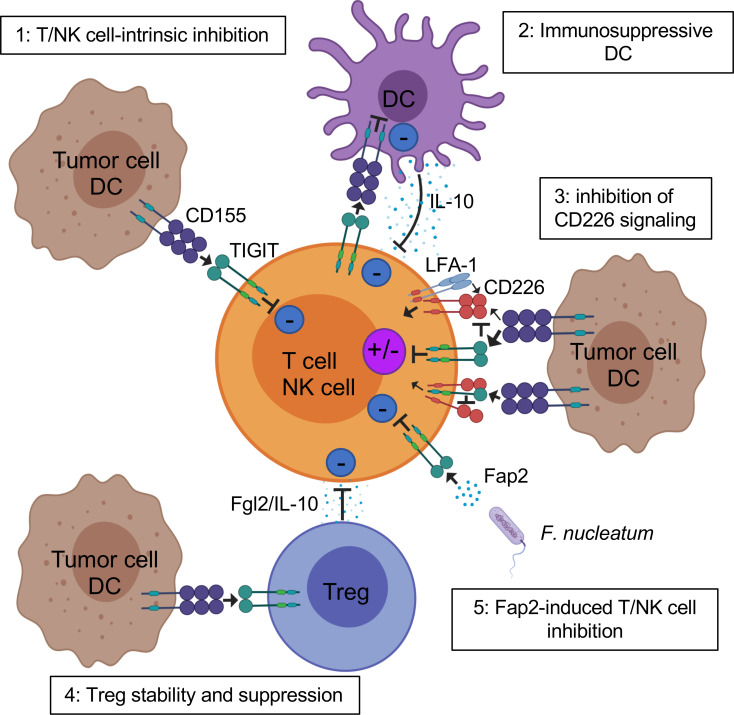
TIGIT potently inhibits innate and adaptive immunity through multiple mechanisms (figure 2). First, in mouse models, TIGIT indirectly impedes T cell function by binding to CD155 on DCs.9 TIGIT engagement on DCs induces CD155 phosphorylation and triggers a signaling cascade promoting tolerogenic DCs with decreased production of interleukin (IL)-12 and increased production of IL-10.9
Figure 2.
Mechanisms of TIGIT inhibition of T cells in the TME. TIGIT displays multiple inhibitory mechanisms in T cells. 1: TIGIT binds CD155 and triggers direct inhibitory signals in T cells. 2: TIGIT binds CD155 on APCs to trigger IL-10 production and decrease IL-12 production, which indirectly inhibits T cells. 3: TIGIT binds CD155 with higher affinity than CD226 or disrupts CD226 homodimerization to impede CD226-mediated T cell activation. 4: TIGIT signaling in Tregs enhances their immunosuppressive functions. 5: Fap2 protein from the gut bacteria Fusobacterium nucleatum binds TIGIT to trigger inhibitory signals. APCs, antigen-presenting cells; IL, interleukin; ITIM, immunoreceptor tyrosine-based inhibitory motif; TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domain; Tregs, regulatory T cells.
