Abstract
Background
Enhanced Recovery After Surgery (ERAS) is a multimodal, multidisciplinary approach to optimizing the postsurgical recovery process through preoperative, perioperative, and postoperative interventions. ERAS protocols are emerging quickly within orthopedic spine surgery, yet there is a lack of consensus on optimal ERAS practices.
Objective
The aim of this systematic review is to identify and discuss the trends in spine ERAS protocols and the associated outcomes.
Methods
A literature search on PubMed was conducted to identify clinical studies that implemented ERAS protocols for various spine procedures in the adult population. The search included English-language literature published through December 2019. Additional sources were retrieved from the reference lists of key studies. Studies that met inclusion criteria were identified manually. Data regarding the study population, study design, spine procedures, ERAS interventions, and associated outcome metrics were extracted from each study that met inclusion criteria.
Results
Of the 106 studies identified from the literature search, 22 studies met inclusion criteria. From the ERAS protocols in these studies, common preoperative elements include patient education and modified preoperative nutrition regimens. Perioperative elements include multimodal analgesia and minimally invasive surgery. Postoperative elements include multimodal pain management and early mobilization/rehabilitation/nutrition regimens. Outcomes from ERAS implementation include significant reductions in length of stay, cost, and opioid consumption. Although these trends were observed, there remained great variability among the ERAS protocols, as well as in the reported outcomes.
Conclusions
ERAS may improve cost-effectiveness to varying degrees for spinal procedures. Specifically, the use of multimodal analgesia may reduce overall opioid consumption. However, the benefits of ERAS likely will vary based on the specific procedure.
Clinical Relevance
This review contributes to the assessment of ERAS protocol implementation in the field of adult spine surgery.
Keywords: Enhanced Recovery After Surgery, ERAS, fast-track surgery, rapid recovery program, spine surgery, orthopedics, multimodal analgesia
INTRODUCTION
Enhanced Recovery After Surgery (ERAS; also known as enhanced or rapid recovery program, fast-track surgery, enhanced perioperative care/EPOC) refers to a multimodal care pathway to accelerate patient recovery after surgery. The goal of ERAS is to mitigate the surgical stress response while also enhancing the recovery of bodily functions, ultimately increasing cost-effectiveness without compromising the quality of care.1 An ERAS protocol typically contains preoperative, perioperative, and/or postoperative elements that are standardized across all patients undergoing a certain procedure.1 The implementation of such a protocol is a multidisciplinary effort involving surgery, anesthesia, nursing, and other disciplines. This concept was first introduced by Kehlet1 and adapted for colonic resection and colorectal procedures.2 Since then, ERAS care pathways have been developed for a variety of other surgical specialties.3 As evidence for and adoption of ERAS have grown, multiple societies also have been established (see the global society http://erassociety.org, the UK society http://www.erasuk.net/, and the American society http://enhancedrecovery.org/) to curate ERAS guidelines worldwide.
The stress response to surgical insult can be particularly devastating for a patient, resulting in a proinflammatory, procatabolic state that can drive metabolic changes and insulin resistance.4–6 Such changes in turn may significantly impact postoperative morbidity and mortality.5,6 Additionally, blood loss and fluid homeostasis are major factors that can affect surgical outcomes.2
To address these concerns, ERAS pathways are being implemented with increasing frequency. Compared with traditional care pathways, which are often unstandardized and according to surgeon preference, ERAS pathways have been associated with improved outcomes within various surgical fields2; for example, ERAS protocols developed for high-volume total knee and hip replacements have been associated with reductions in length of stay (LOS) and hospital costs, as well as in readmission, complication, and mortality rates to varying degrees.7–11
ERAS implementation may become a mainstay for orthopedic spine surgery. The volume of patients undergoing elective spine surgery is increasing, as is the population of aging patients who may become surgical candidates.3,12 Moreover, some spine procedures are associated with significant postoperative pain, LOS, and complications.3 Thus, there is room to grow in terms of optimizing clinical and economic outcomes.
Research on spine ERAS protocols is still in the early stages, but it has been gaining traction.12 Recent reviews by Dietz et al,13 Elsarrag et al,14 and Corniola et al15 highlight the various spine procedures for which ERAS pathways have been designed and implemented. However, there remains a lack of consensus on which specific ERAS elements may be relatively more effective. Indeed, there are no official ERAS guidelines for spine surgery, although many spine ERAS protocols contain elements that are present in the guidelines for other surgical specialties (see http://erassociety.org.loopiadns.com/guidelines/list-of-guidelines). Moreover, existing ERAS spine protocols vary significantly in their preoperative, perioperative, and postoperative elements, rendering it difficult to assess their individual effectiveness. Thus, the aim of this review is to discuss the trends in spine ERAS protocols—the commonly adopted interventions and prior evidence for their implementation in other surgical specialties—as well as the associated outcome metrics.
METHODS
Electronic Literature Search
A literature review of PubMed was conducted as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16 Keywords were combined with Boolean operators to generate the following search term: [“ERAS”] OR [“enhanced recovery after surgery”] OR [“rapid recovery”] OR [“accelerated pathway”] OR [“fast-track surgery”] OR [“same-day discharge”] AND [“spine surgery”], in order to retrieve relevant English-language literature published through December 2019. The literature search was performed independently by 2 reviewers, and a consensus was reached for which articles met inclusion criteria. The last search was performed on December 30, 2019. Later, additional articles were identified from the reference lists of key articles.
Inclusion and Exclusion Criteria
Titles, abstracts, and full-text articles were screened by 2 independent reviewers. Inclusion criteria included: (1) adult patients undergoing (2) any type of elective spine surgery, and (3) randomized, nonrandomized, controlled, noncontrolled, retrospective, and prospective study designs. Exclusion criteria included: (1) pediatric patients, (2) articles not available in English, (3) hypothetical ERAS designs, (4) studies that introduced only a single ERAS element (eg, not in full protocol), and (5) review articles and case reports. Duplicate articles were accounted for. Data from the articles that fit our criteria were extracted and assessed. This review does not incorporate the pediatric population because of the variability in protocols, parameters, and discharge criteria that significantly differ from those for adult populations.
Data Extraction and Quality Assessment
Where available, the following data were extracted from each study that met criteria: sample size, study design, spine procedure(s), quantity and types of ERAS interventions per protocol, and all associated outcomes that were reported—which commonly included LOS, cost, method(s) of pain control, and complication and readmission rates. If the number of ERAS interventions in a study was not itemized explicitly (eg, in a data table), the information was extracted from the article text. Assessment of quality and risk bias was performed according to the Centre for Evidence-Based Medicine guidelines for therapeutic studies,17 as well as the Newcastle-Ottawa Scale,18 which is a 9-point scale designed to assess nonrandomized and case-control studies for sample selection, comparability, and outcome/exposure. A higher score on the scale indicates a higher-quality study.18
Data Analyses
There was great heterogeneity among the studies; for example, there was a wide range of study designs, and several studies did not provide a comparative analysis of pre- and post-ERAS data. A meta-analysis would have been limited by the nature of this source data. As such, data points of interest were reported when available in the literature, but statistical analyses were not performed for this review.
RESULTS
Study Selection
A total of 106 articles were identified from the initial literature search. The 85 nonduplicate articles underwent abstract and title review for inclusion, of which 36 were excluded based on our exclusion criteria and nonapplicability. A total of 49 articles underwent full-text review, and 27 of those articles were excluded because of a lack of evidence pertaining to ERAS protocols. The remaining 22 articles met inclusion criteria.19–40
Of the 22 ERAS studies that met criteria, 12 were controlled “before-and-after” studies,22,25–30,32–36 8 were prospective noncontrolled trials,19,21,23,24,31,37,39,40 1 was a retrospective matched cohort study,38 and 1 was a retrospective noncontrolled study.20 Publication dates ranged from 2004 to 2019.
The literature search process is presented in the PRISMA flow diagram (Figure). Table 1 presents an overview of the 22 studies and their associated outcomes.
Figure.
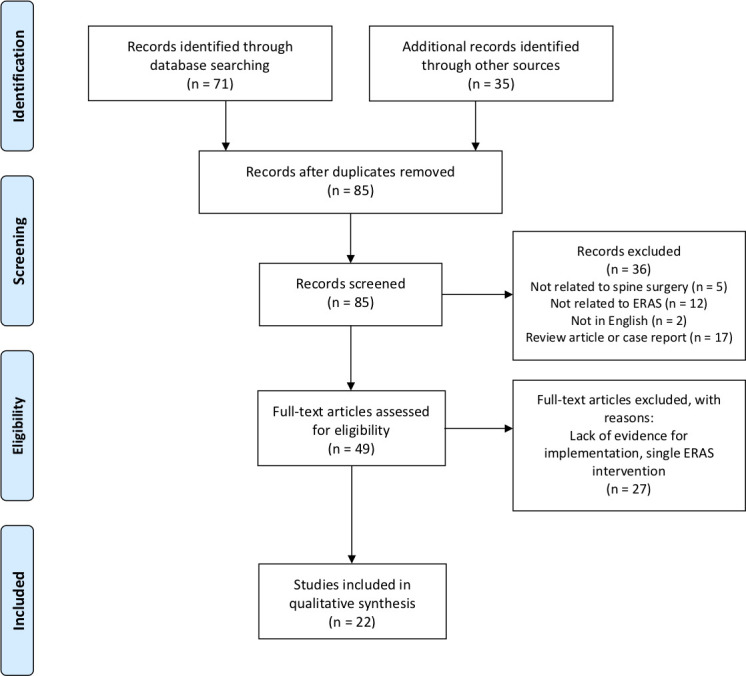
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for systematic review of Enhanced Recovery After Surgery (ERAS) protocols in adult spine surgery.
Table 1.
