Abstract
Two 59-year-old male patients with COVID-19 pneumonia developed pulmonary cavitation with air-fluid level, accompanied by right-sided chest pain several weeks after first onset of symptoms. Considering a possible bacterial abscess formation, both patients were started on antibiotics. No microbiological pathogen was detected in further investigations (sputum analysis, bronchoscopy with bronchoalveolar lavage and CT-guided drainage of the cavitation). Histopathological analysis of the drained fluid was non-specific, and the aetiology remained not fully understood. We report pulmonary cavitation as a rare finding in late stage COVID-19 pneumonia. As both our patients presented with localised chest pain prior to detection of the lesions, new onset of this symptom should warrant further investigation.
Keywords: pneumonia (infectious disease), radiology, pneumonia (respiratory medicine)
Background
Up until the submission of this paper over 12 million people worldwide and over 30 000 people in Switzerland have been diagnosed with SARS-CoV-2 infection. At time of publication, the reference standard diagnostic test according to the WHO is real-time reverse transcriptase PCR (RT-PCR) of a pharyngeal swab. In addition to rising serological testing of serum antibodies, CT of the chest is often performed to further support diagnosis and characterise pulmonary affection. So far, only few case reports describe the appearance of an air-containing space in CT scans in the early stages of COVID-19 infection.1–5 In some review articles,6 7 cavitation is mentioned as a rare finding in chest radiography in patients with COVID-19. However, to the best of our knowledge, there has not been any reported case of spontaneous large pulmonary cavities with air-fluid levels in the late phase of COVID-19. Therefore, we will discuss two patients who developed such lesions approximately 1 month after first onset of symptoms.
Case presentation
Patient 1
A 59-year-old man was admitted to our institution on 20 March 2020 with a 3-week history of fever, dry cough and sore throat starting 1 March after a holiday in Northern Italy. In the following weeks, he reported a weight loss of 10 kg due to reduced appetite and altered sense of taste. Other than for psoriasis vulgaris, his medical history was unremarkable, and he took no medication, especially no immunosuppressant. He had a smoking history of 38 pack-year but stopped 9 years ago.
On physical examination, vital signs were normal except for oxygen saturation (SpO2) of 91% breathing ambient air (table 1). Pulmonary examination revealed distant breath sounds on the right side of the chest, with unremarkable cardiac, abdominal and neurological examination. Laboratory studies showed normal leucocyte count with borderline lymphopenia, C reactive protein (CRP) 162 mg/L and low procalcitonin (PCT). Nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 were negative on 2 consecutive days. Blood cultures showed no growth. Chest radiography on admission displayed extensive bilateral peripheral patchy and irregular infiltrates with subpleural sparing. CT of the chest (figure 1A) demonstrated small patches of paraseptal emphysema. Some of the infiltrates included small areas of minor ground-glass change. There was relative sparing of the anterior and central parts, as well as the basal parts of both lungs, but neither enlarged lymph nodes nor effusion.
Table 1.
Changes in key indicators of laboratory results and vital signs—patient 1
| Day after symptom onset | CRP (mg/L) | Leucocyte count (×109/L) | Lymphocytes (×109/L) | PCT (ng/mL) | SpO2 (%) | Supplemental oxygen (L/min) | RR (breaths/min) | HR (beats/min) | BP (mmHg) | T (°C) | Comment |
| 10 | 78 | 3.1 | 0.6 | Primary care physician’s appointment | |||||||
| 20 | 162 | 6.18 | 1.05 | 0.05 | 91 | 14 | 81 | 137/98 | 36.7 | First admission | |
| 23 | 29 | 5.4 | 1.11 | 95 | 68 | 114/76 | 36.8 | First discharge | |||
| 33 | 37 | 6.18 | 1.11 | 92 | 0–2 | 24 | 90 | 133/75 | 37.2 | Second admission | |
| 36 | 10 | 4.89 | 91 | 12 | 67 | 111/80 | 36.6 | ||||
| 37 | 97 | 14 | 64 | 118/81 | 36.8 | Second discharge | |||||
| 67 | 1.1 | 4.4 | 1.45 | <0.05 | 97 | 61 | 116/65 | 36.3 | Third admission | ||
| 72 | 14.1 | 4.12 | 96 | 63 | 126/78 | 36.3 | |||||
| 73 | 94 | 60 | 123/78 | 36.5 | Third discharge | ||||||
| 137 | 0.5 | 3.94 | 1.14 | 97 | 79 | 120/80 | Follow-up appointment |
BP, blood pressure; CRP, C reactive protein; HR, heart rate; PCT, procalcitonin; RR, respiratory rate; SpO2, oxygen saturation; T, temperature.