Second, TIGIT exhibits direct immune cell-intrinsic inhibitory effects. Agonistic anti-TIGIT antibodies inhibit T cell proliferation and function by attenuating T cell receptor (TCR)-driven activation signals.10 14 24 In mice and humans, TIGIT inhibits NK cell degranulation, cytokine production, and NK cell-mediated cytotoxicity of CD155-expressing tumor cells.8 30–32 Further, interaction of TIGIT+ NK cells with MDSCs expressing CD155 decreases phosphorylation of ZAP70/Syk and ERK1/2, reducing the cytolytic capacity of NK cells.27
Third, multiple lines of evidence show that TIGIT impedes CD155-mediated CD226 activation. CD226 is a costimulatory receptor widely expressed by immune cells, including T cells, NK cells, monocytes, and platelets.33 34 CD226 associates with LFA-1 to promote cell contact and triggers TCR signaling.35 This receptor also fosters production of proinflammatory cytokines by CD4+ T cells on binding to CD155.36 CD226 is directly involved in tumor recognition by T cells and NK cells in mice and humans,33 37 and CD226-deficient mouse CD8+ T cells and NK cells display immunological synapse defects impairing antitumor immunity.38 39 TIGIT binds CD155 with higher affinity than CD226, thus limiting CD226-mediated activation.8–10 TIGIT also directly binds CD226 in cis, disrupting its homodimerization and binding capacity to CD155.17
Fourth, the balance of TIGIT/CD226 expression regulates the effector function of T cells and NK cells. Abrogation of TIGIT expression with shRNA in TCR-activated CD4+ T cells increases T-bet expression and IFN-γ production, which are abolished on CD226 or CD155 blockade. In contrast, CD226 knockdown decreases T-bet expression and IFN-γ production.24 Further, CD226 blockade abrogates the effects of dual PD-1 and TIGIT blockade on proliferation and cytokine production of tumor antigen-specific CD8+ T cells in melanoma.16 Similarly, in CT26 tumor-bearing mice, the antitumor effects of dual programmed cell death-ligand 1 (PD-L1)/TIGIT blockade occur in a CD226-dependent fashion and are abolished on CD226 blockade.17 Interestingly, blocking anti-PD-1 and agonistic anti-GITR mAbs increases overall survival of MC38 tumor-bearing mice. In this model, PD-1 inhibition rescues CD8+ T cell dysfunction by inhibiting SHP2-mediated CD226 dephosphorylation, while anti-GITR mAbs decrease TIGIT expression.40 These important findings support that beyond PD-1 and TIGIT blockade, other ICBs enhance T cell-mediated tumor rejection by favorably tipping the balance between CD226 and TIGIT in CD8+ T cells.
Fifth, TIGIT acts in Tregs to augment immunosuppressive function and stability. TIGIT is highly expressed by a subset of natural Tregs in mice18 and the majority of Tregs in humans,18 19 41 and TIGIT upregulation in Tregs is associated with hypomethylation and Foxp3 binding at the TIGIT locus.20 TIGIT+ Tregs are more suppressive than TIGIT− Tregs in healthy donors and patients with melanoma.19 41 Further, TIGIT+ Tregs in the periphery and at tumor sites upregulate many Treg gene signature markers as compared with TIGIT− Tregs,18 including Foxp3, Helios, neuropilin-1, CTLA-4, PD-1, and LAG-3.18 19 TIGIT+ Tregs also suppress proinflammatory Th1 and Th17 but not Th2-type T cell responses.18 42 On TIGIT ligation, TIGIT+ Tregs produce IL-10 and fibrinogen-like protein 2, which mediate T cell suppression.18
Interestingly, human Foxp3+ Tregs exhibit lower CD226 expression than Foxp3− CD4+ T cells.19 41 CD226 is also downregulated by Tregs in metastatic melanoma as compared with the periphery, resulting in an increased TIGIT/CD226 ratio.19 TIGIT and CD226 oppose each other to augment or disrupt, respectively, Treg suppression and stability.19 A high TIGIT/CD226 ratio in Tregs appears to correlate with increased Treg frequencies in tumors and poor clinical outcome on ICB. Additional studies are needed to determine whether the TIGIT/CD226 ratio in Tregs may represent a biomarker of clinical response to ICB in patients with solid tumors.
Mice-bearing tumors with CD155 loss on host cells or tumor cells exhibit reduced tumor growth and enhanced effector functions of CD8+ and NK cells.43 While CD155 loss on host cells appears to act in a CD226-dependent fashion, CD155 loss in tumor cells promotes tumor growth and metastasis through tumor-intrinsic mechanisms. Further, CD155 deletion on both host and tumor cells results in greater tumor inhibition and increased effects of ICB. In addition, tumor CD155 expression is associated with increased tumor-infiltrating PD-1+ CD8+ T cells and resistance to anti-PD-1 immunotherapy in metastatic melanoma.44 Collectively, these findings suggest that targeting the CD155 pathway with combinatorial ICB (TIGIT and CD96) may improve response to PD-1 blockade.
TIGIT in cancer immunotherapy
Dual PD-1 and TIGIT blockade is a promising combinatorial immunotherapy of cancer. While each single blockade does not significantly impede growth of CT26 tumors in mice, dual TIGIT and PD-1/PD-L1 blockade synergizes to augment proliferation and function of antitumor CD8+ T cells, resulting in protective memory T cells, complete tumor rejection, and prolonged overall survival.17 45 46 These effects are abrogated on CD8+ T cell depletion, supporting a critical role of CD8+ T cell-mediated tumor reactivity.