Overview of spine Enhanced Recovery After Surgery (ERAS) studies.
|
ERAS Study |
Surgical Procedure |
Study Type |
Sample Size(s) |
No. of ERAS Interventions/ Protocol |
| Scanlon and Richards,19 2004 | Lumbar laminectomy ± discectomy | Prospective, noncontrolled | n = 27 | 13 |
| Chin et al,20 2015 | Single-level instrumented PLIF | Retrospective, noncontrolled | n = 16 | 8 |
| Bednar,21 2017 | Unilateral and bilateral lumbar decompression and interbody fusion, ACDF, ACDA, miscellaneous | Prospective, noncontrolled | n = 124 | 11 |
| Bradywood et al,22 2017 | Lumbar spinal fusion | Controlled before-and-after study | ERAS protocol, n = 244 Control, n = 214 | 38 |
| Debono et al,23 2017 | Lumbar microdiscectomy | Prospective, noncontrolled | n = 201 | 13 |
| Wang et al,24 2017 | Lumbar spinal fusion | Prospective, noncontrolled | n = 42 | 17 |
| Grasu et al,25 2018 | Metastatic tumor resection | Controlled before-and-after study | ERAS protocol, n = 41 Control, n = 56 | 15 |
| Wang et al,26 2018 | MIS TLIF | Controlled before-and-after study | ERAS protocol, n = 38 Control, n = 15 | 6 |
| Ali et al,27 2019 | Elective spine or peripheral nerve surgery | Controlled before-and-after study | ERAS protocol, n = 201 Control, n = 74 | 16 |
| Angus et al,28 2019 | Complex spine surgery | Controlled before-and-after study | ERAS protocol, n = 214 Control, n = 412 | 19 |
| Brusko et al,29 2019 | Posterior lumbar fusion (open and MIS, 1–3 levels) | Controlled before-and- after study | ERAS protocol, n = 57 Control, n = 40 | 3 |
| Carr et al,30 2019 | Arthrodesis for spinal deformity, anterior or posterior fusion, corpectomy, pelvic fixation | Controlled before-and-after study | ERAS protocol, n = 620 Traditional, n = 183 Control, n = 129 | 15 |
| Chakravarthy et al,31 2019 | ACDF/PCDF, decompression, microdiscectomy, ALIF, TLIF, XLIF, anterior/posterior fusion ± instrumentation, pedicle subtraction osteotomy, tumor corpectomy/debulking | Prospective, noncontrolled | n = 1770 | 14 |
| Dagal et al,32 2019 | Elective major spine surgery | Controlled before-and-after study | ERAS protocol, n = 267 Traditional pathway, n = 183 No intervention, n = 108 | 36 |
| Debono et al,33 2019 | ACDF, ALIF, PLIF | Controlled before-and-after study | ERAS protocol, n = 1920 Control, n = 1563 | 23 |
| Feng et al,34 2019 | MIS-TLIF | Controlled before-and-after study | ERAS protocol, n = 30 Control, n = 44 | 12 |
| Sivaganesan et al,35 2019 | Elective degenerative spine disease | Controlled before-and-after study | ERAS protocol, n = 151 Control, n = 1596 | 9 |
| Smith et al,36 2019 | 1- to 2-level primary open lumbar fusion | Controlled before-and-after study | ERAS protocol, n = 123 Control, n = 230 | 40 |
| Soffin et al,37 2019 | ACDF and CDA | Prospective, noncontrolled | n = 33 (25 ACDF, 8 CDA) | 18 |
| Soffin et al,38 2019 | Lumbar decompression (laminectomy, laminotomy, microdiscectomy) | Retrospective, matched cohort | ERAS protocol, n = 18 Control, n = 18 | 30 |
| Soffin et al,39 2019 | MIS lumbar decompression | Prospective, noncontrolled | n = 61 | 15 |
| Staartjes et al,40 2019 | Elective tubular microdiscectomy, mini-open decompression, MIS, ALIF, and PLIF | Prospective, noncontrolled | n = 2592 | 22 |
Abbreviations: ACDA, anterior cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion; ALIF, anterior lumbar interbody fusion; CDA, cervical disc arthroplasty; ICU, intensive care unit; LOS, length of stay; MIS, minimally invasive surgery; N/A, not applicable; PCA, patient-controlled analgesia; PCDF, posterior cervical discectomy and fusion; PLIF, posterior lumbar interbody fusion; POD, postoperative day; PONV, postoperative nausea and vomiting; TLIF, transforaminal lumbar interbody fusion; XLIF, extreme lateral interbody fusion.
Table 1.
Extended.
|
LOS/Time to Discharge for ERAS |
“Changed” Outcomes Compared With Traditional Pathway |
“No Change” Outcomes Compared With Traditional Pathway |
| 4–6 hr in PACU (mean 3.95 hr) | No control group | No control group |
| Same-day discharge | No control group | No control group |
| Same-day discharge | No control group | No control group |
| Mean 3.4 days | Reduced LOS Improved discharge disposition/readiness | No significant difference in patient satisfaction |
| Mean total inpatient time 10 hr 12 min | No control group | No control group |
| Mean 1.29 ± 0.9 nights | No control group | No control group |
| Mean 1.5 ± 1.9 days | Reduced postoperative opioid consumption | No significant difference in LOS No significant difference in complication rate No significant difference in readmission rate No significant difference in postoperative pain scores |
| Mean 1.23 ± 0.8 days | Reduced LOS Reduced intraoperative time Reduced complication rate (12% ERAS vs 21% control) Reduced acute care cost, mean $3444 in savings ∼15.2% reduction | N/A |
| Mean 3.6 ± 2.4 days | Reduced opioid use at 1 mo postoperatively Increased administration of postoperative ketorolac and gabapentin Reduction of PCA (0.5% ERAS vs 54.1% control) Increased administration of nonopioid analgesic agents | No significant difference in LOS No significant difference in complication rate No significant difference in readmission rate No significant difference in postoperative pain scores |
| Mean 8 days for scoliosis; mean 5.2 days for complex fixation | Reduced LOS; 100% patient satisfaction (vs 84% control) | Statistically nonsignificant reduction in readmission rate (2.1% to 1.9%) |
| 2.9 days | Reduced LOS Reduced opioid/analgesia consumption on PODs 0, 1, and 3 Reduced narcotics consumption Reduced PONV medication consumption Reduced postoperative pain scores on POD1 Increased distance of ambulation on POD1 | N/A |
| 1.8 days for intensive care LOS; 5.4 days for hospital LOS | Reduced ICU and overall hospital LOS Reduced total costs ($19,344 reduction) | N/A |
| N/A | No control group Estimated $827 cost savings per patient | No control group |
| Mean hospital LOS 6.1 days Mean ICU stay 1.9 days | Reduced ICU and hospital LOS Reduced cost ($53,355 ERAS vs $62,429 control) | No significant difference in complication rate No significant difference in readmission rate |
| ALIF mean 3.33 days; ACDF mean 1.3 days; PLIF mean 4.8 days | Reduced LOS for ACDF, ALIF, and PLIF Reduced complication rate for PLIF, but NOT for ALIF or ACDF 86.5% of patients satisfied/very satisfied regarding mobile e-health app 82.3% of patients satisfied/very satisfied with perceived optimization of care management | N/A |
| Median 5 days | Reduced LOS Reduced cost (mean $70,467 ERAS vs mean $71,426 control) | No significant difference in intraoperative time No significant difference in complication rate |
| N/A | Reduced LOS for lumbar procedures Reduced complication rate for lumbar procedures | No significant difference in readmission rate No significant difference in patient satisfaction |
| Mean 92 hr (3.83 days) | Reduced long-acting opioid use (5.2% ERAS vs 14% control) Reduced PCA use (0% ERAS vs 7% control) | No significant difference in intraoperative time Clinically (not statistically) significantly reduced LOS |
| Median PACU LOS 416 min (6 hr 56 min) | No control group | No control group |
| Median PACU LOS 237 min (3.95 hr) | Reduced LOS Reduced total perioperative opioid consumption | No significant difference in postoperative pain scores No significant difference in postoperative opioid consumption |
| Median PACU LOS 279 min (4.65 hr), 298 min (4.67 hr) for lumbar decompression, 285 min (4.75 hr) for microdiscectomy | No control group | No control group |
| Mean 1.1 ± 1.2 days | No control group | No control group |
Assessment of Quality and Risk of Bias
As per the Centre for Evidence-Based Medicine guidelines for therapeutic studies,17 the 12 controlled “before-and-after” studies22,25–30,32–36 and the retrospective controlled study38 are graded as level of evidence 2B.17 The remaining noncontrolled retrospective study20 is graded as level of evidence 4. The 8 prospective noncontrolled trials19,21,23,24,31,37,39,40 are graded as level of evidence 4.
According to the Newcastle-Ottawa Scale,18 12 of the nonrandomized, controlled cohort studies22,25–30,32,34–36 received 8 stars out of 9 (4 for selection, 1 for comparability, and 3 for outcome), and 1 study39 received 9 stars out of 9.
Types of Spine Surgery
Associated spine procedures included minor, major, and complex spine surgeries, such as laminectomy/laminotomy, (micro)discectomy, open and minimally invasive posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF), anterior lumbar interbody fusion (ALIF), anterior cervical discectomy and fusion (ACDF), anterior cervical disc arthroplasty (ACDA), and metastatic tumor resection (Table 1). Most spine ERAS protocols were implemented for lumbar spine procedures. Because reporting of the type(s) of spine surgery was not standardized across the 22 studies, quantifying the prevalence of any single type of spine procedure among the ERAS protocols was difficult to perform.
Associated Outcomes
A total of 13 of the 22 studies22,25–30,32–36,38 conducted controlled trials that compared ERAS versus traditional care pathways. The outcomes are variable and are summarized in Table 1. Common outcome metrics reported were LOS, cost, opioid consumption, postoperative pain, intraoperative time, patient satisfaction, complication rate, and readmission rate. The most commonly reported outcomes were reductions in LOS, cost, and opioid consumption. Importantly, no ERAS study was associated with worse patient outcomes compared with traditional pathways.