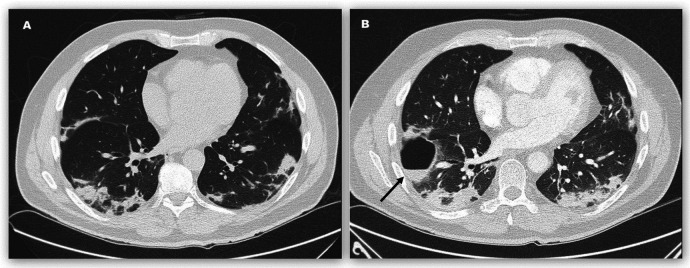
Figure 1.
CT of the chest of patient 1 on 20 March (A) and 2 April (B), days 20 and 33, after symptom onset. The cavity with air-fluid level (B, arrow) was situated anterior to a previously present consolidation patch.
With the presumptive diagnosis of atypical pneumonia, an empirical antibiotic therapy with levofloxacin was initiated. A high clinical and radiological suspicion of COVID-19 pneumonia remained. Until 23 March, the day of discharge, SpO2 improved and CRP declined to almost normal. Levofloxacin was discontinued after a total of 7 days.
Follow-up consultation with the primary care physician on 30 March was unremarkable, laboratory studies displayed normal values. One day later, the patient developed a sudden thoracic pain, worse during inspiration, localised at the lower frontal right-sided rib cage with accompanying dyspnoea. Following an episode of haemoptysis in the morning of 2 April, he was readmitted to our hospital. Conventional radiography of the chest showed a roundish cavity with an air-fluid level in the right lower lobe. On physical examination, vital signs were normal except for an elevated respiratory rate (RR) and slightly impaired SpO2. Pulmonary examination revealed distant breath sounds at the bases bilaterally, with the remainder of the physical examination being unremarkable.
Laboratory studies showed normal leucocyte count and CRP 37 mg/L. Screening for vasculitis (antinuclear and antineutrophil cytoplasmic antibody) and HIV was negative but antibody testing for SARS-CoV-2 was positive for IgM and IgG. CT of the chest (figure 1B) showed a thin-walled cavity, 7.3×4×2.5 cm, with an air-fluid level in the anterior segment of the right lower lobe, bordering the minor fissure. The previously present bilateral infiltrates showed minor progression but there was no association between the new cavity and previous areas of consolidation or emphysema and no pulmonary embolism.
Antibiotic therapy with levofloxacin was re-established and on 3 April bronchoscopy with bronchoalveolar lavage was performed. Macroscopically, no specific abnormalities were detected, and the cytological and microbiological results were negative for Mycobacteria, Mycoplasma, Chlamydia, Legionella, Aspergilla and a respiratory virus panel, including SARS-CoV-2.
One day after initiation of antibiotic therapy, the patient started feeling better and the thoracic pain disappeared. CRP dropped to almost normal on 5 April. He received intermittent supplemental oxygen up to a maximum of 2 L/min and on 6 April, the day of discharge, his SpO2 breathing ambient air and RR were back to normal.
Antibiotic therapy with levofloxacin was continued for a total of 4 weeks until 29 April. Follow-up chest radiography (figure 2) in the primary care setting on 4 May showed mild enlargement of the cavity in the right lower lobe with an increase in fluid content. Thereupon, the patient was readmitted to the hospital for further investigations. He reported mild exertional dyspnoea and mild fatigue. Physical examination was unremarkable, vital signs normal and laboratory studies showed no signs of inflammation.