Dual PD-1/TIGIT blockade also enhances proliferation and function of tumor antigen-specific CD8+ T cells and TILs isolated from patients with melanoma as compared with single blockade.16 47 Interestingly, dual PD-L1/TIGIT blockade (atezolizumab/tiragolumab) appears to provide superior clinical benefits as compared with PD-L1 blockade alone as a first-line therapy for patients with PD-L1-positive non-small cell lung cancers, despite similar toxicity profiles.48 However, these observations need to be confirmed in large randomized clinical trials.
The effects of dual PD-1/TIGIT blockade in mouse tumor models and in vitro are abrogated on CD226 blockade, suggesting that TIGIT blockade acts primarily by tipping CD155-mediated signaling towards CD226 activation.16 17 In addition, PD-1 induces SHP2-mediated CD226 dephosphorylation, supporting the need for dual PD-1/TIGIT blockade to promote CD226 signaling.40 Along this line, CD8+ TILs downregulate CD226 expression in multiple solid tumors, including melanoma, which may represent a significant obstacle limiting the effects of dual PD-1/TIGIT blockade in patients with cancer.16 49 Membrane-bound CD155 plays a critical role in mediating CD226 downregulation by immune cells in the TME via CD226 internalization and degradation, supporting the role of CD155-mediated immune dysfunction.22
In experimental models, TIGIT blockade or TIGIT deletion promotes NK cell-mediated antitumor reactivity in vitro and in vivo.8 27 30 32 50 Strikingly, one recent study of B16 melanoma and CT26 lung metastasis mouse models suggests that TIGIT blockade alone or in combination with PD-1 blockade acts primarily on NK cells to augment CD8+ T cell-mediated antitumor responses and impede tumor growth. In these experimental models, NK cell-specific TIGIT deficiency and NK cell depletion compromised the effects of TIGIT blockade.45 These findings are at odds with many observations supporting that TIGIT blockade alone fails to significantly augment CD8+ T cell immunity and promote tumor rejection in wild-type mice transplanted with solid tumors17 51 and in expanding tumor antigen-specific CD8+ T cell responses16 as compared with combined dual PD-1/TIGIT blockade. The mechanisms supporting potential helper effects of NK cells on CD8+ TILs as well as the relevance of these findings to patients with cancer remain elusive. Whether and how NK cells contribute to environmental cues guiding CD8+ T cell priming, maturation, and memory differentiation needs to be thoroughly determined. Interestingly, IL-15 together with TIGIT blockade increases NK cell-mediated melanoma cytotoxicity in vitro and decreases tumor metastasis in mouse melanoma models.22 These findings support development of novel combinatorial immunotherapy with IL-15 and TIGIT blockade to promote NK cell-mediated destruction of MHC class I-deficient melanoma, which is refractory to CD8+ T cell-mediated immunity.