A total of 10 studies reported significantly reduced hospital and/or intensive care unit LOS22,26,28–30,32–35,38; however, some studies found no significant change in LOS compared with control.25,27,36 Additionally, there was large variation in mean/median LOS, ranging from several hours to several days.
Four studies reported a reduction in hospital costs.26,30,32,34 Wang et al26 calculated a mean acute care cost reduction of $3,444 (15.2%) for ERAS minimally invasive TLIF (MIS TLIF) procedures; Carr et al30 reports a $19,344 reduction in cost. Dagal et al32 calculated a mean cost reduction of $62,429 (control) to $53,355 (ERAS) for several types of major spine surgery; and Feng et al34 found a mean cost decrease from $71,426 to $70,467, also for MIS TLIF. Although the study was not a controlled trial, Chakravarthy et al31 also estimated a per-patient decrease of $827 in hospital costs.
A reduction in intraoperative time has also been noted,26 but conversely no change in intraoperative time has been reported as well.34,36 Here, a decrease in LOS and/or intraoperative time may translate to decreased cost; in fact, Wang et al26 reported reductions in LOS and intraoperative time, as well as cost.
For postoperative opioid consumption, the bulk of evidence suggests a reduction in opioid use after ERAS implementation,25,27,29,36,38 with no reports of increased opioid use. Interestingly, Soffin et al38 reported both reduced and no change in opioid consumption at different time points, with reduced perioperative opioid consumption but no difference in postoperative opioid consumption.
Common ERAS Elements
Preoperative ERAS elements are defined here as interventions that occur any time before the day of surgery (postoperative day 0 [POD0]). They are designed to optimize the patient's condition prior to surgery. The most common interventions were patient education (72.7% of studies), assessment of patient health and comorbidities (45.5%), carbohydrate loading (40.9%), and a modified preoperative fasting regimen (31.8%). Perioperative ERAS elements refer to interventions that occur from POD0 until patient extubation and transfer to the postanesthesia care unit (PACU). Common interventions were perioperative multimodal analgesia (MMA) (68.2%), infection prophylaxis (54.5%), “preemptive” analgesia (50%), catheter and surgical drain sparing (50%), and minimally invasive surgery (MIS; 40.9%). Postoperative ERAS elements are defined as interventions that occur during and after admission to the recovery area (eg, PACU). Common postoperative elements include postoperative MMA (81.8%), early mobilization/rehabilitation (77.3%), and early nutrition (54.5%).
The number of ERAS elements per protocol ranged from 3 to 40 items (Table 1); however, it is important to note that there was great variability across studies in how the ERAS elements were itemized and the degree of detail to which the protocols were presented.
Tables 2, 3, and 4 summarize the percentage prevalence of preoperative, perioperative, and postoperative ERAS elements that were common among the 22 studies, respectively. Table 5 details the postoperative discharge criteria reported by several spine ERAS protocols. Other studies did not report discharge criteria.
Table 2.
Common preoperative Enhanced Recovery After Surgery (ERAS) interventions implemented in spine surgery procedures.
|
ERAS Study |
Surgical Procedure |
Patient Education |
Comorbidity Assessment and Health Optimization |
Modified Preoperative Fasting |
Carbohydrate Loading |
| Scanlon and Richards,19 2004 | Lumbar laminectomy ± discectomy | X | |||
| Chin et al,20 2015 | Single-level instrumented PLIF | X | |||
| Bednar,21 2017 | Unilateral and bilateral lumbar decompression and interbody fusion, ACDF, ACDA, miscellaneous | X | X | ||
| Bradywood et al,22 2017 | Lumbar spinal fusion | X | X | ||
| Debono et al,23 2017 | Lumbar microdiscectomy | X | X | ||
| Wang et al,24 2017 | Lumbar spinal fusion | X | X | X | |
| Grasu et al,25 2018 | Metastatic tumor resection | X | X | X | |
| Wang et al,26 2018 | MIS TLIF | ||||
| Ali et al,27 2019 | Elective spine or peripheral nerve surgery | X | X | X | |
| Angus et al,28 2019 | Complex spine surgery | X | X | X | |
| Brusko et al,29 2019 | Posterior lumbar fusion (open and MIS, 1–3 levels) | ||||
| Carr et al,30 2019 | Arthrodesis for spinal deformity, anterior or posterior fusion, corpectomy, pelvic fixation | X | X | ||
| Chakravarthy et al,31 2019 | ACDF/PCDF, decompression, microdiscectomy, ALIF, TLIF, XLIF, anterior/posterior fusion ± instrumentation, pedicle subtraction osteotomy, tumor corpectomy/debulking | X | |||
| Dagal et al,32 2019 | Elective major spine surgery | X | X | X | |
| Debono et al,33 2019 | ACDF, ALIF, PLIF | X | X | ||
| Feng et al,34 2019 | MIS TLIF | X | X | X | |
| Sivaganesan et al,35 2019 | Elective degenerative spine disease | ||||
| Smith et al,36 2019 | 1- to 2-level primary open lumbar fusion | X | X | ||
| Soffin et al,37 2019 | ACDF and CDA | X | X | X | |
| Soffin et al,38 2019 | Lumbar decompression (laminectomy, laminotomy, microdiscectomy) | X | |||
| Soffin et al,39 2019 | MIS lumbar decompression | X | X | X | |
| Staartjes et al,40 2019 | Elective tubular microdiscectomy, mini-open decompression, MIS, ALIF, and PLIF | X | X | ||
| Percentage (n/total) | 72.7 (16/22) | 45.5 (10/22) | 31.8 (7/22) | 40.9 (9/22) |
Abbreviations: ACDA, anterior cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion; ALIF, anterior lumbar interbody fusion; CDA, cervical disc arthroplasty; MIS, minimally invasive surgery; PCDF, posterior cervical discectomy and fusion; PLIF, posterior lumbar interbody fusion; TLIF, transforaminal lumbar interbody fusion; XLIF, extreme lateral interbody fusion.
Table 3.
Common perioperative Enhanced Recovery After Surgery (ERAS) interventions implemented in spine surgery procedures.
|
ERAS Study |
Surgical Procedure |
Preemptive Analgesia |
Intraoperative MMA/ Analgesia |
MIS |
Blood Loss Management |
Fluid Management |
Temperature Management |
DVT Prophylaxis |
Infection Prophylaxis |
PONV Prophylaxis |
Catheter and Surgical Drain Sparing |
| Scanlon and Richards,19 2004 | Lumbar laminectomy ± discectomy | X | |||||||||
| Chin et al,20 2015 | Single-level instrumented PLIF | X | |||||||||
| Bednar,21 2017 | Unilateral and bilateral lumbar decompression and interbody fusion, ACDF, ACDA, miscellaneous | X | X | X | |||||||
| Bradywood et al,22 2017 | Lumbar spinal fusion | X | X | X | |||||||
| Debono et al,23 2017 | Lumbar microdiscectomy | X | |||||||||
| Wang et al,24 2017 | Lumbar spinal fusion | X | X | X | X | X | X | X | |||
| Grasu et al,25 2018 | Metastatic tumor resection | X | X | X | X | X | X | ||||
| Wang et al,26 2018 | MIS TLIF | X | X | ||||||||
| Ali et al,27 2019 | Elective spine or peripheral nerve surgery | X | X | X | |||||||
| Angus et al,28 2019 | Complex spine surgery | X | X | ||||||||
| Brusko et al,29 2019 | Posterior lumbar fusion (open & MIS, 1–3 levels) | X | X | ||||||||
| Carr et al,30 2019 | Arthrodesis for spinal deformity, anterior or posterior fusion, corpectomy, pelvic fixation | X | X | X | X | X | X | X | |||
| Chakravarthy et al,31 2019 | ACDF/PCDF, decompression, microdiscectomy, ALIF, TLIF, XLIF, anterior/posterior fusion ± instrumentation, pedicle subtraction osteotomy, tumor corpectomy/debulking | X | X | X | X | X | X | ||||
| Dagal et al,32 2019 | Elective major spine surgery | X | X | X | X | X | X | X | |||
| Debono et al,33 2019 | ACDF, ALIF, PLIF | X | X | X | X | ||||||
| Feng et al,34 2019 | MIS TLIF | X | X | X | X | X | X | X | |||
| Sivaganesan et al,35 2019 | Elective degenerative spine disease | X | X | ||||||||
| Smith et al,36 2019 | 1- to 2-level primary open lumbar fusion | X | X | X | X | X | |||||
| Soffin et al, 37 2019 | ACDF and CDA | X | X | X | X | X | |||||
| Soffin et al,38 2019 | Lumbar decompression (laminectomy, laminotomy, microdiscectomy) | X | X | X | X | ||||||
| Soffin et al,39 2019 | MIS lumbar decompression | X | X | X | X | X | X | X | |||
| Staartjes et al,40 2019 | Elective tubular microdiscectomy, mini-open decompression, MIS, ALIF, and PLIF | X | X | X | X | X | X | X | X | ||
| Percentage (n/total) | 50% (11/22) | 68.2% (15/22) | 40.9% (9/22) | 31.8 (7/22) | 40.9 (9/22) | 36.4 (8/22) | 22.7 (5/22) | 54.5 (12/22) | 22.7 (5/22) | 50 (11/22) |
Abbreviations: ACDA, anterior cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion; ALIF, anterior lumbar interbody fusion; CDA, cervical disc arthroplasty; DVT, deep venous thrombosis; MIS, minimally invasive surgery; MMA, multimodal analgesia; PCDF, posterior cervical discectomy and fusion; PLIF, posterior lumbar interbody fusion; PONV, postoperative nausea and vomiting; TLIF, transforaminal lumbar interbody fusion; XLIF, extreme lateral interbody fusion.