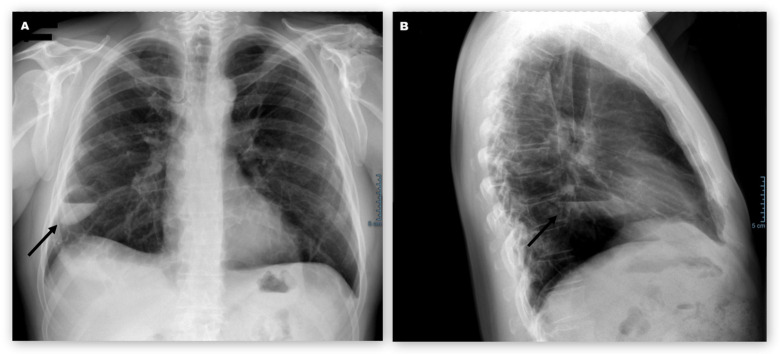
Figure 2.
Posteroanterior (A) and lateral (B) chest radiography of patient 1 on 4 May, day 65, after first symptoms and following a 4-week therapy with levofloxacin. Despite the antibiotics, the cavity (arrows) showed mild enlargement with an increase in fluid content.
CT-guided puncture of the cavity revealed 20 mL of red-brownish, turbid liquid, with pH of 7.23 and a cell count of 10×109/L with predominantly neutrophil granulocytes (more than 50%), scattered monocytes (25%) and few lymphocytes (10%). Microbiological results, including Ziehl-Neelsen stain, were negative, cultures showed no growth and RT-PCR for SARS-CoV-2 was negative. Diluted material was sent for further cytological analysis, disclosing degenerated blood with foam cells and blood residues, resembling an organising haematoma. No malignant cells were detected. Pre-emptive antibiotic therapy with amoxicillin/clavulanate was administered for a total of 7 days. The patient left the hospital on 12 May.
Patient 2
A 59-year-old man was admitted to our hospital on 26 March 2020 with a 10-day history of fever, cough and shortness of breath. Nasopharyngeal swab for SARS-CoV-2 was positive 8 days prior. He had a history of arterial hypertension and gastro-oesophageal reflux, taking a calcium-channel blocker for hypertension, atorvastatin and ezetimibe for hypercholesterinaemia, as well as a proton pump inhibitor. He had a smoking history of 20 pack-year but stopped 5 years prior. On physical examination, blood pressure and pulse were normal, temperature was 38.6°C, RR was 27 breaths/min and SpO2 was 93% breathing supplemental oxygen at a rate of 4 L/min (table 2). Pulmonary examination revealed crackles at the right lung base, with the remainder of the physical examination being unremarkable. Laboratory studies showed increased leucocyte count with lymphopenia and CRP 157 mg/L. Liver enzymes and parameters for cholestasis were elevated. Blood cultures showed no growth and serological testing for HIV and hepatitis B and C was negative. Chest radiography on admission showed bilaterally diffused and discretely increased density of the lung parenchyma.
Table 2.
