Abstract
Background
In solid tumours, antibiotic use during immune checkpoint inhibitor (ICI) treatment is associated with shorter survival. Following allogeneic haematopoietic cell transplantation (allo-HCT), antibiotic-induced gut microbiome alterations are associated with risk of relapse and mortality. These findings suggest that the gut microbiota can modulate antitumour immune response across tumour types, though it is not clear if the impact on outcomes is specific to immune therapy. An important limitation of previous studies is that the analysis combined all antibiotic exposures irrespective of the antibiotic spectrum of activity. Whether antibiotic exposure during induction chemotherapy in acute myeloid leukaemia (AML) affects risk of relapse is also unknown.
Patients and methods
We performed a single-centred retrospective analysis of antibiotic exposures in metastatic/advanced non-small cell lung cancer (NSCLC) and renal cell cancer (RCC) receiving ICI and newly diagnosed AML patients receiving induction chemotherapy achieving a complete remission 1. Antibiotic use within 4 weeks before and 6 weeks after the ICI initiation were included. In AML patients, antibiotic exposures between days 1 and 28 of induction were collected. Antibiotics were a priori stratified based on spectrum of activity. Primary outcomes of interest were progression-free survival (PFS), overall survival (OS) in NSCLC and RCC and relapse-free survival (RFS) in AML.
Results
140 patients with NSCLC, 55 with RCC and 143 with AML were included. In multivariable analysis, PFS and OS were shorter in NSCLC patients who received broad-spectrum anti-anaerobes (PFS, HR=3.2, 95% CI 1.6 to 6.2, p<0.01; OS, HR=1.7, 95% CI 0.8 to 3.6, p=0.19) or ‘other’ antibiotics (vancomycin-predominant) (PFS, HR=2.4, 95% CI 1.3 to 4.6, p<0.01; OS, HR=2.4, 95% CI 1.2 to 4.7, p=0.01). In RCC, patients who received penicillins/penicillin-class/early-generation cephalosporins had shorter PFS (HR=3.6, 95% CI 1.7 to 7.6, p<0.01) but similar OS (p=0.37). In the AML cohort, none of the exposures were associated with RFS.
Conclusion
In contrast to AML, antibiotic exposures in solid tumours affected clinical outcomes. The presence of an allogeneic effect (allo-HCT) or an augmented immune system (checkpoint blockade) may be necessary for microbiota mediation of relapse risk.
Keywords: immunotherapy, antibiotics, acute myeloid leukaemia, non-small cell lung cancer, renal cell cancer
Key questions.
What is already known?
Antibiotics have been previously shown to negatively affect treatment outcomes of immune checkpoint blockers in solid tumours and associated with risk of leukaemia relapse post-allogeneic haematopoietic cell transplant.
What does this study add?
We tested the effect of exposure to different antibiotic subclasses based on spectrum of activity on clinical outcomes of immune checkpoint blocker in non-small cell lung cancer (NSCLC) and renal cell cancer (RCC) and tested the risk of relapse-free survival (RFS) in acute myeloid leukaemia (AML) following induction chemotherapy.
We found that NSCLC patients who received broad-spectrum anti-anaerobes (most commonly piperacillin-tazobactam) or intravenous vancomycin had shorter progression-free survival (PFS) and overall survival.
RCC patients who received penicillins, penicillin-class or early-generation cephalosporins had the greatest impact on PFS. In AML, none of the antibiotic exposures were associated with RFS.
How might this impact on clinical practice?
Antibiotics-induced gut microbiome dysbiosis can influence clinical outcomes of immunotherapy in solid tumours. The impact might be dependent on the type of antibiotic used and its spectrum of activity.
Introduction
The commensal gut microbiota has been increasingly recognised as a key constituent orchestrating the activity and repertoire of host immunity.1–5 Antibiotic use among cancer patients is common and can lead to disruption of the microbiome community structure and function (‘dysbiosis’). In allogenic haematopoietic cell transplant (allo-HCT) recipients, early post-transplant gut microbiota alterations predict mortality.6 Similarly, several studies have shown that antibiotic use just before initiation and during immune checkpoint inhibitors (ICI) use was associated with shorter progression-free survival (PFS) and overall survival (OS) in patients with non-small cell lung cancer (NSCLC), renal cell cancer (RCC), urothelial cancer and melanoma.7–26 In contrast to solid tumours treated with ICI, the success of cytotoxic chemotherapy in acute myeloid leukaemia (AML) depends on non-immune direct cytotoxicity, and thus, would not be expected to be changed by potential microbiota-mediated immune effects. However, the intestinal microbiota can modify antineoplastic effects of some chemotherapeutic agents.27 28 We therefore tested the effect of antibiotic exposure on immunotherapy outcomes in NSCLC and RCC and compared it with outcomes of AML patients receiving induction chemotherapy.