Table 4.
Common postoperative Enhanced Recovery After Surgery (ERAS) interventions implemented in spine surgery procedures.
|
ERAS Study |
Surgical Procedure |
Postoperative MMA/Analgesia |
Early Mobilization and Physical Rehabilitation |
Early Nutrition |
Early Discharge Criteria |
| Scanlon and Richards,19 2004 | Lumbar laminectomy ± discectomy | X | X | ||
| Chin et al,20 2015 | Single-level instrumented PLIF | X | X | ||
| Bednar,21 2017 | Unilateral and bilateral lumbar decompression and interbody fusion, ACDF, ACDA, misc. | ||||
| Bradywood et al,22 2017 | Lumbar spinal fusion | X | X | X | X |
| Debono et al,23 2017 | Lumbar microdiscectomy | X | X | X | |
| Wang et al,24 2017 | Lumbar spinal fusion | X | X | X | |
| Grasu et al,25 2018 | Metastatic tumor resection | X | X | X | |
| Wang et al,26 2018 | MIS TLIF | ||||
| Ali et al,27 2019 | Elective spine or peripheral nerve surgery | X | X | ||
| Angus et al,28 2019 | Complex spine surgery | X | X | ||
| Brusko et al,29 2019 | Posterior lumbar fusion (open and MIS, 1–3 levels) | X | |||
| Carr et al,30 2019 | Arthrodesis for spinal deformity, anterior or posterior fusion, corpectomy, pelvic fixation | X | X | ||
| Chakravarthy et al,31 2019 | ACDF/PCDF, decompression, microdiscectomy, ALIF, TLIF, XLIF, anterior/posterior fusion ± instrumentation, pedicle subtraction osteotomy, tumor corpectomy/debulking | X | X | ||
| Dagal et al,32 2019 | Elective major spine surgery | X | X | X | |
| Debono et al,33 2019 | ACDF, ALIF, PLIF | X | X | X | |
| Feng et al,34 2019 | MIS-TLIF | X | X | X | |
| Sivaganesan et al,35 2019 | Elective degenerative spine disease | X | X | ||
| Smith et al,36 2019 | 1- to 2-level primary open lumbar fusion | X | X | X | |
| Soffin et al,37 2019 | ACDF and CDA | X | X | X | X |
| Soffin et al,38 2019 | Lumbar decompression (laminectomy, laminotomy, microdiscectomy) | X | X | X | X |
| Soffin et al,39 2019 | MIS lumbar decompression | X | X | X | |
| Staartjes et al,40 2019 | Elective tubular microdiscectomy, mini-open decompression, MIS, ALIF, and PLIF | X | X | X | |
| Percentage (n/total) | 81.8 (18/22) | 77.3 (17/22) | 54.5 (12/22) | 27.3 (6/22) |
Abbreviations: ACDA, anterior cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion; ALIF, anterior lumbar interbody fusion; CDA, cervical disc arthroplasty; MIS, minimally invasive surgery; MMA, multimodal analgesia; PCDF, posterior cervical discectomy and fusion; PLIF, posterior lumbar interbody fusion; TLIF, transforaminal lumbar interbody fusion; XLIF, extreme lateral interbody fusion.
Table 5.
Summary of early discharge criteria from spine Enhanced Recovery After Surgery (ERAS) protocols.
|
ERAS Study |
Surgical Procedure |
Discharge Criteria |
| Scanlon and Richards,19 2004 | Lumbar laminectomy for discectomy | PACU stay of at least 4 hr Respiratory, energy, alertness, circulation, and temperature (REACT) score Controlled nausea and/or vomiting Controlled pain managed with oral medications Has ambulated and is steady on feet Stable neurologic status Stable hemodynamic status Has voided Ride is available to take patient home Discharge instruction provided to patient and caregiver Neurosurgeon and anesthesiologist each approve discharge |
| Bradywood et al,22 2017 | Lumbar spinal fusion | Has had x-rays Has passed gas Understands medications Understands activity limitations and use of the brace Understands where to go next Understands f/u plan Has had all questions and concerns addressed |
| Debono et al,23 2017 | Lumbar microdiscectomy | Patients discharged upon reaching a score of 9 on the Chung Post-Anesthetic Discharge Scoring System |
| Debono et al,33 2019 | ACDF, ALIF, PLIF | Patients discharged upon reaching a score of 9 on the Chung Post-Anesthetic Discharge Scoring System |
| Soffin et al,37 2019 | ACDF and CDA | 5 hr of observation in PACU, after which patient evaluated for discharged by anesthesia using Aldrete criteria (tolerating regular diet, ambulating without assistance, adequate pain control on oral analgesics, clear understanding of f/u plan, contact details in case of event/questions/complications) |
| Soffin et al,38 2019 | Lumbar decompression (laminectomy, laminotomy, microdiscectomy) | Discharge from PACU when patients achieved a modified Aldrete score ≥9 |
Abbreviations: ACDF, anterior cervical discectomy and fusion; ALIF, anterior lumbar interbody fusion; CDA, cervical disc arthroplasty; f/u, follow-up; PACU, postanesthesia care unit; PLIF, posterior lumbar interbody fusion.
DISCUSSION
The most commonly implemented preoperative, perioperative, and postoperative spine ERAS elements are discussed here, both with regard to how they were implemented in the ERAS studies and in the context of prior evidence from other surgical specialties or non-ERAS spine studies. ERAS elements that were not popular among the reviewed studies are not discussed.
Preoperative ERAS Elements
Patient Education
Educating the patient prior to surgery is a common component of the ERAS preoperative pathway. Although traditional care pathways may include patient education, they are less structured and standardized in implementation. ERAS patient education for spine surgery includes setting realistic goals and expectations for surgery and recovery,21–25,28,30,32–34,36,37,39,40 recommending preoperative nutrition and exercise regimens,25 educating about the ERAS components,23,34,39 and educating about pain management (perioperative and postoperative MMA, how to grade pain, how pain scores translate to analgesia selection, etc.).25,33,34,37–39 In addition to providing videos, handouts, and oral presentations to educate patients, some research groups also implemented a helpline and enlisted 24-hour on-call nurses to aid in preoperative patient education.23,28,33 Many of the ERAS patient education guidelines share similar features but differed in their degree of detail and implementation.
Despite the frequent inclusion of patient education in ERAS protocols, there is a lack of evidence regarding its impact on patient outcomes. Previously, patient education was not found to significantly improve surgical outcomes or reduce patient anxiety; in a review of preoperative education for hip and knee replacement procedures, McDonald et al41 concluded that patient education may only benefit patients with preexisting depression, anxiety, or unrealistic expectations. It is thus unclear whether standardized patient education guidelines are necessary in most cases. However, some research groups34,39 have based their rationale for including ERAS patient education on the McDonald et al41 review article, claiming that educating patients can enhance postoperative and recovery outcomes.
Comorbidity Assessment and Health Optimization
Patients who undergo elective spine surgery often have other comorbidities, such as diabetes or cardiovascular disease, especially as the population of elderly patients desiring spine surgery continues to increase. Considering the physiologic stress placed on the body by surgery, several institutions opted to evaluate and address patient comorbidities as part of their preoperative ERAS protocol (Table 2). In this way, the patient's health may be optimized prior to surgery to reduce the risk of postoperative complications.40
Several ERAS spine protocols required screening for risk factors like diabetes, cardiovascular disease, obesity (body mass index), smoking status, narcotic/alcohol use, anemia, nutritional status, and obstructive sleep apnea. The American Society of Anesthesiologists classification, which indicates the severity of a patient's comorbidities, was also calculated in some protocols.20,21,36,40 For patients who presented with comorbidities or who did not meet the health criteria, one of several forms of intervention were taken. Some studies built in exclusion criteria for surgery, such as cutoff body mass index and American Society of Anesthesiologists classification values20,31; some delayed the procedure and/or offered counseling and medical services for diabetes, weight loss/nutrition, exercise, smoking cessation, obstructive sleep apnea, and narcotics/alcohol use21,22,28,31,37,40; and some developed their own “pre-habilitation” exercise regimens for patients to follow.22,27
Although it is unclear how health assessment and optimization independently contributed to patient outcomes, previous studies in other surgical specialties have shown a link between preoperative comorbidities and postoperative outcomes. In a study of geriatric patients undergoing noncardiac surgery, Leung and Dzankic42 showed that patients with neurologic or cardiovascular comorbidities were at higher risk of adverse postoperative events. Oresanya et al43 similarly reported a correlation between cognitive impairment, malnutrition, and frailty with adverse outcomes in geriatric surgical patients.43
Modified Preoperative Fasting With Carbohydrate Loading
Traditionally, patients are instructed to begin fasting the night prior to surgery. But this practice not only lacks scientific backing but also can exacerbate the surgical stress response and the development of postsurgical insulin resistance.2 As such, several spine ERAS protocols modified their preoperative fasting guidelines, instead permitting consumption of solid foods up to 6 to 12 hours prior to surgery and liquids up to 2 to 8 hours prior to surgery.24,25,33,34,37,39 There is evidence that clear fluids are safe to ingest up to 2 hours prior to surgery, and so fasting guidelines have been changing accordingly since the mid-1990s.2
Additionally, carbohydrate loading—which involves the oral (PO) or intravenous (IV) administration of a carbohydrate fluid (eg, Nutricia preOp)—usually 2 hours prior to surgery—was incorporated into many spine ERAS protocols (Table 2). The carbohydrate fluid was often but not always administered in conjunction with the shortened preoperative fasting regimen. Carbohydrate loading has become a staple practice in ERAS surgery protocols and is thought to help mitigate postsurgical insulin resistance.44 A review by Smith et al44 of preoperative carbohydrate loading treatments from ERAS surgery protocols found an association with reduced LOS, but not with postoperative complications. Similarly, spine ERAS protocols that featured carbohydrate loading have not yielded significant changes in postoperative complication and/or readmission rates.25,27,30,32 However, some protocols that included carbohydrate loading did report reduced LOS,28,30,32,34 whereas others denied any significant change in outcomes.25,27
Perioperative ERAS Elements
“Preemptive” Analgesia
The consumption of nonopioid analgesics prior to induction has been correlated independently with decreased pain levels and reduced opioid consumption (known as an opioid sparing effect) after spine surgery.45,46 Notably, Kim et al46 administered a cocktail of 200 mg of celecoxib, 75 mg of pregabalin, 500 mg of acetaminophen, and 10 mg of extended-release oxycodone—as opposed to conventional morphine for the control group—at least 1 hour prior to surgery. Patients who received this preemptive cocktail demonstrated lower visual analog scale (VAS) and Oswestry Disability Index values at nearly all POD time points measured. Furthermore, there were no significant differences in postoperative complication rates.46
For spine ERAS protocols, the combination of acetaminophen PO (usually 1000 mg) and gabapentin PO (usually 300 mg) was commonly administered to patients on the morning of surgery,25,30,32,36–39 and also the night before surgery.30,32 Other oral analgesics, like 200 mg of celecoxib34 and 150 or 75 mg of pregabalin,25,34 were also administered. These analgesic options have been shown to effectively reduce pain45–47 and opioid consumption45 following spine surgery. For the acetaminophen and gabapentin combination specifically, Syal et al48 previously reported improved postoperative pain scores in patients who underwent open cholecystectomy, which suggests that this combination of premedication may be effective for other types of surgeries.