Besides PD-1 blockade, other ICBs combined with TIGIT blockade also enhance antitumor immune responses. TIGIT blockade has been tested together with ICB targeting IRs outside of the TIGIT network. For example, TIGIT and TIM-3 synergize to suppress antitumor immune responses in mice.42 Adoptive transfer of mixed T cell subsets in subcutaneous B16F10-bearing Rag−/− mice, including CD8+ T cells, CD4+ T cells, and Tregs from wild-type and Tigit−/− mice, show that TIGIT depletion in Tregs but not CD8+ T cells decreases tumor growth. These data suggest that TIGIT can act primarily in Tregs to impede antitumor CD8+ T cell responses and promote tumor growth. TIGIT+ Treg-infiltrating tumors upregulate TIM-3, and blocking TIM-3 in Tigit−/− mice further decreases tumor size and increases overall survival.42 Because TIGIT competes with the IRs CD96 and CD112R for binding to its ligands, multiple studies have investigated the immunological and clinical effects of combinatorial therapies targeting PD-1 together with TIGIT and/or other IRs within the TIGIT network, including CD96 and CD112R. TIGIT synergizes with CD96 to inhibit antitumor responses—in tumor-bearing mouse models with lung metastasis, antitumoral effects of CD96 blockade are higher in Tigit−/− mice.52 CD96 blockade appears more effective in combination with anti-CTLA-4 or anti-PD-1, and its effects depend on NKs, CD226 signaling, and IFN-γ production. Further, TIGIT blockade alone or in combination with PD-1 blockade adds to CD96 blockade to significantly reduce B16 melanoma growth in wild-type and Cd155−/− mouse models.43 Notably, the role of CD96 as an IR remains controversial because there is also evidence that it can act as a costimulatory receptor in CD8+ T cells.53 Multiple experimental studies in mice and in vitro suggest that CD112R blockade combined with TIGIT blockade increases antitumor immune responses. CD112R blockade synergizes with TIGIT blockade to enhance human NK cell-triggered antibody-dependent cellular cytotoxicity (ADCC) against breast tumor cell lines in vitro.50 Dual CD112R/PD-L1 blockade also confers improved outcomes as compared with single blockade in mice with MC38 tumors.54 Further, CD112R blockade alone or combined with either TIGIT blockade or PD-1 blockade or both increases cytokine production by TILs from ovarian, endometrial, and lung tumors in the presence of allogeneic melanoma cells expressing surface-bound anti-CD3 antibody.55 However, the relevance of these findings to human cancer and autologous CD8+ T cell responses against well-defined tumor antigens remains to be demonstrated.
Multiple studies have suggested that anti-CTLA-4 mAbs act through ADCC-mediated Treg depletion.56–59 Because Tregs highly express TIGIT in the TME, one wonders whether anti-TIGIT mAbs with Fc-binding capability induce Treg depletion. Interestingly, in mouse tumor models, ICB with Fc variants of anti-TIGIT mAbs shows that selective FcγR coengagement on APCs enhances antigen-specific T cell responses and tumor reactivity without evidence of Treg depletion.60 Whether the antitumor effects of anti-TIGIT antibodies in patients with cancer are Fc-dependent remains to be determined. The answer to this critical question may be provided by multiple phase I and II clinical trials (table 2) that are testing Fc-engineered anti-TIGIT mAbs: IgG1 (MTIG7192/Genentech, MK-7684/Merck, and OP-313M32/Oncomed), inert-Fc IgG1 (BMS-986207/Bristol-Myers Squibb; AB-154/Arcus), and IgG4 (ASP8374/Potenza/Astellas).
Table 2.
Clinical trials targeting TIGIT, CD112R, and CD226
| Target | Drug (manufacturer) | Drug type | Protocol and tumor types | Therapeutic combinations |
| TIGIT | BMS-986207 (Bristol Myers Squibb) | TIGIT blocking human IgG1 mAb | Phase I/II in patients with multiple myeloma with relapse | BMS-986207 or Elotuzumab (anti-SLAMF7) or Relatimab (anti-LAG-3) +Potomalidimide +Dexamethasone |
| BGB-A1217 (BeiGene) | TIGIT blocking humanized IgG1 mAb | Phase I/Ib in patients with metastatic solid tumors | BGB-A1217 +Tislelizumab (anti-PD-1) |
|
| Tiragolumab, MTIG7192A (Genentech) | TIGIT blocking human IgG1 mAb | Phase II in chemotherapy-naive patients with locally advanced unresectable or metastatic PD-L1-selected non-small cell lung cancer | Tiragolumab or placebo +Atezolizumab (anti-PD-L1) |
|
| Tiragolumab, MTIG7192A (Genentech) | TIGIT blocking human IgG1 mAb | Phase III in patients with untreated extensive-stage small lung cell cancer | Tiragolumab or placebo +Atezolizumab (anti-PD-L1) +Etoposide +Carboplatin |
|