Methods
NSCLC and RCC cohorts
After Institutional Review Board (IRB) approval, we retrospectively identified consecutively diagnosed patients with relapsed or refractory advanced or metastatic NSCLC and RCC who received any single-agent ICI at any of the M-Health Fairview health system sites in the Minneapolis region between May 2015 and December 2017. Follow-up was completed as of August 2019. Patients who received at least two doses of ICI as a part of standard-of-care treatment were included. Patients receiving combination chemoimmunotherapy were excluded. Data on systemic antibacterial antibiotic use (inpatient or outpatient; oral or intravenous; any duration) within 1 month before and 6 weeks after the first dose of ICI were reviewed. Only patients surviving at least 6 weeks after the first ICI dose were included in the analysis. Antibiotic use was classified as any antibiotic use (yes vs no). A subclassification was performed for different antibiotic classes: group 1 (fluroquinolones: levofloxacin, ciprofloxacin, macrolides: azithromycin, tetracyclines: doxycycline, minocycline); group 2 (broad-spectrum anti-anaerobes (AA): piperacillin-tazobactam, clindamycin, metronidazole, meropenem); group 3 (third-generation and fourth-generation cephalosporins: cefdinir, cefpodoxime, ceftazidime, ceftriaxone, cefepime); group 4 (penicillins, penicillin-class or first-generation and second-generation cephalosporins: cefazolin, cephalexin, cefuroxime, amoxicillin, penicillin, ampicillin, nafcillin, oxacillin, ampicillin-sulbactam and group 5 (others: vancomycin, nitrofurantoin, rifampin, rifaximin, tobramycin, trimethoprim-sulfamethoxazole). PFS was defined as the time from first dose of ICI until disease progression or death as assessed by Response Evaluation Criteria in Solid Tumors (V.1.1). OS was measured as time from first dose of ICI to death.
AML cohort
After IRB approval, we retrospectively identified newly diagnosed, consecutive AML patients achieving a complete remission (CR1) with one or two rounds of standard-of-care intensive chemotherapy (including the 7+3 regimen: cytarabine plus daunorubicin or idarubicin) at the University of Minnesota Medical Center between December 2011 and June 2018. Antibacterial antibiotic exposures (oral or intravenous; prophylactic or therapeutic; any duration) between day 1 of induction and day 28 were extracted. The recommended antibiotic prophylaxis was levofloxacin, acyclovir and an azole. Cefepime was the recommended front-line empiric antibiotic for neutropenic fever. Since antibiotic types and exposure patterns are different between AML and NSCLC or RCC, we used a different classification system herein: fluoroquinolones (FQN): ciprofloxacin, levofloxacin; AA: piperacillin-tazobactam, clindamycin, metronidazole, imipenem, meropenem; third-generation or higher-generation cephalosporins (CPN3+): cefepime, ceftriaxone, ceftazidime; intravenous vancomycin (IV vanc) and oral vancomycin (oral vanc). Relapse-free survival (RFS) was measured as the time from achieving CR1 to disease relapse or death.
Statistical analysis
NSCLC and RCC cohorts
The primary end points were PFS per and OS at 1 year. The secondary end point was clinical benefit rate (CBR) defined as the proportion of patients with best response as CR, partial response or stable disease. Analysis was performed using any antibiotic exposures and in predefined antibiotic subgroups based on different antibiotic subclasses. Fisher’s exact test was used for univariate analysis of CBR. Multivariable logistic regression was used to assess the independent association of antibiotics with CBR. Kaplan-Meier (K-M) curves were used to estimate OS and PFS. Cox regression was used to assess the independent association of antibiotics with PFS and OS. Prespecified potential confounders in the NSCLC cohort were age, gender, Eastern Cooperative Oncology Group (ECOG) performance status (0–1 vs 2+), histology (adenocarcinoma vs squamous vs other) and prior lines of therapy (<2 vs ≥2). The same variables were used in the RCC cohort except that histology was excluded as 96% patients had clear cell histology.
AML cohort
The primary end point was RFS. Secondary end points included response (CR vs primary induction failure (PIF)), OS, relapse and non-relapse mortality (NRM). OS, relapse and NRM were measured from day 1 of chemotherapy in time-to-event analysis. Covariates for multivariable analysis included predetermined covariates important for relapse and RFS: age, AML risk (based on ELN-2017)29 and number of inductions (1 vs 2).
All reported p values were two-sided. SAS V.9.4 (SAS Institute) and R V.3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for analyses.
Results
NSCLC and RCC cohorts
Median follow-up was 11 months in NSCLC and 18.7 months in RCC. Of note, 148 metastatic NSCLC patients and 55 metastatic RCC patients received at least two doses of ICI. Eight patients with NSCLC were excluded from the final analysis because they died within 6 weeks of the first ICI dose. Final analysis included 140 patients with metastatic NSCLC and 55 with metastatic RCC. Table 1 shows clinical characteristics of the patient population. Median age was 66 years (range 39–92) and 62 years (range 23–86) in NSCLC and RCC cohorts, respectively. Almost all patients were Caucasian (93% of NSCLC; 95% of RCC) and most had adenocarcinoma (64% of NSCLC) or clear cell histology (93% of RCC). Most patients received nivolumab in the second or later line setting (88% of NSCLC and 93% of RCC). Of note, 54/140 (39%) and 24/55 (44%) patients received at least one antibiotic dose within 1 month before or 6 weeks after the first dose of ICI in NSCLC and RCC cohorts, respectively (online supplementary table S1).
