Abstract
Prevotella genus comprises of obligate anaerobic, gram-negative bacteria that are commensal organisms of oral cavity, gut and vaginal mucosa. Although many Prevotella species have well-established pathogenicity with respect to pulmonary infections, rarely has Prevotella pleuritidis been isolated as a cause of lung abscess. We present a rare case of left lower lobe lung abscess due to P. pleuritidis identified using next-generation sequencing of microbial cell-free DNA testing. A brief review of the literature regarding Prevotella species pulmonary infections, use of next-generation cell-free DNA testing early in the evaluation, antibiotic susceptibility and resistance is also a part of this report.
Keywords: pneumonia (infectious disease), pneumonia (respiratory medicine)
Background
Prevotella species, a known commensal of oral cavity, has been implicated in a variety of infections. We present a case of lung abscess due to Prevotella pleuritidis. This case highlights the need to recognise this organism as a possible cause, the importance of use of next-generation cell-free DNA testing to facilitate diagnosis and growing concern for the rising prevalence of antibiotic resistance in clinical isolates.
Case presentation
A 30- year-old man with medical history of recurrent sinusitis, presented with 1-month history of intermittent fever, gradually worsening cough that produced yellow-coloured phlegm and a single episode of haemoptysis. His episodes of cough were associated with left-sided chest and mid-thoracic back pain. He also had problem of night sweats and weight loss over the same period of time. The patient denied recent travel outside the USA, any history of incarceration, recent ill contacts or exposure to tuberculosis. Other medical history was significant for asthma and bipolar disorder for which the patient was chronically on lithium, quetiapine and olanzapine. He worked as a carpenter with no known exposure to moulds. He denied smoking, vaping, alcohol use or any injection drug use. Family history was negative for malignancy. The patient reported drug allergy to cephalosporins, resulting in rash.
Before presenting to our facility, the patient had already undergone an unsuccessful therapeutic course of outpatient oral antimicrobial therapy with amoxicillin-clavulanic acid, azithromycin and levofloxacin for suspected pneumonia. He then visited an outlying hospital, where after initial lab work and imaging, he was eventually transferred to our facility for further evaluation and management. Physical examination on presentation was significant for decreased breath sounds throughout lung fields, more prominent on the left side. The patient was noted to have fairly decent dentition and oral hygiene with no visible dental caries or periodontal abscess.
Investigations
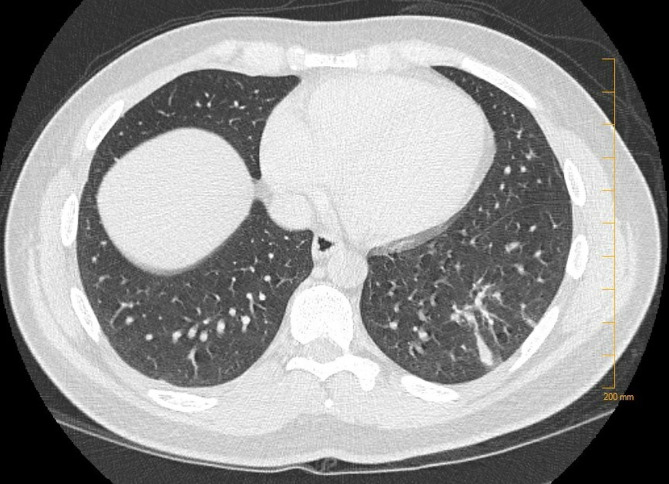
C-reactive protein was elevated at 9.7 mg/dL, complete blood count showed leucocytosis with neutrophil predominance and anaemia. Serological test was positive for Mycoplasma IgM and IgG. Rest of the serology including antinuclear antibody, antineutrophil cytoplasmic antibodies, (1-3)-β-d-glucan in serum, HIV, Brucella and antigen testing for Histoplasma, Pneumococcus and Legionella was unremarkable. Sputum culture showed Candida albicans with mixed oral flora. Acid-fast bacilli smear and mycobacterium tuberculosis culture were negative too. Blood cultures showed no evidence of bacteraemia. CT imaging of chest showed left lower lobe abscess as shown in figure 1.
Figure 1.
CT chest showing abscess within the posterolateral left lower lobe with surrounding consolidation.