| Tiragolumab, MTIG7192A (Genentech) | TIGIT blocking human IgG1 mAb | Phase III in patients with untreated locally advanced, unresectable, or metastatic PD-L1-selected non-small cell lung cancer | Tiragolumab or placebo +Atezolizumab (anti-PD-L1) |
|
| Tiragolumab, MTIG7192A (Genentech) | TIGIT blocking human IgG1 mAb | Phase Ib/II in patients with locally advanced unresectable or metastatic gastro-esophageal junction cancer or esophageal cancer | Tiragolumab +Atezolizumab (anti-PD-L1) or Tiragolumab +Atezolizumab (anti-PD-L1) +Cisplatin +5-Fluorouracil or combinations without Tiragolumab |
|
| AB154 (Arcus Biosciences) |
TIGIT blocking humanized IgG1 mAb | Phase I in patients with advanced solid malignancies | AB154 +Zimberelimab (anti-PD-1) or Zimberelimab alone |
|
| AB154 (Arcus Biosciences) |
TIGIT blocking humanized IgG1 mAb | Phase II in patients with PD-L1 positive, locally advanced or metastatic non-small cell lung cancer | AB154 +Zimberelimab (anti-PD-1) AB154 +Zimberelimab +AB928 (anti-A2a/bR antagonist) or Zimberelimab alone |
|
| ASP8374 (Astella Pharma Global Development) |
TIGIT blocking human IgG4 mAb | Phase Ib in patients with advanced tumors | ASP8374 +Pembrolizumab (anti-PD-1) |
|
| MK-7684 (Merck Sharp & Dohme) | TIGIT blocking humanized IgG1 mAb | Phase I/II in patients with melanoma | MK-7684 +Pembrolizumab (anti-PD-1) or Pembrolizumab alone |
|
| MK-7684 (Merck Sharp & Dohme) | TIGIT blocking humanized IgG1 mAb | Phase I/II in patients with PD-1 refractory melanoma | MK-7684 or Lenvatinib +Pembrolizumab (anti-PD-1) +MK-1308 (anti-CTLA-4) |
|
| CD112R | COM701 (Compugen) | CD112R/PVRIG inhibitor | Phase I in patients with advanced solid tumors | COM701 +Nivolumab (anti-PD-1) or COM701 alone |
| CD226 | LY3435151 (Eli Lilly and Company) | CD226 agonist | Phase Ia/Ib in patients with advanced solid tumors | LY3435151 +Pembrolizumab (anti-PD-1) or LY3435151 alone |
Antibodies targeting TIGIT and drugs targeting CD112R or CD226 found on ClinicalTrials.gov (as of April 2020) that are currently active in clinical trials for the indicated tumor types, with the therapeutic combination listed.
IgG, immunoglobulin; mAb, monoclonal antibody; PD-1, programmed cell death receptor 1.
Concluding remarks, challenges, and critical questions
TIGIT is a promising target in cancer immunotherapy, particularly in combination with PD-1 blockade. Moving forward with ongoing TIGIT-based clinical trials in patients with cancer, however, we need to address many key questions and challenges. First, what mechanisms drive the effects of TIGIT blockade in patients with cancer? Are these effects primarily mediated by its direct activity in CD8+ T cells, Tregs, or both? Can TIGIT blockade reprogram APCs in the TME to increase T cell priming/activation? Can these effects be context-dependent and vary according to the disease stage? Can TIGIT blockade mediate NK cell-mediated tumor reactivity against MHC class I-deficient human tumors in vivo, and will this be an opportunity to provide clinical benefits to a subset of PD-1-refractory patients with cancer? And, in addition to dual PD-1/TIGIT blockade, is there any potential synergy/additive effect of CD112R or CD96 blockade as suggested by mouse tumor models and in vitro studies? In this regard, one has to keep in mind that the role of CD96 as an IR remains controversial.53 Further, evidence that CD112R blockade can potently enhance autologous human tumor antigen-specific CD8+ T cells is still missing. The answer to this important question may come from the phase I clinical trial evaluating the effects of one CD112R inhibitor alone or in combination with anti-PD-1 mAbs in patients with advanced solid tumors (NCT03667716, table 2).
In addition, CD226 plays a critical role as a master regulator of dual PD-1/TIGIT blockade. Its downregulation by CD8+ T cells and NK cells in the TME may represent a major obstacle for the success of dual PD-1/TIGIT blockade in the clinic. Therefore, it appears essential to design novel strategies to augment CD226 expression and signaling or prevent its downregulation in the TME. It is noteworthy that one ongoing clinical trial is testing agonistic anti-CD226 in multiple cancers (NCT04099277, table 2). Because of the role of CD226 in mediating platelet adhesion and activation, however, potential hematological adverse events will need to be monitored carefully.34 Finally, the many ongoing clinical trials using different Fc-engineered anti-TIGIT mAbs will likely help determine the role of FcγR coengagement in promoting the effects of TIGIT blockade in patients with cancer.