Table 1.
Patient characteristics NSCLC and RCC cohort
| RCC | NSCLC | |
| N | 55 | 140 |
| Gender: male | 40 (73%) | 67 (48%) |
| Age | ||
| Median (range) | 62 (23–86) | 66 (39–92) |
| Race | 62 (23–86) | 66 (39–92) |
| Caucasian | 52 (95%) | 131 (93%) |
| African American | 2 (4%) | 3 (2%) |
| Others | 1 (2%) | 6 (3%) |
| Pathology | ||
| Adenocarcinoma | 0 | 91 (64%) |
| Squamous | 0 | 41 (29%) |
| Others | 0 | 8 (6%) |
| Clear cell | 53 (96%) | 0 |
| Papillary | 2 (4%) | 0 |
| Drug | ||
| Nivolumab | 51 (93%) | 123 (88%) |
| Pembrolizumab | 0 | 11 (8%) |
| Others | 4 (7%) | 6 (4%) |
| ECOG | ||
| 0–1 | 36 (66%) | 99 (71%) |
| >2 | 19 (34%) | 36 (25%) |
| Missing | 0 | 5 (4%) |
| Number of doses | ||
| Median (range) | 8 (2–63) | 7 (2–89) |
| Ongoing doses | ||
| NA | 1 (2%) | 0 |
| Yes | 11 (20%) | 12 (9%) |
| No | 43 (78%) | 128 (91%) |
| Months of therapy | ||
| Median (range) | 5 (1–35) | 3.9 (1–47) |
| Previous lines of therapy | ||
| Median (range) | 1 (0–5) | 1 (1–4) |
| Smoking status | ||
| Never | Not available | 15 (11%) |
| Former | Not available | 88 (63%) |
| Current | Not available | 30 (21%) |
ECOG, Eastern Cooperative Oncology Group; NSCLC, non-small cell lung cancer; RCC, renal cell cancer.
esmoopen-2020-000803supp001.pdf (92.3KB, pdf)
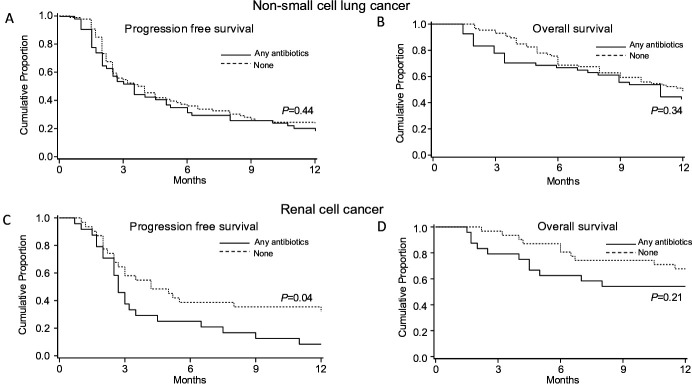
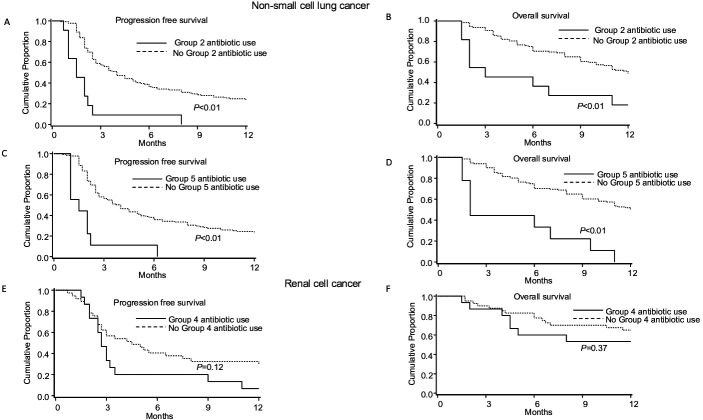
The results of univariate analysis for NSCLC are shown in online supplementary table S2. In multivariable analysis, any antibiotic use was not with PFS (median 3.5 vs 3.9 months, p=0.44) and OS (median 17 vs 11 months, p=0.34) (figure 1). Patients who received AA had shorter PFS (median 1.5 vs 4.0 months, HR=3.2, 95% CI 1.6 to 6.2; p<0.01) and OS (median 3.0 vs 12.0 months; HR=1.7, 95% CI 0.8 to 3.6; p=0.19) (figure 2 and table 2). Patients who received ‘other’ antibiotics had shorter PFS (median 2.0 vs 4.0 months, HR=2.4, 95% CI 1.3 to 4.6; p<0.01) and OS (median 2.0 vs 12.0 months; HR=2.4, 95% CI 1.2 to 4.7; p=0.01) (figure 2 and table 2). Antibiotic groups 1, 3 and 4 were not associated with PFS or OS (online supplementary table S1).
Figure 1.
Kaplan-Meier curves of progression free survival and overall survival in non-small cell lung cancer and renal cell cancer with any antibiotic use.
Figure 2.
Kaplan-Meier curves of progression free survival and overall survival in non-small cell lung cancer and renal cell cancer with specific antibiotic class based on spectrum of activity.