Indeed, some spine ERAS studies that implemented preemptive analgesia showed decreased use of postoperative patient-controlled analgesia (PCA) and/or opioid consumption, even as their associated surgical procedures varied greatly.25,27,36,38 These studies did not indicate reduced pain scores, however.25,27 In fact, Soffin et al38 implemented the acetaminophen-gabapentin combination yet found neither reduced pain scores nor reduced opioid consumption postoperatively—even with the contribution of other ERAS elements. It seems that more research regarding the effects of preemptive analgesia on postoperative outcomes in spine surgery is required.
Intraoperative MMA
The use of intraoperative medications to minimize postoperative pain is another integral component of optimizing patient outcomes. A variety of intraoperative MMA regimens have been implemented in the spine ERAS protocols (Table 3). For example, for spinal metastatic tumor resection surgeries, Grasu et al25 implemented infusions of propofol, dexmedetomidine, ketamine, and lidocaine, along with a single upfront IV dose of methadone (0.1–0.2 mg/kg) in opioid-tolerant patients. In contrast, Ali et al27 administrated intraoperative NSAIDs, dexamethasone, and local bupivacaine in addition to conventional opioids for various elective spine procedures.27 Other common analgesic medications were used intraoperatively, like acetaminophen30,32 and other local analgesics.24,31,34,39,40 Particular attention was given to modulating the choice of anesthetics; several studies avoided using general anesthesia and long-acting agents. When general anesthesia was used, propofol appeared to be preferred among the ERAS studies.25,30,32,38–40
Moreover, numerous non-ERAS studies have investigated whether a multimodal approach to pain management can limit postoperative pain and opioid use in spine surgery.49–52 Loftus et al49 studied the effect of intraoperative ketamine (initial bolus of 0.5 mg/kg, followed by infusion at 10 μg/kg/min). In this study, the treatment group received a loading dose of ketamine prior to induction and a continuous drip during surgery.49 Patients in this treatment group required 24% less intraoperative opiate medication; these patients also consumed 37% less morphine in the 48 hours following surgery compared with the control group.49 Moreover, at the 6-week follow-up, the treatment group patients reported the same reduction in pain despite a 71% reduction in opioid consumption.49 Tunali et al50 similarly studied the efficacy of IV paracetamol (1 g) and dexketoprofen (50 mg) on postoperative pain and morphine consumption following lumbar disc surgery. Here, Tunali and colleagues50 measured VAS scores and total morphine consumption in the first 24 hours after surgery, respectively. The authors reported that pain scores were significantly reduced with dexketoprofen administration but not with paracetamol.50 However, there was no difference in morphine consumption with either medication when compared to placebo.50 Later, in a randomized controlled study, Nielsen et al51 investigated the administration of preoperative dexamethasone (16 mg) combined with intraoperative paracetamol, reporting that although pain scores were significantly reduced in the first 24 hours following surgery, this difference dissipated at the 3-month follow-up. Notably, there was a 6.5% incidence of postoperative infection in the dexamethasone treatment group, which limits the benefit that this medication can have on patients.51 Finally, Garg et al52 administered ketamine (bolus 0.25 mg/kg followed by 10 mg/kg/hr infusion) or dexmedetomidine (0.5 mg/kg followed by 0.3 mg/kg/hr infusion) to patients during induction for various elective spine procedures.52 The authors reported that patients who received ketamine and dexmedetomidine experienced greater pain-free periods during the first 48 hours after surgery, as well as reduced consumption of rescue pain medication, compared with the control group.52
Minimally Invasive Surgery
The MIS technique is being adopted rapidly in spine surgery and involves minimizing intraoperative soft tissue disruption and blood loss.53 The inclusion of MIS in ERAS pathways is aimed at improving cost-effectiveness and enhancing postoperative recovery. Prior research comparing MIS TLIF/PLIF with the traditional open approach suggests that MIS results in reduced hospital costs,26,53,54 LOS,53,55,56 and blood loss.53,56 However, the benefits of MIS on overall clinical outcomes remain controversial,3,55 with some studies achieving reduced short-term readmission and complication rates,56 and others finding no additional short- or long-term clinical benefits compared with the open surgical approach.53–55 From current evidence, it seems that MIS procedures are more cost-effective and yield comparable clinical outcomes.
The MIS approach was adopted by several ERAS protocols for lumbar fusion or decompression procedures (Table 3). Grasu et al25 also employed MIS technique for an ERAS metastatic tumor resection protocol. Brusko et al29 employed MIS technique for 1- to 3-level posterior-only lumbar fusion surgeries. Debono et al23 and Soffin et al38 pursued a protocol for lumbar microdiscectomy.23,38 In ERAS studies, MIS technique involved a small incision, tubular working channels and endoscopy, percutaneous screw insertion, expandable cage implants,24,26,38–40 and robotic and microscope guidance.38–40
Thus far, the use of MIS in spine ERAS studies has been associated with various positive outcomes, especially for MIS TLIF procedures (Table 1). For example, Wang et al26 reported reduced intraoperative time, LOS, complication rate, and acute care costs, and Feng et al34 reported reduced LOS and mean costs but no difference in intraoperative time or complication and readmission rates. Interestingly, Wang et al26 were able to enhance the cost-effectiveness of MIS TLIF using only 6 perioperative ERAS interventions, 3 of which pertained to an MIS surgical approach (working channel endoscope, expandable cage, small-caliber percutaneous screws). Results by Wang and colleagues26 suggest that the use of MIS may account significantly for the benefits of ERAS pathways.
Maintaining Homeostasis (Blood Loss, Fluids, Temperature)
Managing body homeostasis during surgery helps to reduce postoperative complications and enhance the return of normal bodily functions.2 In particular, the management of blood loss, normovolemia, and normothermia has emerged as a popular inclusion in ERAS pathways. Minimizing blood loss in turn can reduce the risk of hypotension, end-organ damage, and coagulopathy, as well as complications related to blood transfusions. Maintaining normovolemia for patients undergoing major spine surgery has been associated with reduced blood loss and transfusion, improved postoperative respiratory function, faster return of bowel function, and reduced LOS.57 Conversely, overhydration and underhydration of the patient intraoperatively have been associated with increased complications.2 Monitoring and maintaining body temperature during surgery is similarly important for postoperative recovery, because hypothermia increases the risk of wound infection after spine surgery.58
For blood loss management, tranexamic acid—which has been reported to decrease bleeding and transfusions during spine surgery59—was administered intraoperatively in several spine ERAS pathways (Table 3). In the studies, tranexamic acid was given as an IV bolus before skin incision and as an infusion through the procedure. Bolus dosage varied (2 g, 20 mg/kg, 0.5 g/hr, or 1 g prior to incision and another 1 g over 8 hours)21,30,31 because optimal dosing for surgery has not yet been determined. Other methods of minimizing blood loss include minimal use of blood transfusions and preoperative arterial embolization for high-risk bleeding tumors,25 making available autologous cell-salvage transfusions,40 and administering oral iron supplements or iron transfusions for anemic patients prior to surgery.34 Consequently, Feng et al34 and Dagal et al32 were able to significantly reduce intraoperative blood loss for MIS TLIF and various major spine procedures, respectively. Chakravarthy et al31 also reported decreased blood transfusions for ERAS patients undergoing major and complex spine surgeries. Other studies that administered tranexamic acid did not report a comparative blood loss measure.25
For maintaining normovolemia intraoperatively, a combination of hemodynamic monitoring and as-needed fluid replacement therapy was used (Table 3). This type of intervention is known as goal-directed fluid therapy (GDFT).60 According to the American Society for Enhanced Recovery, the goal of GDFT is to achieve a net “zero-fluid balance” (euvolemia) perioperatively in order to reduce postoperative complications and LOS.60 Thiele et al60 recommend the use of isotonic IV crystalloids (salt solution with small molecules) for fluid replacement, which was employed by Chakravarthy et al.31 Other ERAS protocols that implemented GDFT did not specify their method of fluid therapy. Ljungqvist2 further recommends the use of a combination of colloids (which contain larger molecules) and crystalloids, and warns against the use of 0.9% saline, which can cause prolonged postoperative fluid retention. Of note, both Thiele et al60 and Ljungqvist2 formulated their guidelines based on data from ERAS colorectal surgery. But there seems to be no consensus for optimal GDFT for spine surgery.2,60
Finally, normothermia was monitored and maintained intraoperatively in a number of studies (Table 3). Optimal body temperature was set between 36°C and 37°C and was monitored throughout the procedure. Various methods of body warming were used intraoperatively, like hot air blankets, fluid warmers, and a convective warming device.