Next-generation sequencing of microbial cell-free DNA (‘Karius Test’, Karius, Redwood City, California, USA) detected significant levels of Prevotella pleuritidis at 435 molecules/μL of plasma.
Treatment
The patient was started empirically on intravenous vancomycin and piperacillin–tazobactam. Once the Karius test result was available, the antibiotic coverage was narrowed down to piperacillin–tazobactam only. This was continued at 3.375 g every 8 hours extended infusion over 4 hours instead of standard infusion every 6 hours over 30 min as per institutional protocol.
Outcome and follow-up
The patient was discharged home with skilled nursing services, after placing a peripherally inserted central catheter (PICC) in the right upper extremity. The initial plan was to continue piperacillin–tazobactam for 4 weeks starting from the day of admission. At discharge, dosing of piperacillin–tazobactam was switched to 13.5 g per 24 hours infused continuously via continuous ambulatory delivery device (CADD).
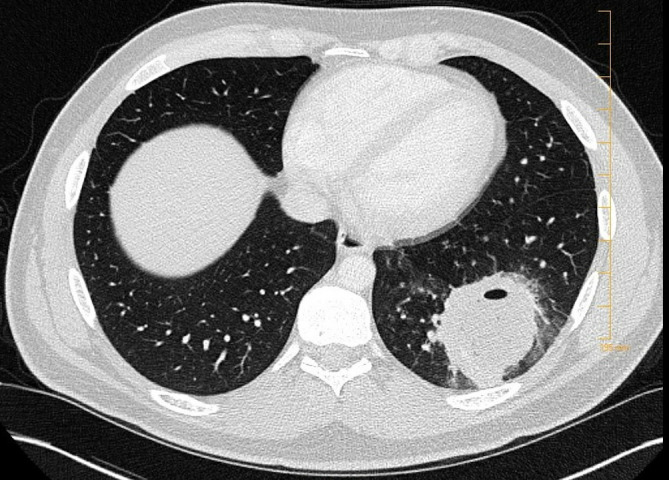
He was followed up outpatient around a month after discharge. His symptoms had completely resolved and C-reactive protein levels had normalised. A repeat CT chest reported resolution of the left lower lobe abscess with a residual 2.2×3.7 cm area of consolidative pneumonia in the left lower lobe as shown in figure 2. At this time, the patient wished to stop intravenous antimicrobial therapy as he wanted to return to work. PICC was removed and he successfully completed a 10-day course of oral levofloxacin and metronidazole.
Figure 2.
Follow-up CT chest in 1 month showed resolution of abscess.
Discussion
Prevotella genus comprises a group of obligate-anaerobic, moderately saccharolytic, bile-sensitive, gram-negative bacteria that produce melanin on blood agar to form black colonies. These are common commensals of oral, gastrointestinal and vaginal microbiota and usually associated with periodontal disease/abscess and anaerobic infections of respiratory tract, for example, lung abscess, empyema, aspiration pneumonia, sinusitis and so on.1 Other associations include osteomyelitis, brain abscesses, urinary tract infections and bacterial vaginosis. P. melaninogenica and P. intermedia are some of the well-known species of the group, but other lesser known species have emerged as pathogens over the past few years.2–5
P. pleuritidis was identified as a novel species in a paper published in 2007 after it was isolated from the pleural fluid of a patient with suppurative pleuritis.1 As per our literature review using PubMed, there have been no case reports of pulmonary abscess due to P. pleuritidis since its discovery and identification in 2007. We did find multiple cases reporting involvement of P. pleuritidis in non-pulmonary infections or of pulmonary infections due to Prevotella species other than P. pleuritidis, like P. dentalis and P. intermedia as sole causes or contributing causes to other bacterial infections.6–8 Most of these reports presented cases due to Prevotella species in the setting of poor oral hygiene, oral infections or following dental procedure. This was not the case in our patient as he had no signs or symptoms of dental infection and denied recent history of dental procedure.3 4
A novel case published in 2017 about liver abscess due to Fusobacterium, identified P. pleuritidis by 16s ribosomal RNA sequencing of the fluid from the abscess. It went on to conclude that gingival/oropharyngeal flora like Prevotella spp was identified as copathogens in around 18% of liver abscess caused by Fusobacterium nucleatum.9 Shaker et al10 published a case of unilateral frontal sinusitis complicated by orbital cellulitis, subperiosteal abscess, subdural empyema and superior sagittal sinus thrombosis where 16s RNA/DNA sequencing revealed P. pleuritidis, Streptococcus intermedius and F. nucleatum as responsible organisms.