Table 2.
Multivariable analysis of PFS and OS in group 2 and group 5 antibiotics in NSCLC
| Group 2 (NSCLC) | Group 5 (NSCLC) | |||||||
| PFS, HR (95% CI) | P | OS, HR (95% CI) | P | PFS, HR (95% CI) | P | OS, HR (95% CI) | P | |
| 3.2 (1.6 to 6.2) | <0.01 | 1.7 (0.8 to 3.6) | 0.19 | 2.4 (1.3 to 4.6) | <0.01 | 2.4 (1.2 to 4.7) | 0.01 | |
| ECOG (0–1 vs 2+) | 1.6 (1.1 to 2.5) | 0.02 | 2.6 (1.6 to 4.3) | <0.01 | 1.6 (1.1 to 2.5) | 0.02 | 2.6 (1.2 to 4.7) | <0.01 |
| Histology | NS | NS | NS | NS | ||||
| A vs S | 1.0 (0.7 to 1.6) | 1.3 (0.7 to 2.1) | 1.1 (0.7 to 1.7) | 1.2 (0.7 to 2.1) | ||||
| A vs others | 1.3 (0.6 to 2.9) | 1.3 (0.5 to 3.4) | 1.4 (0.6 to 3.1) | 1.4 (0.6 to 3.6) | ||||
| Prior therapy (0–1 vs 2+) | 0.9 (0.6 to 1.2) | NS | 0.7 (0.4 to 1.2) | NS | 0.8 (0.5 to 1.2) | NS | 0.7 (0.4 to 1.2) | NS |
A, adenocarcinoma; ECOG, Eastern Cooperative Oncology Group; NS, non-significant; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; S, squamous.
The results of univariate analysis for RCC are shown in (online supplementary table S3). In multivariable analysis, any antibiotic use was associated with shorter PFS (median 2.7 vs 4.2 months, HR=2.7, 95% CI 1.3 to 5.9; p=0.01) and OS (median 17 vs 22 months, HR=4.2, 95% CI 1.5 to 12.2; p<0.01) (figure 1). Patients who received penicillins, penicillin-class or first-generation and second-generation cephalosporins had shorter PFS (median 2.7 vs 4.2 months, HR=3.6, 95% CI 1.7 to 7.6; p<0.01) but similar OS (p=0.37) (table 3 and figure 2). None of the other antibiotic groups were associated with PFS or OS (online supplementary table S3).
Table 3.
Multivariable analysis of PFS in group 4 antibiotics in RCC
| HR (95% CI) | P | |
| Variables | 3.6 (1.7 to 7.6) | <0.01 |
| Age | 0.9 (0.92 to 0.98) | <0.01 |
| Gender | 1.0 (0.5 to 2.1) | NS |
| ECOG (0–1 vs 2+) | 8.5 (3.8 to 18.8) | <0.01 |
ECOG, Eastern Cooperative Oncology Group; NS, non-significant; PFS, progression-free survival; RCC, renal cell carcinoma.
In multivariable analysis, in the NSCLC cohort, CBR was similar in patients who received any antibiotics compared with patients who were antibiotic-free (49% vs 54%; p=0.96). In contrast, patients who received AA or ‘other’ antibiotics had lower CBR (11% vs 55%; p=0.06 and 20% vs 54%; p=0.04), respectively. Results of CBR analysis in NSCLC are shown in online supplementary table S4. In the RCC cohort, patients who received any antibiotics had lower CBR compared with patients who were antibiotic-free (30% vs 59%; p=0.10). In addition, patients who received penicillins, penicillin-class or first-generation and second-generation cephalosporins had lower CBR (20% vs 57%; p<0.01). Results of CBR analysis in RCC are shown in online supplementary table S5. Indications for antibiotics in NSCLC and RCC are shown in online supplementary table S6.
AML cohort
Of note, 143 patients achieved CR1 and 27 patients had PIF. First, we analysed the CR1 subgroup. Median (range) age was 59 (19–75) years and 83 (58%) were men. ELN-2017 risk was poor, intermediate and favourable in 29 (20%), 80 (56%) and 34 (24%) patients, respectively. Forty-six (32%) patients received two inductions (table 4). All patients received antibacterial antibiotics for prophylaxis and/or treatment. Exposure to FQN occurred in 122 (85%), AA antibiotics in 116 (81%) patients, CPN3+ in 110 (77%) patients, IV vanc in 108 (76%) patients and oral vanc in 14 (10%) of patients. None of the antibiotic groups were associated with RFS (FQN: HR 0.75, 95% CI 0.42 to 1.36, p=0.35; AA antibiotics: HR 1.02, 95% CI 0.59 to 1.76, p=0.94; CPN3+: HR 1.29, 95% CI 0.77 to 2.16, p=0.34; IV vanc: HR 1.1, 95% CI 0.67 to 1.82, p=0.71; oral vanc: HR 0.63, 95% CI 0.28 to 1.45, p=0.28) (table 5). Censoring at the time of allo-HCT did not change the results. Figure 3 shows K-M curves for RFS.
Table 4.