Deep Venous Thrombosis, Infection, and Postoperative Nausea and Vomiting Prophylaxis
Deep venous thrombosis, infection, and postoperative nausea and vomiting (PONV) are postoperative complications that can significantly hinder patient recovery. To avoid such complications, prophylactic measures have been incorporated into spine ERAS protocols (Table 3). For antithrombotic prophylaxis, compression stockings/devices and low–molecular weight heparin were used intraoperatively.24,35,40 Postoperative thromboprophylaxis has been implemented as well, also in the form of compression devices, heparin, and early and frequent ambulation.31,35 Notably, Sivaganesan et al35 reserved the use of chemoprophylaxis for uncommon cases, namely for cases involving combined anterior and posterior approach, trauma, spinal cord injury, tumor, and hypercoagulable-state patients; only mechanical prophylaxis was used for common elective spine surgeries.
To reduce the risk of infection, several types of antimicrobial agents and routines have been documented in the literature. For example, Bradywood et al22 required that patients take chlorhexidine showers or baths prior to surgery, given that preoperative bathing is believed to reduce bacterial load; Feng et al34 administered 1.5 g cefuroxime close to time of incision34; and Smith et al36 administered 2 to 3 g of Ancef, 900 mg of Clindamycin, or 15 mg/kg Vancomycin.36 These interventions were mostly perioperative in nature, with the exception of the chlorhexidine baths.
For PONV prophylaxis, Soffin and colleagues37–39 employed both perioperative prophylaxis and postoperative treatments in a series of spine ERAS protocols. Perioperatively, patients at high risk for PONV were given a scopolamine patch (1.5 g transdermal) in the surgical holding area,37,39 as well as 4 to 8 mg of dexamethasone on induction and 4 to 8 mg of ondansetron 30 minutes prior to emergence from anesthesia.38 Postoperatively, PONV was treated with IV metoclopramide (10 mg) or ondansetron (4 mg), plus a scopolamine patch (1.5 mg transdermal) if PONV was refractory.38,39 Carr et al30 also administered IV ondansetron intra-operatively as prophylaxis. Bednar21 administered IV/PO dexamethasone 4 mg every 6 hours postoperatively until discharge.
Catheter and Surgical Drain Sparing
Several spine ERAS protocols emphasized sparing the use of Foley catheters and surgical site drains—either avoiding use or performing early removal (Table 3). The use of Foley catheters and surgical drains has been associated with postsurgical infection of the urinary tract and wound site, respectively. To minimize such complications and to facilitate patient mobility, Ali et al27 reported significantly decreased Foley catheter placements intraoperatively, as well as postoperatively for patients without bed restrictions; however, there was no significant difference in complication rate (which included infection rate) in pre-ERAS intervention versus post-ERAS intervention patients in this study. Staartjes et al40 used surgical drains only for patients who underwent mini-open decompression or MIS PLIF procedures; the authors also stressed the removal of catheters and drains as soon as possible postoperatively. Likewise, Debono et al23 and Sivaganesan et al35 reported no drain use for lumbar microdiscectomy and various elective spine surgery procedures, respectively; Debono et al33 described a drastic reduction in drain use for ACDF, ALIF, and PLIF procedures; Soffin et al37 omitted the use of catheters or drains for ACDF and cervical disc arthroplasty (CDA) surgeries.37 Wang et al24 avoided the use of a Foley catheter altogether. In studies that did not emphasize catheter or wound drain sparing, most documented removal on POD131,36 or POD2/322,30,32 in order to facilitation mobilization.
Postoperative ERAS Elements
Postoperative Pain Modulation
In addition to perioperative medication regimens for patients undergoing spine surgery (see the “Preemptive Analgesia” and “Intraoperative MMA” sections), postoperative MMA measures have been implemented. A variety of MMA combinations have been administered to supplement PCA opioid consumption. Common elements of a postoperative MMA cocktail include gabapentin/pregabalin,22,24,25,32,34–36,38,39 acetaminophen,22,24,25,27,30,32,37–39 and celecoxib and other NSAIDs.22,25,27,33–40 Other nonopioid options include dexamethasone,27 paracetamol,40 and ketamine.28 To maximize opioid sparing, some studies also avoided the use of long-acting opioids24,40 and implemented early discontinuation of PCA.22 For various types of lumbar decompression surgeries, Soffin et al38 stratified postoperative MMA and opioid use based on numeric rating scale pain scores: patients with numeric rating scale scores of 4 or less only received nonopioid interventions (acetaminophen, ketorolac, gabapentin); patients with scores 5 to 7 could receive 50 mg of tramadol; and patients with scores 8 to 10 received 5 mg of oxycodone.38 Interestingly, Soffin and colleagues found decreased perioperative opioid consumption, but no significant changes in postoperative pain scores or opioid consumption compared with the pre-ERAS control group. For 1- or 2-level lumbar fusion procedures, Smith et al36 administered a postoperative cocktail of 200 mg of celecoxib PO, 300 mg of gabapentin PO, and 975 mg of acetaminophen PO; the authors reported a decrease from 7% to 0% in PCA use and a decrease from 14% to 5.2% in the use of long-acting opioids.
Importantly, several research groups have investigated the effects of postoperative MMA for non-ERAS spine surgery, which yielded varied outcomes.45,47,61,62 In a randomized controlled trial, Korkmaz et al61 studied the effect of either IV 1 g of metamizol, 1 g of paracetamol, or 8 mg of lornoxicam supplementation after wound closure for lumbar disc surgery. These analgesics were administered in tandem with conventional postoperative PCA of morphine.61 The authors reported decreased VAS pain scores in the first 24 hours after surgery for patients who received metamizol and paracetamol, but not lornoxicam; PCA use in the paracetamol group also significantly decreased over time.61 Other studies demonstrated the postoperative use of MMA pain management with nonsteroidal anti-inflammatories (NSAIDs) and GABA-acting drugs. Mathiesen et al45 compared the administration of a cocktail of acetaminophen, NSAIDs, gabapentin, S-ketamine, and dexamethasone versus PCA morphine in patients undergoing spinal fusion surgery. Results suggest that patients who received the MMA cocktail required less opioid consumption and could mobilize earlier with physical therapy compared with control patients who only depended on PCA morphine.45 Likewise, Garcia et al62 studied the effect of giving celecoxib, pregabalin, and extended-release oxycodone supplementation for PCA morphine compared with the IV morphine infusion alone. Total morphine consumption and VAS scores were lower in patients receiving the MMA regimen compared with control.62 Finally, a meta-analysis by Yu et al47 analyzing the use of pregabalin and gabapentin in the postoperative period demonstrated that gabapentin PO was efficacious in the management of postoperative pain at all time points on POD1, and thus may significantly reduce total opioid consumption without increasing the incidence of adverse effects. Pregabalin also was found to be efficacious in the management of postoperative pain, but more trials were needed to further evaluate its effectiveness.47
Early Mobilization and Physical Rehabilitation
Early mobilization of the patient is a critical prerequisite for achieving early discharge, which is one of the goals of ERAS. Here, early mobilization is defined as getting the patient out of bed on POD0 or POD1,63 which may involve physical therapy, occupational therapy, or other exercises. In a review of the effects of early mobilization after various types of surgeries, Epstein63 described correlations with reduced morbidity and LOS. However, research on mobilization/rehabilitation following spine surgery remains scarce. Nielsen et al64 implemented a pre-habilitation (6- to 8-week exercise regimen and smoking/drinking cessation) and early rehabilitation program for patients undergoing surgery for degenerative lumbar disease; the authors reported reduced LOS and improved patient satisfaction for patients who received this intervention, although postoperative complication rate and pain did not significantly change.
Although only 1 ERAS protocol included a “pre-habilitation” regimen,28 many other protocols encourage early mobilization and provided physical rehabilitation services (Table 4). In the ERAS protocols, patient mobilization started as early as less than 90 minutes after arrival at the PACU following a laminectomy/laminotomy/microdiscectomy procedure.38 Early mobilization goals included independence in performing log roll,22 movement out of bed and to chair 3 times per day,25,39 working with physical therapy/occupational therapy,25,31,36–39 or even being discharged after surgery.20 Most protocols did not provide much detail beyond when early mobilization began and whether physical therapy/occupational therapy was offered; even then, there was considerable variety in early mobilization requirements. Because of the paucity of research on this topic, there seems to be no consensus on which features of early mobilization and physical rehabilitation are most beneficial to patients.
Early Nutrition
Early commencement of postoperative enteral nutrition is an established ERAS intervention in other surgical specialties. For instance, in the ERAS guidelines for colonic resection2 and rectal/pelvic surgery,65 early oral intake of liquids, solids, or nutritional supplements is recommended.2 Indeed, early postoperative enteral nutrition may decrease postsurgical infection rates, reduce LOS and hospital cost, and reduce postoperative ileus when compared to late enteral or parenteral feeding.66–70
Early nutrition was commonly advised for spine surgery patients treated under ERAS guidelines (Table 4). In the spine ERAS protocols, a subset of studies initiated a clear liquid diet at POD0 or POD1 that advanced to a regular diet as tolerated,22,25,34,40 whereas others permitted a regular diet of solids and liquids on POD0 or POD1.30,32,36,38–40 Some protocols initiated oral intake as soon as the patient recovered from anesthesia.34,37–39 These early nutrition guidelines were similar across the different ERAS protocols, regardless of whether the patients had undergone major or minor surgery.