Diagnosis is frequently delayed and complicated by inability to identify pathogens if present in numbers below traditional culture lower detection limit or due to organism survival and recovery rate being adversely affected by specimen handling.11 Such limitations of traditional cultures were likely the reason behind inability to grow any pathogens in our patient’s blood and sputum cultures. Next-generation sequencing of microbial cell-free DNA helps overcome such limitations and provides better diagnostic yield. This is well illustrated in our patient’s case, where only Karius test was able to isolate the underlying pathogen, P. pleuritidis, which helped direct and target treatment.12 Karius test result is reported in molecules per microlitre (MPM). MPM refers to the number of microorganism DNA fragments present in 1 μL of plasma and the result is significant only if it is higher than the 97.5th percentile for the pathogen in asymptomatic individuals. Karius test also proves beneficial in cases where samples are drawn after antibiotic administration. Pathogens that are reported in high concentrations are likely associated with true infection and low concentrations may result from both true infection or contaminant. Clinical interpretation of this test is strongly recommended in conjunction with other clinical data such as physical examination, imaging findings and other laboratory results.
Lung abscess is a potential complication of pneumonia. Mycoplasma pneumoniae usually causes atypical pneumonia in children and adolescents.13 It is extremely rare for it to cause lung abscess in adults. In our patient, detection of Mycoplasma antibody on initial serology could be either a false positive or indicate preceding Mycoplasma infection. Prior treatment with azithromycin and levofloxacin (both of which act against Mycoplasma) failed to produce a clinical response in this patient.
Antimicrobial coverage with piperacillin–tazobactam was initiated and continued in our patient. This was widely based on review of the following literature. A multicentre survey which measured antimicrobial sensitivity of 19 Prevotella species (508 clinical isolates) using E-test reported susceptibility to piperacillin–tazobactam, carbapenems, tigecycline and metronidazole. A significantly high percentage of isolates were non-susceptible to ampicillin, clindamycin, tetracycline and moxifloxacin.14 Another study used E-test to investigate antibiotic susceptibility and resistance in a series of 33 Prevotella strains isolated from head and neck abscesses of Romanian patients. This study reported susceptibility of almost all 33 strains to amoxicillin-clavulanic acid, metronidazole and clindamycin but increased resistance to penicillin-G and ampicillin due to beta lactamase activity.15
Multiple studies have also been conducted to identify the presence of antimicrobial resistance genes within Prevotella species. One such study by Arredondo et al16 was conducted on 100 Prevotella spp isolates to determine susceptibility to azithromycin and erythromycin using the Etest method and to screen presence of macrolide resistance genes therein. The study concluded that the presence of either erm(F) and erm(B) gene in Prevotella spp was associated with a higher degree of resistance to azithromycin and erythromycin.16 Another study conducted in the Netherlands by Veloo et al17 described prevalence of three antimicrobial resistance genes in 99 Prevotella spp and 101 Bacteroides spp isolates. The study reported prevalence of 9.1% of the ermF gene, 30.3% of tetQ gene and 50.5% of the cfxA gene. Some of the isolates harboured all three resistance genes. The study expressed concern over presence of multiple resistance genes in a single isolate and increase in prevalence of tetQ gene, which could define changes in treatment strategies in the future.17
Learning points.
Prevotella species commonly colonises the oral cavity. Lung abscess due to Prevotella pleuritidis is rare.
There is growing need for utilisation of next-generation sequencing of microbial cell-free DNA testing early in the evaluation, for targeted treatment and management.
Lung abscesses can be a result of concomitant infection by multiple organisms. Next-generation sequencing of microbial cell-free DNA testing proves to be of higher diagnostic yield in such situations where it can detect organisms present in low numbers or in samples obtained after antibiotic administration.
There is growing concern for treatment failure due to increasing prevalence of antibiotic resistance which requires further research.