Characteristics of the AML cohort who achieved first complete remission
| Total | 143 |
| Males, N (%) | 83 (58%) |
| Age (years), median (range) | 59 (19–75) |
| AML type, n (%) | |
| t-AML/s-AML | 16 (11%) |
| AML-MDS/MPN/haematological disorder | 27 (19%) |
| De-novo AML | 100 (59%) |
| ELN-2017 risk, n (%) | |
| Favourable | 34 (24%) |
| Intermediate | 80 (56%) |
| Adverse | 29 (20%) |
| Re-induction, n (%) | 46 (32%) |
AML, acute myeloid leukaemia; AML-MDS, AML with myelodysplastic changes; s-AML, secondary AML; t-AML, therapy-related AML.
Table 5.
Analysis of outcomes in the AML cohort
| Antibiotic | OS, HR (95% CI), p | RFS, HR (95% CI), p | Relapse, HR (95% CI), p | NRM, HR (95% CI), p |
| FQN (yes vs no) | 0.95 (0.47–1.92), 0.89 | 0.65 (0.35 to 1.2), 0.17 | 0.89 (0.43 to 1.84), 0.76 | 0.65 (0.18 to 2.3), 0.5 |
| Risk | ||||
| Favourable vs high | 0.64 (0.33 to 1.26), 0.2 | 0.55 (0.24 to 1.26), 0.16 | ||
| Intermediate vs high | 0.71 (0.41 to 1.23), 0.22 | 0.67 (0.36 to 1.28), 0.23 | ||
| Age | ||||
| 52–65 vs <52 | 1.53 (0.88 to 2.66), 0.13 | 1.47 (0.77 to 2.84), 0.25 | ||
| 66–75 vs <52 | 1.56 (0.88 to 2.76), 0.13 | 1.17 (0.59 to 2.36), 0.65 | ||
| Re-induction vs induction | 1.08 (0.68 to 1.7), 0.75 | 0.8 (0.45 to 1.43), 0.46 | ||
| AA antibiotics (yes vs no) | 1.02 (0.56–1.87), 0.94 | 1.12 (0.64 to 1.96), 0.68 | 1.39 (0.67 to 2.87), 0.38 | 0.92 (0.26 to 3.27), 0.9 |
| Risk | ||||
| Favourable vs high | 0.64 (0.32 to 1.26), 0.19 | 0.54 (0.24 to 1.24), 0.15 | ||
| Intermediate vs high | 0.72 (0.41 to 1.24), 0.24 | 0.65 (0.34 to 1.24), 0.2 | ||
| Age | ||||
| 52–65 vs <52 | 1.47 (0.85 to 2.54), 0.17 | 1.47 (0.77 to 2.82), 0.24 | ||
| 66–75 vs <52 | 1.49 (0.84 to 2.62), 0.17 | 1.15 (0.58 to 2.29), 0.68 | ||
| Re-induction vs induction | 1.06 (0.67 to 1.68), 0.81 | 0.85 (0.47 to 1.51), 0.57 | ||
| CPN3+ (yes vs no) | 1.07 (0.61–1.87), 0.82 | 1.19 (0.7 to 2.03), 0.51 | 1.49 (0.75 to 2.99), 0.26 | 0.79 (0.25–2.48), 0.69 |
| Risk | 0.56 (0.24 to 1.29), 0.17 | |||
| Favourable vs high | 0.65 (0.33 to 1.29), 0.22 | 0.71 (0.37 to 1.35), 0.3 | ||
| Intermediate vs high | 0.75 (0.43 to 1.3), 0.3 | |||
| Age | ||||
| 52–65 vs <52 | 1.45 (0.84 to 2.52), 0.18 | 1.43 (0.74 to 2.76), 0.29 | ||
| 66–75 vs <52 | 1.45 (0.82 to 2.57), 0.21 | 1.1 (0.54 to 2.21), 0.8 | ||
| Re-induction vs induction | 1.04 (0.66 to 1.64), 0.87 | 0.8 (0.45 to 1.43), 0.45 | ||
| IV vanc (yes vs no) | 1.25 (0.69–2.26), 0.46 | 1.08 (0.65 to 1.79), 0.78 | 0.74 (0.42 to 1.3), 0.3 | 4.85 (0.64 to 36.92), 0.13 |
| Risk | ||||
| Favourable vs high | 0.65 (0.33 to 1.27), 0.21 | 0.55 (0.24 to 1.26), 0.16 | ||
| Intermediate vs high | 0.73 (0.42 to 1.26), 0.26 | 0.67 (0.35 to 1.27), 0.22 | ||
| Age | ||||
| 52–65 vs <52 | 1.46 (0.84 to 2.52), 0.18 | 1.5 (0.78 to 2.89), 0.22 | ||
| 66–75 vs <52 | 1.49 (0.84 to 2.62), 0.17 | 1.17 (0.59 to 2.32), 0.65 | ||
| Re-induction vs induction | 1.03 (0.65 to 1.63), 0.89 | 0.83 (0.47 to 1.48), 0.53 | ||
| Oral vanc (yes vs no) | 0.69 (0.28–1.71), 0.42 | 0.58 (0.25 to 1.35), 0.2 | 1.02 (0.43 to 2.42), 0.97 | 0 (0 to Inf), 1 |
| Risk | ||||
| Favourable vs high | 0.64 (0.33 to 1.27), 0.2 | 0.55 (0.24 to 1.25), 0.15 | ||
| Intermediate vs high | 0.72 (0.41 to 1.24), 0.23 | 0.67 (0.35 to 1.28), 0.23 | ||
| Age | ||||
| 52–65 vs <52 | 1.51 (0.88 to 2.61), 0.14 | 1.46 (0.76 to 2.81), 0.26 | ||
| 66–75 vs <52 | 1.46 (0.82 to 2.58), 0.19 | 1.16 (0.58 to 2.31), 0.68 | ||
| Re-induction vs induction | 1.09 (0.69 to 1.73), 0.7 | 0.8 (0.45 to 1.43), 0.45 |
AA, broad-spectrum anti-anaerobes; AML, acute myeloid leukaemia; CPN3+, third-generation or higher-generation cephalosporins; FQN, fluoroquinolones; IV vanc, intravenous vancomycin; NRM, non-relapse mortality; oral vanc, oral vancomycin; OS, overall survival; RFS, relapse-free survival.