Footnotes
Contributors: J-MC and HMZ wrote the manuscript.
Funding: This work was supported by NIH/NCI grants R01CA228181 and R01CA222203 (to HMZ), a research grant by Bristol-Myers Squibb (to HMZ), a cancer vaccine collaborative clinical strategy team grant (to HMZ), NCI grant P50CA121973 (to JMK), and the James W and Frances G McGlothlin Chair in Melanoma Immunotherapy (to HMZ).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 1998;188:2205–13. 10.1084/jem.188.12.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009;10:29–37. 10.1038/ni.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33. 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res 2016;22:1856–64. 10.1158/1078-0432.CCR-15-1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, et al. Five-Year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or Non–Small cell lung cancer treated with nivolumab. JAMA Oncol 2019;5. 10.1001/jamaoncol.2019.2187. [Epub ahead of print: 25 Jul 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid O, Robert C, Daud A, et al. Five-Year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019;30:582–8. 10.1093/annonc/mdz011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boles KS, Vermi W, Facchetti F, et al. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur J Immunol 2009;39:695–703. 10.1002/eji.200839116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanietsky N, Simic H, Arapovic J, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A 2009;106:17858–63. 10.1073/pnas.0903474106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu X, Harden K, Gonzalez LC, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 2009;10:48–57. 10.1038/ni.1674 [DOI] [PubMed] [Google Scholar]
- 10.Levin SD, Taft DW, Brandt CS, et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol 2011;41:902–15. 10.1002/eji.201041136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottino C, Castriconi R, Pende D, et al. Identification of PVR (CD155) and nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med 2003;198:557–67. 10.1084/jem.20030788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seth S, Maier MK, Qiu Q, et al. The murine pan T cell marker CD96 is an adhesion receptor for CD155 and nectin-1. Biochem Biophys Res Commun 2007;364:959–65. 10.1016/j.bbrc.2007.10.102 [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Paniccia A, Schulick AC, et al. Identification of CD112R as a novel checkpoint for human T cells. J Exp Med 2016;213:167–76. 10.1084/jem.20150785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joller N, Hafler JP, Brynedal B, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol 2011;186:1338–42. 10.4049/jimmunol.1003081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu H, Chen Y, Liu H, et al. Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice. Eur J Immunol 2016;46:1152–61. 10.1002/eji.201546094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chauvin J-M, Pagliano O, Fourcade J, et al. Tigit and PD-1 impair tumor antigen-specific CD8⁺ T cells in melanoma patients. J Clin Invest 2015;125:2046–58. 10.1172/JCI80445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014;26:923–37. 10.1016/j.ccell.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 18.Joller N, Lozano E, Burkett PR, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014;40:569–81. 10.1016/j.immuni.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fourcade J, Sun Z, Chauvin J-M, et al. Cd226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight 2018;3. 10.1172/jci.insight.121157. [Epub ahead of print: 26 Jul 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Maksimovic J, Naselli G, et al. Genome-Wide DNA methylation analysis identifies hypomethylated genes regulated by FOXP3 in human regulatory T cells. Blood 2013;122:2823–36. 10.1182/blood-2013-02-481788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Hou H, Wu S, et al. Tigit expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J Immunol 2015;45:2886–97. 10.1002/eji.201545480 [DOI] [PubMed] [Google Scholar]