Early Discharge Criteria
Currently, there does not seem to be a consensus on the criteria for early discharge in spine surgery (Table 5). This diversity may be due in part to the different spine procedures for which the protocols were developed. Nevertheless, current ERAS discharge criteria include physical therapy clearance, pain control, mobility, tolerating diet, and scoring systems such as the Aldrete37 and Chung postanesthetic discharge scoring system.23,33,71
CONCLUSIONS
Research on ERAS protocols for spine surgery is still in the nascent stages. Although there are commonalities between existing spine ERAS protocols, there is also much diversity. Notably, studies included in this review varied in their methodology, the number of ERAS interventions per protocol, the types of spine surgery, and the degree to which their data were analyzed and reported. In turn, this review was limited in its ability to assess the efficacy of ERAS protocols.
Overall, ERAS protocols seem to improve cost-effectiveness without compromising the quality of patient care. MMA administration as an ERAS intervention may be linked directly to decreased opioid consumption, which was reported in several studies included in this review. Moreover, MMA administration has been associated independently with decreased opioid consumption in a non-ERAS setting. Study outcomes suggest that improved cost-effectiveness may be achievable through ERAS, in particular via reductions in LOS, cost, and opioid consumption.
Currently, it is difficult to isolate the effect of any one ERAS element on patient outcome; it is also difficult to determine whether ERAS would be more successful for particular spine surgeries. Different spine procedures vary in their expected surgical stress levels and recovery rates, and the age and comorbidity status of patients vary as well; different ERAS interventions may then disproportionately benefit certain patient populations compared with others. However, these distinctions have not been made yet in the literature; in fact, in most studies, the same ERAS protocol was implemented across a number of different spine procedures.
From a review of the relevant studies, it is clear that much investigation is still needed to determine the ideal ERAS elements that are more effective for particular patient populations, spine surgeries, and pathology—such as deformity, tumor resection, and degenerative spine. Focused studies in these areas may allow for more accurate analyses of the effectiveness of particular ERAS elements. As research on spine ERAS pathways grows, greater consensus and protocol standardization may be achieved to benefit patient care.
REFERENCES
- 1.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 2.Ljungqvist O. ERAS—Enhanced Recovery After Surgery. JPEN J Parenter Enteral Nutr. 2014;38(5):559–566. doi: 10.1177/0148607114523451. [DOI] [PubMed] [Google Scholar]
- 3.Wainwright TW, Immins T, Middleton RG. Enhanced recovery after surgery (ERAS) and its applicability for major spine surgery. Best Pract Res Clin Anaesthesiol. 2016;30(1):91–102. doi: 10.1016/j.bpa.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Desborough J. The stress response to trauma and surgery. Br J Anaesth. 2000;85(1):109–117. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 5.Giannoudis PV, Dinopoulos H, Chalidis B, Hall GM. Surgical stress response. Injury. 2006;38(10):1224. doi: 10.1016/S0020-1383(07)70005-0. [DOI] [PubMed] [Google Scholar]
- 6.Carli F. Physiologic considerations of Enhanced Recovery After Surgery (ERAS) programs: implications of the stress response. Can J Anaesth. 2014;62(2):110–119. doi: 10.1007/s12630-014-0264-0. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim MS, Twaij H, Giebaly DE, Nizam I, Haddad FS. Enhanced recovery in total hip replacement. Bone Joint J. 2013;95-B(12):1587–1594. doi: 10.1302/0301-620X.95B12.31303. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim M, Alazzawi S, Nizam I, Haddad F. An evidence-based review of enhanced recovery interventions in knee replacement surgery. Ann R Coll Surg Engl. 2013;95(6):386–389. doi: 10.1308/003588413X13629960046435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones E, Wainwright T, Foster J, Smith J, Middleton R, Francis N. A systematic review of patient reported outcomes and patient experience in enhanced recovery after orthopaedic surgery. Ann R Coll Surg Engl. 2014;96(2):89–94. doi: 10.1308/003588414X13824511649571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761–772. doi: 10.2106/JBJS.G.01472. [DOI] [PubMed] [Google Scholar]
- 11.Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthopaedica. 2013;85(1):26–31. doi: 10.3109/17453674.2013.874925. doi: 10.3109/17453674.2013.874925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wainwright TW, Wang MY, Immins T, Middleton RG. Enhanced recovery after surgery (ERAS)—concepts, components, and application to spine surgery. Semin Spine Surg. 2018;30(2):104–110. [Google Scholar]
- 13.Dietz N, Sharma M, Adams S, et al. Enhanced Recovery After Surgery (ERAS) for spine surgery: a systematic review. World Neurosurg. 2019;139:415–426. doi: 10.1016/j.wneu.2019.06.181. [DOI] [PubMed] [Google Scholar]
- 14.Elsarrag M, Soldozy S, Patel P, et al. Enhanced recovery after spine surgery: a systematic review. Neurosurg Focus. 2019;46(4):E3. doi: 10.3171/2019.1.FOCUS18700. [DOI] [PubMed] [Google Scholar]
- 15.Corniola MV, Debono B, Joswig H, Lemée JM, Tessitore E. Enhanced recovery after spine surgery: review of the literature. Neurosurg Focus. 2019;46(4):E2. doi: 10.3171/2019.1.FOCUS18657. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–310. doi: 10.1097/PRS.0b013e318219c171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute Web site. 2019 Dec; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed.
- 19.Scanlon J, Richards B. Development of a same day laminectomy program. J Perianesth Nurs. 2004;19(2):84–88. doi: 10.1016/j.jopan.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Chin KR, Coombs AV, Seale JA. Feasibility and patient-reported outcomes after outpatient single-level instrumented posterior lumbar interbody fusion in a surgery center. Spine. 2015;40(1):E36–E42. doi: 10.1097/BRS.0000000000000604. [DOI] [PubMed] [Google Scholar]
- 21.Bednar DA. Description and results of a comprehensive care protocol for overnight-stay spine surgery in adults. Spine. 2017;42(14) doi: 10.1097/BRS.0000000000001987. doi: 10.1097/brs.0000000000001987. [DOI] [PubMed] [Google Scholar]
- 22.Bradywood A, Farrokhi F, Williams B, Kowalczyk M, Blackmore CC. Reduction of inpatient hospital length of stay in lumbar fusion patients with implementation of an evidence-based clinical care pathway. Spine. 2017;42(3):169–176. doi: 10.1097/BRS.0000000000001703. [DOI] [PubMed] [Google Scholar]
- 23.Debono B, Sabatier P, Garnault V, et al. Outpatient lumbar microdiscectomy in France: from an economic imperative to a clinical standard—an observational study of 201 cases. World Neurosurg. 2017;106:891–897. doi: 10.1016/j.wneu.2017.07.065. [DOI] [PubMed] [Google Scholar]
- 24.Wang MY, Chang PY, Grossman J. Development of an Enhanced Recovery After Surgery (ERAS) approach for lumbar spinal fusion. J Neurosurg Spine. 2017;26(4):411–418. doi: 10.3171/2016.9.SPINE16375. [DOI] [PubMed] [Google Scholar]
- 25.Grasu RM, Cata JP, Dang AQ, et al. Implementation of an Enhanced Recovery After Spine Surgery program at a large cancer center: a preliminary analysis. J Neurosurg Spine. 2018;29(5):588–598. doi: 10.3171/2018.4.SPINE171317. [DOI] [PubMed] [Google Scholar]
- 26.Wang MY, Chang HK, Grossman J. Reduced acute care costs with the ERAS® minimally invasive transforaminal lumbar interbody fusion compared with conventional minimally invasive transforaminal lumbar interbody fusion. Neurosurgery. 2018;83(4):827–834. doi: 10.1093/neuros/nyx400. [DOI] [PubMed] [Google Scholar]
- 27.Ali ZS, Flanders TM, Ozturk AK, et al. Enhanced recovery after elective spinal and peripheral nerve surgery: pilot study from a single institution. J Neurosurg Spine. 2019;30(4):532–540. doi: 10.3171/2018.9.SPINE18681. [DOI] [PubMed] [Google Scholar]
- 28.Angus M, Jackson K, Smurthwaite G, et al. The implementation of enhanced recovery after surgery (ERAS) in complex spinal surgery. J Spine Surg. 2019;5(1):116–123. doi: 10.21037/jss.2019.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brusko GD, Kolcun JPG, Heger JA, et al. Reductions in length of stay, narcotics use, and pain following implementation of an enhanced recovery after surgery program for 1- to 3-level lumbar fusion surgery. Neurosurg Focus. 2019;46(4) doi: 10.3171/2019.1.FOCUS18692. doi: 10.3171/2019.1.focus18692. [DOI] [PubMed] [Google Scholar]
- 30.Carr DA, Saigal R, Zhang F, Bransford RJ, Bellabarba C, Dagal A. Enhanced perioperative care and decreased cost and length of stay after elective major spinal surgery. Neurosurg Focus. 2019;46(4) doi: 10.3171/2019.1.FOCUS18630. doi: 10.3171/2019.1.focus18630. [DOI] [PubMed] [Google Scholar]
- 31.Chakravarthy VB, Yokoi H, Coughlin DJ, Manlapaz MR, Krishnaney AA. Development and implementation of a comprehensive spine surgery enhanced recovery after surgery protocol: the Cleveland Clinic experience. Neurosurg Focus. 2019;46(4):E11. doi: 10.3171/2019.1.FOCUS18696. [DOI] [PubMed] [Google Scholar]