Figure 3.
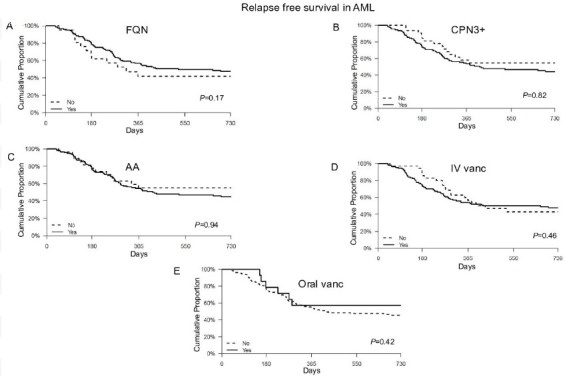
Kaplan-Meier curves of relapse free survival outcomes in acute myeloid leukemia with use of specific antibiotic class based on spectrum of activity. AA, broad-spectrum anti-anaerobic antibiotics; CPN3+, third-generation or higher-generation cephalosporins; FQN, fluroquinolone; IV vanc, intravenous vancomycin; oral vanc, oral vancomycin.
Twenty-seven patients had PIF. There were no differences between CR1 and PIF rates in patients with or without exposure to each antibiotic group except IV vanc, where PIF was higher in the exposed group (19% vs 3%; p=0.018). There was no association of OS, relapse and NRM with exposure to any of the antibiotic groups (table 5).
Discussion
In this study, we compared the association of antibiotic use with clinical outcomes among two distinct cohorts, that is, patients with solid tumours (RCC and NSCLC) receiving immunotherapy and patients with AML receiving induction chemotherapy. We systematically evaluated the impact of exposure to different antibacterial antibiotic classes and explored the effect of exposure duration on clinical outcomes. We found that in contrast to chemotherapy-treated AML, exposure to some antibiotic groups during immunotherapy-treated NSCLC/RCC was associated with clinical outcomes. To our knowledge, this is the first study to test the association of antibiotic use with RFS in the non-transplant setting. Previous studies in allo-HCT recipients showed an association between early post-transplant gut microbiota alterations and transplant-related mortality30 or relapse of haematological malignancies.6 Our study only looked at the time period during induction chemotherapy which may be one reason for the observed difference. Alternatively, our study may suggest that for microbiota-mediated increased risk of relapse, an augmented immune system by allogeneic effect may be necessary. Further studies directly evaluating microbiome profile during chemotherapy and after allo-HCT may be informative.
In contrast to previously published studies in NSCLC,8–13 16 21–23 26 31 our study did not show an association of antibiotic use with PFS or OS. One reason for this discrepancy could be related to the differences in the chosen window of antibiotic exposure among different studies. With respect to NSCLC, our study is the only one to assess antibiotic use during the window of 10 weeks around ICI use. We chose the 6-week post-ICI initiation window to allow at least 2–3 doses of nivolumab, the most commonly used ICI in the study population. In comparison, other studies in NSCLC assessed a range of different antibiotic exposure windows. Some of the studies tested only during the period before immunotherapy (up to 2 months).12 22 26 Others chose arbitrary time windows anywhere from up to 2 months before beginning immunotherapy7–11 13 21 23 26 or through the entire course of immunotherapy,8 23 the most common time frame being less than 2 months before initiation or 1 month after ICI therapy.7 9 13 Some of the studies also included patients with exposure to antibiotics even several months after initiation of immunotherapy8 23 which also includes antibiotic exposure at times in which maximal clinical benefit had already been obtained. Nonetheless, a pooled analysis of studies suggested that antibiotic use up to 30 days before ICI initiation has the greatest potential effect on ICI outcomes,8 14 15 further suggesting that stratification by timing of antibiotic exposure is critical.