- 22.Chauvin J-M, Ka M, Pagliano O, et al. IL15 stimulation with TIGIT blockade reverses CD155-mediated NK-cell dysfunction in melanoma. Clin Cancer Res 2020. 10.1158/1078-0432.CCR-20-0575. [Epub ahead of print: 26 Jun 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs A, Cella M, Giurisato E, et al. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155). J Immunol 2004;172:3994–8. 10.4049/jimmunol.172.7.3994 [DOI] [PubMed] [Google Scholar]
- 24.Lozano E, Dominguez-Villar M, Kuchroo V, et al. The TIGIT/CD226 axis regulates human T cell function. J Immunol 2012;188:3869–75. 10.4049/jimmunol.1103627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pende D, Bottino C, Castriconi R, et al. Pvr (CD155) and nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol 2005;42:463–9. 10.1016/j.molimm.2004.07.028 [DOI] [PubMed] [Google Scholar]
- 26.Ardolino M, Zingoni A, Cerboni C, et al. Dnam-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK-T cell interaction. Blood 2011;117:4778–86. 10.1182/blood-2010-08-300954 [DOI] [PubMed] [Google Scholar]
- 27.Sarhan D, Cichocki F, Zhang B, et al. Adaptive NK cells with low TIGIT expression are inherently resistant to myeloid-derived suppressor cells. Cancer Res 2016;76:5696–706. 10.1158/0008-5472.CAN-16-0839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015;42:344–55. 10.1016/j.immuni.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stengel KF, Harden-Bowles K, Yu X, et al. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc Natl Acad Sci U S A 2012;109:5399–404. 10.1073/pnas.1120606109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Zhang H, Li M, et al. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ 2013;20:456–64. 10.1038/cdd.2012.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Xia P, Du Y, et al. T-Cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (Pvr) ligand engagement suppresses interferon-γ production of natural killer cells via β-arrestin 2-mediated negative signaling. J Biol Chem 2014;289:17647–57. 10.1074/jbc.M114.572420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanietsky N, Rovis TL, Glasner A, et al. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur J Immunol 2013;43:2138–50. 10.1002/eji.201243072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibuya A, Campbell D, Hannum C, et al. Dnam-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 1996;4:573–81. 10.1016/S1074-7613(00)70060-4 [DOI] [PubMed] [Google Scholar]
- 34.Kojima H, Kanada H, Shimizu S, et al. Cd226 mediates platelet and megakaryocytic cell adhesion to vascular endothelial cells. J Biol Chem 2003;278:36748–53. 10.1074/jbc.M300702200 [DOI] [PubMed] [Google Scholar]
- 35.Shibuya K, Lanier LL, Phillips JH, et al. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity 1999;11:615–23. 10.1016/S1074-7613(00)80136-3 [DOI] [PubMed] [Google Scholar]
- 36.Lozano E, Joller N, Cao Y, et al. The CD226/CD155 interaction regulates the proinflammatory (Th1/Th17)/anti-inflammatory (Th2) balance in humans. J Immunol 2013;191:3673–80. 10.4049/jimmunol.1300945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lakshmikanth T, Burke S, Ali TH, et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest 2009;119:1251–63. 10.1172/JCI36022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsbottom KM, Hawkins ED, Shimoni R, et al. Cutting edge: DnaX accessory molecule 1-deficient CD8+ T cells display immunological synapse defects that impair antitumor immunity. J Immunol 2014;192:553–7. 10.4049/jimmunol.1302197 [DOI] [PubMed] [Google Scholar]
- 39.Kim JS, Shin BR, Lee HK, et al. Cd226-/- natural killer cells fail to establish stable contacts with cancer cells and show impaired control of tumor metastasis in vivo. Oncoimmunology 2017;6:e1338994. 10.1080/2162402X.2017.1338994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang B, Zhang W, Jankovic V, et al. Combination cancer immunotherapy targeting PD-1 and GITR can rescue CD8+ T cell dysfunction and maintain memory phenotype. Sci Immunol 2018;3. 10.1126/sciimmunol.aat7061. [Epub ahead of print: 02 Nov 2018]. [DOI] [PubMed] [Google Scholar]