Changes in key indicators of laboratory results and vital signs—patient 2
| Day after symptom onset | CRP (mg/L) | Leucocyte count (×109/L) | Lymphocytes (×109/L) | PCT (ng/mL) | SpO2 (%) | Supplemental oxygen (L/min) | RR (breaths/min) | HR (beats/min) | BP (mm Hg) | T (°C) | Comment |
| 11 | 157 | 14.7 | 0.47 | 93 | 4 | 27 | 76 | 141/83 | 38.6 | First admission | |
| 15 | 173 | 11.9 | 93 | 6–8 | 22 | 75 | 144/91 | 38 | |||
| 18 | 152 | 10.24 | 0.13 | 94 | 10–15 | 24 | 84 | 154/88 | 37.9 | ||
| 22 | 74 | 10.8 | 92 | 7–8 | 19 | 82 | 115/69 | 37 | |||
| 25 | 116 | 9.25 | <0.05 | 92 | 6 | 19 | 84 | 118/76 | 36.8 | ||
| 30 | 88 | 7.35 | 90 | 4 | 26 | 103 | 136/94 | 37.3 | |||
| 33 | 92 | 2 | 93 | 127/89 | 36.5 | First discharge | |||||
| 36 | 47 | 6.7 | 1.07 | Pulmonary rehabilitation | |||||||
| 37 | 77 | 9.9 | 1.54 | Pulmonary rehabilitation | |||||||
| 38 | 83 | 8.94 | 1.51 | <0.05 | 91 | 0–2 | 25 | 92 | 138/90 | 36.7 | Second admission |
| 40 | 37 | 6.13 | 1.5 | 93 | 0–2 | 84 | 128/92 | 36.5 | |||
| 43 | 16 | 6.13 | 93 | 78 | 150/95 | 36.5 | |||||
| 44 | 94 | 87 | 133/94 | 36.5 | Second discharge | ||||||
| 129 | 0.5 | 5.23 | 1.73 | 99 | 64 | 155/95 | Follow-up appointment |
BP, blood pressure; CRP, C reactive protein; HR, heart rate; PCT, procalcitonin; RR, respiratory rate; SpO2, oxygen saturation; T, temperature.
We diagnosed COVID-19 pneumonia and, according to the national recommendations at time, experimental therapy with hydroxychloroquine was administered for 5 days. While fever resolved within 3 days, dyspnoea worsened, and an increasing amount of supplemental oxygen with a flow rate of up to 15 L/min administered by non-rebreather reservoir mask was required. CT of the chest (figure 3A) on 9 April showed bilateral confluent irregular infiltrates with varying density and subpleural sparing. Neither emphysema, nor pulmonary embolism, nor enlarged mediastinal lymph nodes were visible.
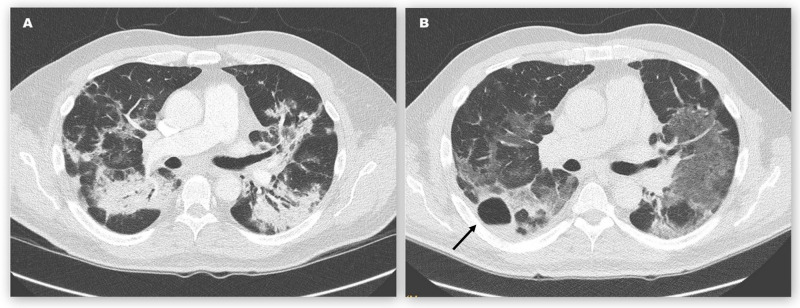
Figure 3.
CT of the chest of patient 2 on 9 April (A) and 22 April (B), days 25 and 38, after symptom onset. The cavity with air-fluid level (B, arrow) was situated anterior and lateral to a previously present consolidation patch.
On 13 April, the patient described a sudden onset of right-sided chest pain, worse during inspiration. After 4 days, the pain dissolved spontaneously, and SpO2 and RR started improving. CRP levels decreased without further treatment. He was discharged for pulmonary rehabilitation on 17 April with continuous oxygen therapy at a flow rate of 2 L/min.
Five days later. the patient was readmitted after radiological detection of a right-sided pulmonary cavity with air-fluid level and rising CRP. Leucocyte count remained normal. The patient, however, denied worsening of his symptoms. On physical examination, vital signs were normal except for RR of 25 breaths/min and SpO2 of 91% breathing supplemental oxygen at a rate of 2 L/min. Pulmonary examination revealed rales over the right upper lung zone with the remainder of the examination being unremarkable. Additional laboratory studies showed normal PCT. Sputum was RT-PCR negative for Mycobacterium tuberculosis and SARS-CoV-2, and showed no bacterial growth. Antibody testing for SARS-CoV-2 was positive for IgM and IgG. CT of the chest (figure 3B) confirmed a thin-walled cavity, 3.8 cm in diameter, with air-fluid level centred in the apical segment of the right lower lobe, bordering the major fissure. The previously present dense infiltrates had significantly improved but widespread ground-glass opacities in both lungs remained. There was no association between the new cavity and previous areas of consolidation.