Unlike the previous studies, which combined all antibiotic exposures as a single event, we tested the effect of antibiotic subgroups depending on the spectrum of antimicrobial activity. In NSCLC, we found significant association of concurrent use of AA antibiotics and ‘other’ antibiotics on CBR, PFS and OS. Piperacillin-tazobactam was the most commonly used anti-anaerobic and IV vanco was the most commonly used ‘other’ antibiotics. It has been shown that broad-spectrum antibiotic use may cause sustained impairment of mucosal and systemic immunity by affecting macrophages and cytotoxic T cells.31 32 Our findings, while exploratory, underscore the importance of distinguishing between concomitant exposure to broad-spectrum combination antibiotic treatments from single-agent or narrow-spectrum antibiotics and sequential exposures to individual antibiotics which may allow enough time for microbiome repopulation between exposures.
Our study in RCC showed a significant association of any antibiotic exposure to PFS, CBR and OS which is in agreement with previous studies.11 15 22 24 In addition, we showed that use of penicillins, penicillin-class or early-generation cephalosporins was associated with inferior outcomes (CBR and PFS). None of the previous studies looked at the effect of specific antibiotic classes. Notably, cefazolin given as prophylaxis periprocedurally was the most frequently used antibiotic in this group. It can be speculated that the gut microbiome integrity is critical to immunotherapy response in the period leading to immunotherapy and that any derangements even by narrow-spectrum antibiotics can impair its therapeutic efficacy. The difference between the type and pattern of usage of antibiotics between RCC and NSCLC suggests distinctive infection patterns and clinical indication of antibiotics between the two tumour types. An alternative explanation is heterogeneity in the baseline microbiome profile of RCC and NSCLC. The influence of microbiota-induced antitumour immune response may be context-dependent, that is, different species of bacteria may modulate the host immunity depending on the type of cancer. Supporting this argument is that microbiome profiling in preclinical and clinical models of melanoma and NSCLC has shown that bacterial species associated with outcomes were different between studies.17 19
Our findings are limited by the retrospective nature of this study and modest sample size. The gut microbiome is dynamic in nature and our study did not assess the relationship between antibiotic use and its immediate and long-term effect on gut microbiome composition. Previously, a study also showed that antibiotic use was associated with reduced rate of immune-mediated diarrhoea or colitis and was also associated with increased risk of severity.33 In our study, we did not specifically collect data on immune-related toxicities associated with antibiotic exposure in the solid tumour population. Prospective serial assessment of microbiome while on treatment with immunotherapy and following antibiotic exposure should be explored in future studies. Other factors with a potential to affect microbiome include diet, concomitant medications and probiotics; these factors were not captured in our study. It is possible that our observed associations between antibiotic use and worse survival are not reflective of microbiota–immunity interaction, but due to more antibiotic exposure in more seriously infected patients with higher infection-related mortality. We speculate that this could explain some of our findings in the NSCLC cohort. However, antibiotic use in our RCC cohort was independently associated with worse outcomes after adjustment for other markers of adverse prognosis. Despite these limitations, our findings in RCC are consistent with other studies and further validate the need for systematic investigation of the clinical outcomes of antibiotics stratified by spectrum of antimicrobial activity in the context of immunotherapy. While we know that broad-spectrum antibiotic exposure is more detrimental than narrow-spectrum antibiotics to the microbiome diversity, their differential effect on immunotherapy outcomes is yet to be investigated. There is a need for a consensus on the most critical antibiotic exposure window around ICI and the minimum duration of antibiotic exposure impacting immunotherapy outcomes.
In conclusion, we show that antibiotic use in patients with AML during induction chemotherapy was not associated with RFS. In NSCLC, use of only certain classes of antibiotics were associated with decreased PFS and OS, while for RCC, in general, antibiotic use was associated with inferior outcomes. Use of penicillins, penicillin-class or early-generation cephalosporins in RCC influenced outcomes the most. Validation in other cohorts, mechanistic studies and prospective microbiome profiling are some of the next steps being explored.