- 41.Fuhrman CA, Yeh W-I, Seay HR, et al. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J Immunol 2015;195:145–55. 10.4049/jimmunol.1402381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurtulus S, Sakuishi K, Ngiow S-F, et al. Tigit predominantly regulates the immune response via regulatory T cells. J Clin Invest 2015;125:4053–62. 10.1172/JCI81187 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Li X-Y, Das I, Lepletier A, et al. Cd155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms. J Clin Invest 2018;128:2613–25. 10.1172/JCI98769 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Lepletier A, Madore J, O'Donnell JS, et al. Tumor CD155 expression is associated with resistance to anti-PD1 immunotherapy in metastatic melanoma. Clin Cancer Res 2020;26:clincanres.3925.2019. 10.1158/1078-0432.CCR-19-3925 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol 2018;19:723–32. 10.1038/s41590-018-0132-0 [DOI] [PubMed] [Google Scholar]
- 46.He W, Zhang H, Han F, et al. CD155T/TIGIT Signaling Regulates CD8+ T-cell Metabolism and Promotes Tumor Progression in Human Gastric Cancer. Cancer Res 2017;77:6375–88. 10.1158/0008-5472.CAN-17-0381 [DOI] [PubMed] [Google Scholar]
- 47.Inozume T, Yaguchi T, Furuta J, et al. Melanoma cells control antimelanoma CTL responses via interaction between TIGIT and CD155 in the effector phase. J Invest Dermatol 2016;136:255–63. 10.1038/JID.2015.404 [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Abreu D, Johnson ML, Hussein MA, et al. Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). JCO 2020;38:9503 10.1200/JCO.2020.38.15_suppl.9503 [DOI] [Google Scholar]
- 49.Kong Y, Zhu L, Schell TD, et al. T-Cell immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T-cell exhaustion and poor clinical outcome in AML patients. Clin Cancer Res 2016;22:3057–66. 10.1158/1078-0432.CCR-15-2626 [DOI] [PubMed] [Google Scholar]
- 50.Xu F, Sunderland A, Zhou Y, et al. Blockade of CD112R and TIGIT signaling sensitizes human natural killer cell functions. Cancer Immunol Immunother 2017;66:1367–75. 10.1007/s00262-017-2031-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dixon KO, Schorer M, Nevin J, et al. Functional Anti-TIGIT antibodies regulate development of autoimmunity and antitumor immunity. J Immunol 2018;200:3000–7. 10.4049/jimmunol.1700407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blake SJ, Stannard K, Liu J, et al. Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov 2016;6:446–59. 10.1158/2159-8290.CD-15-0944 [DOI] [PubMed] [Google Scholar]
- 53.Chiang EY, de Almeida PE, de Almeida Nagata DE, et al. CD96 functions as a co-stimulatory receptor to enhance CD8+ T cell activation and effector responses. Eur J Immunol 2020;50:891–902. 10.1002/eji.201948405 [DOI] [PubMed] [Google Scholar]
- 54.Murter B, Pan X, Ophir E, et al. Mouse PVRIG Has CD8 + T Cell–Specific Coinhibitory Functions and Dampens Antitumor Immunity. Cancer Immunol Res 2019;7:244–56. 10.1158/2326-6066.CIR-18-0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whelan S, Ophir E, Kotturi MF, et al. PVRIG and PVRL2 Are Induced in Cancer and Inhibit CD8+ T-cell Function. Cancer Immunol Res 2019;7:257–68. 10.1158/2326-6066.CIR-18-0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arce Vargas F, Furness AJS, Litchfield K, et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell 2018;33:649–63. 10.1016/j.ccell.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X, Song X, Li K, et al. FcγR-Binding is an important functional attribute for immune checkpoint antibodies in cancer immunotherapy. Front Immunol 2019;10:292. 10.3389/fimmu.2019.00292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furness AJS, Vargas FA, Peggs KS, et al. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends Immunol 2014;35:290–8. 10.1016/j.it.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 59.Ha D, Tanaka A, Kibayashi T, et al. Differential control of human Treg and effector T cells in tumor immunity by Fc-engineered anti-CTLA-4 antibody. Proc Natl Acad Sci U S A 2019;116:609–18. 10.1073/pnas.1812186116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waight JD, Chand D, Dietrich S, et al. Selective FcγR Co-engagement on APCs Modulates the Activity of Therapeutic Antibodies Targeting T Cell Antigens. Cancer Cell 2018;33:1033–47. 10.1016/j.ccell.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tahara-Hanaoka S, Shibuya K, Onoda Y, et al. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112). Int Immunol 2004;16:533–8. 10.1093/intimm/dxh059 [DOI] [PubMed] [Google Scholar]