Amoxicillin/clavulanate was administered for a total of 2 weeks. CRP declined and the patient’s respiratory status improved continuously with no further requirement of supplemental oxygen after the third day of readmission. He returned to pulmonary rehabilitation on 28 April.
Differential diagnosis
Following two negative nasopharyngeal and oropharyngeal swabs for SARS-CoV-2, we initially treated patient 1 for atypical pneumonia. A high suspicion of COVID-19 pneumonia remained, and the diagnosis was later postulated from the synopsis of clinical presentation after staying in a high-risk area before symptom onset, typical radiological findings and positive antibody testing more than a month after first symptom onset.
In patient 2, COVID-19 pneumonia was diagnosed with RT-PCR, supported by clinical and radiological findings.
No other pathogens were detected in further investigations. In patient 2, we only performed sputum analysis, whereas in patient 1 we aggressively tried to find underlying pathogens in bronchoalveolar lavage and CT-guided drainage of the cavitation. Histopathological analysis of the drainage was non-specific but could be interpreted as either abscess or organising haematoma.
Treatment
With the presumptive diagnosis of an atypical pneumonia, patient 1 initially received a 7-day empirical antibiotic course of levofloxacin. Patient 2 was, according to the national recommendations at time, experimentally treated with hydroxychloroquine for five days after the diagnosis of COVID-19 pneumonia. Considering a possible bacterial abscess formation, both patients were started on empirical antibiotics for 4 and 2 weeks, respectively, after pulmonary cavitation appeared. The choice of agent was made considering the previous treatments. After CT-guided puncture of patient 1, pre-emptive antibiotic therapy with amoxicillin/clavulanate was administered for a total of 7 days.
Outcome and follow-up
Follow-up consultations were performed about 20 weeks after symptom onset. Physical examination, laboratory studies, spirometry and chest radiography showed complete remission and both patients resumed work full-time.
Discussion
We present two 59-year-old male patients with COVID-19-pneumonia, who developed pulmonary cavitation with air-fluid level, accompanied by right-sided chest pain, several weeks after first onset of symptoms.
Thoroughly researching the current literature, we found reporting of intrapulmonary cavities in CT scans, all small, without air-fluid level and at early stages of COVID-19-infection.1–5 Jacobi et al6 demonstrate cavitation on chest radiography accompanied by spontaneous pneumothorax and without air-fluid level. Other reviews show no cavitation at all.8–17
Sun et al18 describe formation of pneumomediastinum, a giant bulla and multiple smaller bullae in the subpleural lung zone in a non-smoker without prior lung disease. Wu and Li7 show a growing right-sided cavity. In contrast to our cases, these patients received high flow nasal cannula oxygen therapy or invasive ventilation and there was no air-fluid level either. The history of both our patients as former smokers and the radiographically visible paraseptal emphysema in patient 1 suggest a possible underlying lung disease, making the tissue vulnerable for developing cavities. Maximum oxygen support in our patients was 2 L/min and 15 L/min via non-rebreather reservoir mask. Thus, an iatrogenic mechanical reason for their cavitation seems unlikely. However, alveolar rupture through coughing facilitated by diffuse alveolar injury in severe COVID-19 pneumonia is plausible, especially considering the coincidental chest pain. Local haemorrhage with consecutive organisation and cavitation is conceivable. This thesis is supported by cytological findings in patient 1, suggesting organising haematoma. However, the diluted character of the analysed material warrants careful interpretation of these results.
Ye et al5 discuss that a small air-containing space, they call air bubble sign, might be the pathological dilatation of a physiological space or associated with the process of consolidation and resorption. Necrotising processes with development of a cavity and air-fluid level after viral pneumonias in areas with initial consolidation have been described before.19 20 High cell count with predominantly neutrophil granulocytes and low pH in the aspirate from patient 1 suggest infectious aetiology, such as abscess formation. Furthermore, antibiotic therapy seemed to have a positive influence on inflammatory markers. The CT images (figures 1 and 3), however, showed no association between the cavities and previous areas of consolidation. Also, microbiological investigations remained sterile and the pulmonary cavity found in patient 1 developed and progressed despite an extended broad-spectrum antibiotic therapy with levofloxacin.