Footnotes
Contributors: AAK, MRP, AR and ME conceived and planned the project. RS and TD carried out the analysis. SZ, MAM, AAK, DW, CR, AMA and SV contributed to the interpretation of the results. AAK took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: DW: No relevant conflicts, unrelated research support from Incyte and Consultation fees for GVHD adjudication, FATE Therapeutics. CR: Research support from Pfizer and has served on an advisory board for Exelixis. MRP: No relevant conflicts related to this work. Served on Nektar Therapeutics Advisory Board and received research funding from Merck, Vyriad and FATE therapeutics.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Deidentified participant data are available upon reasonable request (patel069@umn.edu or kulkarni@umn.edu)
References
- 1.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature 2016;535:75–84. 10.1038/nature18848 [DOI] [PubMed] [Google Scholar]
- 2.Verdam FJ, Fuentes S, de Jonge C, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013;21:E607–15. 10.1002/oby.20466 [DOI] [PubMed] [Google Scholar]
- 3.Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett 2004;93:97–108. 10.1016/j.imlet.2004.02.005 [DOI] [PubMed] [Google Scholar]
- 4.Kamada N, Seo S-U, Chen GY, et al. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 2013;13:321–35. 10.1038/nri3430 [DOI] [PubMed] [Google Scholar]
- 5.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016;375:2369–79. 10.1056/NEJMra1600266 [DOI] [PubMed] [Google Scholar]
- 6.Peled JU, Gomes ALC, Devlin SM, et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med 2020;382:822–34. 10.1056/NEJMoa1900623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91–7. 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 8.Pinato DJ, Howlett S, Ottaviani D, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol 2019;5:1774–8. 10.1001/jamaoncol.2019.2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouaknine J, Helly De Tauriers P, Dumenil C, et al. MA10.03 plasmatic evaluation of the intestinal barrier and blood microbiota, and antibiotic use in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol 2018;13:S389–90. 10.1016/j.jtho.2018.08.397 [DOI] [Google Scholar]
- 10.Galli G, Poggi M, Fucà G, et al. MA10.02 impact of antibiotics on outcome of metastatic non small cell lung cancer patients treated with immunotherapy. Journal of Thoracic Oncology 2018;13:S389 10.1016/j.jtho.2018.08.396 [DOI] [Google Scholar]
- 11.Ahmed J, Kumar A, Parikh K, et al. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology 2018;7:e1507670. 10.1080/2162402X.2018.1507670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakozaki T, Okuma Y, Omori M, et al. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett 2019;17:2946–52. 10.3892/ol.2019.9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mielgo-Rubio X, Chara L, Sotelo-Lezama M, et al. MA10.01 antibiotic use and PD-1 inhibitors: shorter survival in lung cancer, especially when given intravenously. type of infection also matters. Journal of Thoracic Oncology 2018;13:S389 10.1016/j.jtho.2018.08.395 [DOI] [Google Scholar]
- 14.Wilson BE, Routy B, Nagrial A, et al. The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies. Cancer Immunol Immunother 2020;69:343–54. 10.1007/s00262-019-02453-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sen S, Carmagnani Pestana R, Hess K, et al. Impact of antibiotic use on survival in patients with advanced cancers treated on immune checkpoint inhibitor phase I clinical trials. Ann Oncol 2018;29:2396–8. 10.1093/annonc/mdy453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tinsley N, Zhou C, Tan G, et al. Cumulative antibiotic use significantly decreases efficacy of checkpoint inhibitors in patients with advanced cancer. Oncologist 2020;25:55–63. 10.1634/theoncologist.2019-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359:104–8. 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elkrief A, El Raichani L, Richard C, et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology 2019;8:e1568812:1–6. 10.1080/2162402X.2019.1568812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359:97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X-Z, Gao P, Song Y-X, et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology 2019;8:e1665973. 10.1080/2162402X.2019.1665973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huemer F, Rinnerthaler G, Westphal T, et al. Impact of antibiotic treatment on immune-checkpoint blockade efficacy in advanced non-squamous non-small cell lung cancer. Oncotarget 2018;9:16512–20. 10.18632/oncotarget.24751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 2018;29:1437–44. 10.1093/annonc/mdy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao S, Gao G, Li W, et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer 2019;130:10–17. 10.1016/j.lungcan.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 24.Lalani A-KA, Xie W, Lin X, et al. Antibiotic use and outcomes with systemic therapy in metastatic renal cell carcinoma (mRCC). JCO 2018;36:607 10.1200/JCO.2018.36.6_suppl.607 [DOI] [Google Scholar]
- 25.Hemadri A, Lin H, Lin Y, et al. Association of medication (Med) and antibiotic (abx) use with response and survival in advanced melanoma (MEL) receiving PD-1 inhibitors. JCO 2019;37:9572 10.1200/JCO.2019.37.15_suppl.9572 [DOI] [Google Scholar]
- 26.Schett A, Rothschild SI, Curioni-Fontecedro A, et al. Predictive impact of antibiotics in patients with advanced non small-cell lung cancer receiving immune checkpoint inhibitors : Antibiotics immune checkpoint inhibitors in advanced NSCLC. Cancer Chemother Pharmacol 2020;85:121–31. 10.1007/s00280-019-03993-1 [DOI] [PubMed] [Google Scholar]
- 27.Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013;342:971–6. 10.1126/science.1240537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342:967–70. 10.1126/science.1240527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47. 10.1182/blood-2016-08-733196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber D, Jenq RR, Peled JU, et al. Microbiota disruption induced by early use of broad-spectrum antibiotics is an independent risk factor of outcome after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2017;23:845–52. 10.1016/j.bbmt.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott NA, Andrusaite A, Andersen P, et al. Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci Transl Med 2018;10:aao4755. 10.1126/scitranslmed.aao4755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekmekciu I, von Klitzing E, Fiebiger U, et al. Immune responses to broad-spectrum antibiotic treatment and fecal microbiota transplantation in mice. Front Immunol 2017;8:397. 10.3389/fimmu.2017.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abu-Sbeih H, Herrera LN, Tang T, et al. Impact of antibiotic therapy on the development and response to treatment of immune checkpoint inhibitor-mediated diarrhea and colitis. J Immunother Cancer 2019;7:242. 10.1186/s40425-019-0714-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000803supp001.pdf (92.3KB, pdf)