- 32.Dagal A, Bellabarba C, Bransford R, et al. Enhanced perioperative care for major spine surgery. Spine. 2019;44(13):959–966. doi: 10.1097/BRS.0000000000002968. [DOI] [PubMed] [Google Scholar]
- 33.Debono B, Corniola MV, Pietton R, Sabatier P, Hamel O, Tessitore E. Benefits of Enhanced Recovery After Surgery for fusion in degenerative spine surgery: impact on outcome, length of stay, and patient satisfaction. Neurosurg Focus. 2019;46(4):E6. doi: 10.3171/2019.1.FOCUS18669. [DOI] [PubMed] [Google Scholar]
- 34.Feng C, Zhang Y, Chong F, et al. Establishment and implementation of an Enhanced Recovery After Surgery (ERAS) pathway tailored for minimally invasive transforaminal lumbar interbody fusion surgery. World Neurosurg. 2019;129 doi: 10.1016/j.wneu.2019.05.139. doi: 10.1016/j.wneu.2019.05.139. [DOI] [PubMed] [Google Scholar]
- 35.Sivaganesan A, Chotai S, Cherkesky CM, McGirt MJ, Stephens BF, Devin CJ. A perioperative protocol for elective spine surgery is associated with reduced length of stay and complications. Spine J. 2017;17(10):183–189. doi: 10.5435/JAAOS-D-17-00274. [DOI] [PubMed] [Google Scholar]
- 36.Smith J, Probst S, Calandra C, et al. Enhanced recovery after surgery (ERAS) program for lumbar spine fusion. Perioper Med. 2019;8(1):4. doi: 10.1186/s13741-019-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soffin EM, Wetmore DS, Barber LA, et al. An enhanced recovery after surgery pathway: association with rapid discharge and minimal complications after anterior cervical spine surgery. Neurosurg Focus. 2019;46(4):E9. doi: 10.3171/2019.1.FOCUS18643. [DOI] [PubMed] [Google Scholar]
- 38.Soffin EM, Wetmore DS, Beckman JD, et al. Opioid-free anesthesia within an enhanced recovery after surgery pathway for minimally invasive lumbar spine surgery: a retrospective matched cohort study. Neurosurg Focus. 2019;46(4):E8. doi: 10.3171/2019.1.FOCUS18645. [DOI] [PubMed] [Google Scholar]
- 39.Soffin EM, Vaishnav AS, Wetmore DS, et al. Design and implementation of an Enhanced Recovery After Surgery (ERAS) program for minimally invasive lumbar decompression spine surgery. Spine. 2019;44(9):E561–E570. doi: 10.1097/BRS.0000000000002905. [DOI] [PubMed] [Google Scholar]
- 40.Staartjes VE, Wispelaere MPD, Schröder ML. Improving recovery after elective degenerative spine surgery: 5-year experience with an enhanced recovery after surgery (ERAS) protocol. Neurosurg Focus. 2019;46(4):E7. doi: 10.3171/2019.1.FOCUS18646. [DOI] [PubMed] [Google Scholar]
- 41.McDonald S, Green S. Pre-operative education for hip or knee replacement. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD003526.pub2. CD003526. doi: 10.1002/14651858.cd003526. [DOI] [PubMed]
- 42.Leung JM, Dzankic S. relative importance of preoperative health status versus intraoperative factors in predicting postoperative adverse outcomes in geriatric surgical patients. J Am Geriatr Soc. 2001;49(8):1080–1085. doi: 10.1046/j.1532-5415.2001.49212.x. [DOI] [PubMed] [Google Scholar]
- 43.Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient. JAMA. 2014;311(20):2110. doi: 10.1001/jama.2014.4573. [DOI] [PubMed] [Google Scholar]
- 44.Smith MD, McCall J, Plank L, Herbison GP, Soop M, Nygren J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD009161.pub2. CD009161. doi: 10.1002/14651858.cd009161. [DOI] [PMC free article] [PubMed]
- 45.Mathiesen O, Dahl B, Thomsen BA, et al. A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. Eur Spine J. 2013;22(9):2089–2096. doi: 10.1007/s00586-013-2826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim SI, Ha KY, Oh IS. Preemptive multimodal analgesia for postoperative pain management after lumbar fusion surgery: a randomized controlled trial. Eur Spine J. 2015;25(5):1614–1619. doi: 10.1007/s00586-015-4216-3. [DOI] [PubMed] [Google Scholar]
- 47.Yu L, Ran B, Li M, Shi Z. Gabapentin and pregabalin in the management of postoperative pain after lumbar spinal surgery. Spine. 2013;38(22):1947–1952. doi: 10.1097/BRS.0b013e3182a69b90. [DOI] [PubMed] [Google Scholar]
- 48.Syal K, Goma M, Dogra RK, Ohri A, Gupta AK, Goel A. “Protective premedication”: a comparative study of acetaminophen, gabapentin and combination of acetaminophen with gabapentin for post-operative analgesia. J Anaesthesiol Clin Pharmacol. 2010;26(4):531–536. [PMC free article] [PubMed] [Google Scholar]
- 49.Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639–646. doi: 10.1097/ALN.0b013e3181e90914. [DOI] [PubMed] [Google Scholar]
- 50.Tunali Y, Akçil EF, Dilmen OK, et al. Efficacy of intravenous paracetamol and dexketoprofen on postoperative pain and morphine consumption after a lumbar disk surgery. J Neurosurg Anesthesiol. 2013;25(2):143–147. doi: 10.1097/ANA.0b013e31827464af. [DOI] [PubMed] [Google Scholar]
- 51.Nielsen RV, Siegel H, Fomsgaard JS, et al. Preoperative dexamethasone reduces acute but not sustained pain after lumbar disk surgery: a randomized, blinded, placebo-controlled trial. Pain. 2015;156(12):2538–2544. doi: 10.1097/j.pain.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 52.Garg N, Panda NB, Gandhi KA, et al. Comparison of small dose ketamine and dexmedetomidine infusion for postoperative analgesia in spine surgery–a prospective randomized double-blind placebo controlled study. J Neurosurg Anesthesiol. 2016;28(1):27–31. doi: 10.1097/ANA.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 53.Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82(1–2):230–238. doi: 10.1016/j.wneu.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 54.Sulaiman WA, Singh M. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis grades 1-2: patient-reported clinical outcomes and cost-utility analysis. Ochsner J. 2014;14(1):32–37. [PMC free article] [PubMed] [Google Scholar]
- 55.Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine. 2016;24(3):416–427. doi: 10.3171/2015.2.SPINE14973. doi: 10.3171/2015.2.spine14973. [DOI] [PubMed] [Google Scholar]
- 56.Ge DH, Stekas ND, Varlotta CG, et al. Comparative analysis of two transforaminal lumbar interbody fusion techniques: open TLIF versus Wiltse MIS TLIF. Spine (Phila Pa 1976) 2019;44(9):E555–E560. doi: 10.1097/BRS.0000000000002903. [DOI] [PubMed] [Google Scholar]
- 57.Bacchin MR, Ceria CM, Giannone S, et al. Goal-directed fluid therapy based on stroke volume variation in patients undergoing major spine surgery in the prone position. Spine. 2016;41(18):752–761. doi: 10.1097/BRS.0000000000001601. [DOI] [PubMed] [Google Scholar]
- 58.Guest JD, Vanni S, Silbert L. Mild hypothermia, blood loss and complications in elective spinal surgery. Spine J. 2004;4(2):130–137. doi: 10.1016/j.spinee.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 59.Cheriyan T, Maier SP, Bianco K, et al. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J. 2015;15(4):752–761. doi: 10.1016/j.spinee.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 60.Thiele RH, Raghunathan K, Brudney CS, et al. Correction to: American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on perioperative fluid management within an enhanced recovery pathway for colorectal surgery. Perioper Med Lond. 2018;7(1) doi: 10.1186/s13741-018-0085-8. doi: 10.1186/s13741-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korkmaz Dilmen O, Tunali Y, Cakmakkaya OS, et al. Efficacy of intravenous paracetamol, metamizol and lornoxicam on postoperative pain and morphine consumption after lumbar disc surgery. Eur J Anaesthesiol. 2010;27(5):428–432. doi: 10.1097/EJA.0b013e32833731a4. [DOI] [PubMed] [Google Scholar]
- 62.Garcia RM, Cassinelli EH, Messerschmitt PJ, Furey CG, Bohlman HH. A multimodal approach for postoperative pain management after lumbar decompression surgery: a prospective, randomized study. J Spinal Disord Tech. 2013;26(6):291–297. doi: 10.1097/BSD.0b013e318246b0a6. [DOI] [PubMed] [Google Scholar]
- 63.Epstein N. A review article on the benefits of early mobilization following spinal surgery and other medical/surgical procedures. Surg Neurol Int. 2014;5(4):S66–S73. doi: 10.4103/2152-7806.130674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nielsen PR, Jørgensen LD, Dahl B, Pedersen T, Tønnesen H. Prehabilitation and early rehabilitation after spinal surgery: randomized clinical trial. Clin Rehabil. 2010;24(2):137–148. doi: 10.1177/0269215509347432. [DOI] [PubMed] [Google Scholar]
- 65.Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg. 2012;37(2):285–305. doi: 10.1007/s00268-012-1787-6. [DOI] [PubMed] [Google Scholar]
- 66.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications the results of a meta-analysis. Ann Surg. 1992;216(2):172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Carlo VD. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med. 2001;29(2):242–248. doi: 10.1097/00003246-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Beier-Holgersen R, Boesby S. Influence of postoperative enteral nutrition on postsurgical infections. Gut. 1996;39(6):833–835. doi: 10.1136/gut.39.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD004080.pub2. CD004080. doi: 10.1002/14651858.cd004080.pub2. [DOI] [PubMed]
- 70.Correia MITD, Silva RGD. The impact of early nutrition on metabolic response and postoperative ileus. Curr Opin Clin Nutr Metabol Care. 2004;7(5):577–583. doi: 10.1097/00075197-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 71.Chung F, Chan VW, Ong D. A post-anesthetic discharge scoring system for home readiness after ambulatory surgery. J Clin Anesth. 1995;7(8):500–506. doi: 10.1016/0952-8180(95)00130-a. [DOI] [PubMed] [Google Scholar]