Lastly, several studies show coronaviruses causing severe dysregulation of the host immune reaction, resulting in endothelial cell injuries and apoptosis of cells.19 21 22 Localised destruction leading to cavitation or providing a foundation for both mechanical and infectious complications is conceivable.
In conclusion, we report pulmonary cavitation as a rare finding in late stage COVID-19 pneumonia. The aetiology of the reported pulmonary cavities is not fully understood, and further studies are necessary. Causes could be mechanical, infectious, immunological or a combination thereof. As both our patients presented with localised chest pain prior to detection of the lesions, new onset of this symptom warrants further investigation.
Learning points.
New onset of localised chest pain in patients with COVID-19 warrants further investigation.
Pulmonary cavities are a rare finding in late stage COVID-19 pneumonia.
The aetiology of the pulmonary cavities is not fully understood, and further studies are necessary.
Acknowledgments
We thank Dr Christoph Juli for his contribution to interpreting and reproducing CT scan and X-ray images.
Footnotes
Twitter: @mimuMD
Contributors: MM wrote the main part of the manuscript, supported by ES and FJW. PM contributed to revising and finalising the manuscript. All authors were involved in the patients’ care.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kong W, Agarwal PP. Chest imaging appearance of COVID-19 infection. Radiology: Cardiothoracic Imaging 2020;2:e200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salehi S, Abedi A, Balakrishnan S, et al. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 2020;215:1–7. 10.2214/AJR.20.23034 [DOI] [PubMed] [Google Scholar]
- 3.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425–34. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z, Pan A, Zhou H, et al. Rare CT feature in a COVID-19 patient: cavitation. Diagnostic and Interventional Radiology 2020;26:380–1. 10.5152/dir.2020.20181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Z, Zhang Y, Wang Y, et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobi A, Chung M, Bernheim A, et al. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin Imaging 2020;64:35–42. 10.1016/j.clinimag.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu G, Li X. Mobile x-rays are highly valuable for critically ill COVID patients. Eur Radiol 2020:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung M, Bernheim A, Mei X, et al. Ct imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 2020;295:202–7. 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Q, Guan H, Sun Z, et al. Early CT features and temporal lung changes in COVID-19 pneumonia in Wuhan, China. Eur J Radiol 2020;128:109017–17. 10.1016/j.ejrad.2020.109017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Zeng X, Liu B, et al. COVID-19 infection presenting with CT halo sign. Radiology: Cardiothoracic Imaging 2020;2:e200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T, Huang P, Liu H, et al. Spectrum of chest CT findings in a familial cluster of COVID-19 infection. Radiology: Cardiothoracic Imaging 2020;2:e200025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 2020:200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020;30:3306–9. 10.1007/s00330-020-06731-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song F, Shi N, Shan F, et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020;295:210–7. 10.1148/radiol.2020200274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Z, Guo D, Li C, et al. Coronavirus disease 2019: initial chest CT findings. Eur Radiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol 2020;21:541–4. 10.3348/kjr.2020.0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo HJ, Lim S, Choe J, et al. Radiographic and CT features of viral pneumonia. Radiographics 2018;38:719–39. 10.1148/rg.2018170048 [DOI] [PubMed] [Google Scholar]
- 20.Krutikov M, Rahman A, Tiberi S. Necrotizing pneumonia (aetiology, clinical features and management). Curr Opin Pulm Med 2019;25:225–32. 10.1097/MCP.0000000000000571 [DOI] [PubMed] [Google Scholar]
- 21.Hui DS, Memish ZA, Zumla A. Severe acute respiratory syndrome vs. the middle East respiratory syndrome. Curr Opin Pulm Med 2014;20:233–41. 10.1097/MCP.0000000000000046 [DOI] [PubMed] [Google Scholar]
- 22.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–8. 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]