Footnotes
Twitter: @sharjeel__ahmad
Contributors: AAA wrote the initial manuscript. MR performed initial edit and SA performed the final edit. All authors performed data designing, data collection, image designing and reviewed the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sakamoto M, Ohkusu K, Masaki T, et al. Prevotella pleuritidis sp. nov., isolated from pleural fluid. Int J Syst Evol Microbiol 2007;57:1725–8. 10.1099/ijs.0.64885-0 [DOI] [PubMed] [Google Scholar]
- 2.Kedzia A, Kwapisz E, Wierzbowska M. [Incidence of anaerobic bacteria in respiratory tract infections]. Pneumonol Alergol Pol 2003;71:68–73. [PubMed] [Google Scholar]
- 3.Monteiro R, Alfaro TM, Correia L, et al. [Lung abscess and thoracic empyema: retrospective analysis in an internal medicine department]. Acta Med Port 2011;24(Suppl 2):229–40. [PubMed] [Google Scholar]
- 4.Schimmel T, Trawinski H, Karlas T, et al. [Polymicrobial liver abscesses and pleural empyema in a 40-year-old male after tooth extraction and closed periodontal treatment: A case report]. Z Gastroenterol 2019;57:600–5. 10.1055/a-0829-7017 [DOI] [PubMed] [Google Scholar]
- 5.Bekasiak A, Dammann F, Nader C. A rare cause of a scrotal abscess due to the symbiotic infection of Gardnerella vaginalis and Prevotella bivia in an adult male. Pathogens 2020;9:93 10.3390/pathogens9020093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobo F, Calatrava E, Rodríguez-Granger J, et al. A rare case of pleural effusion due to Prevotella dentalis. Anaerobe 2018;54:144–5. 10.1016/j.anaerobe.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 7.Schindel C, Siepmann U, Han S, et al. Persistent Legionella infection in a patient after bone marrow transplantation. J Clin Microbiol 2000;38:4294–5. 10.1128/JCM.38.11.4294-4295.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyara T, Tokashiki K, Shimoji T, et al. Rapidly expanding lung abscess caused by Legionella pneumophila in immunocompromised patients: a report of two cases. Intern Med 2002;41:133–7. 10.2169/internalmedicine.41.133 [DOI] [PubMed] [Google Scholar]
- 9.Jayasimhan D, Wu L, Huggan P. Fusobacterial liver abscess: a case report and review of the literature. BMC Infect Dis 2017;17:440. 10.1186/s12879-017-2548-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaker R, Reslan L, Al-Amin F. Subdural empyema, and superior sagittal sinus thrombosis: intracranial complications of sinusitis caused In: Clinical cases in microbiology and infectious diseases E-Book. 15, 2017: 6. [Google Scholar]
- 11.Jones MG, De Mel S, Cortes NJ, et al. Cough, confusion and flaccid paralysis in a 46-year old man with left apical consolidation and ring-enhancing lesions on cerebral imaging. Thorax 2009;64:862. 10.1136/thx.2009.116293 [DOI] [PubMed] [Google Scholar]
- 12.Blauwkamp TA, Thair S, Rosen MJ, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol 2019;4:663–74. 10.1038/s41564-018-0349-6 [DOI] [PubMed] [Google Scholar]
- 13.Omae T, Matsubayashi T. Lung abscess caused by Mycoplasma pneumoniae. Pediatr Int 2015;57:773–5. 10.1111/ped.12644 [DOI] [PubMed] [Google Scholar]
- 14.Ulger Toprak N, Veloo ACM, Urban E, et al. A multicenter survey of antimicrobial susceptibility of Prevotella species as determined by Etest methodology. Anaerobe 2018;52:9–15. 10.1016/j.anaerobe.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 15.Bancescu G, Didilescu A, Bancescu A, et al. Antibiotic susceptibility of 33 Prevotella strains isolated from Romanian patients with abscesses in head and neck spaces. Anaerobe 2015;35:41–4. 10.1016/j.anaerobe.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 16.Arredondo A, Blanc V, Mor C, et al. Azithromycin and erythromycin susceptibility and macrolide resistance genes in Prevotella from patients with periodontal disease. Oral Dis 2019;25:860–7. 10.1111/odi.13043 [DOI] [PubMed] [Google Scholar]
- 17.Veloo ACM, Baas WH, Haan FJ, et al. Prevalence of antimicrobial resistance genes in Bacteroides spp. and Prevotella spp. Dutch clinical isolates. Clin Microbiol Infect 2019;25:1156.e9–13. 10.1016/j.cmi.2019.02.017 [DOI] [PubMed] [Google Scholar]