Abstract
Introduction
Diabetic nephropathy (DN) is the leading cause of chronic kidney disease worldwide. The Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway participates in the development and progression of DN. Among the different mechanisms involved in JAK/STAT negative regulation, the family of suppressor of cytokine signaling (SOCS) proteins has been proposed as a new target for DN. Our aim was to evaluate the effect of SOCS1 mimetic peptide in a mouse model of obesity and type 2 diabetes (T2D) with progressive DN.
Research design and methods
Six-week-old BTBR (black and tan brachyuric) mice with the ob/ob (obese/obese) leptin-deficiency mutation were treated for 7 weeks with two different doses of active SOCS1 peptide (MiS1 2 and 4 µg/g body weight), using inactive mutant peptide (Mut 4 µg) and vehicle as control groups. At the end of the study, the animals were sacrificed to obtain blood, urine and kidney tissue for further analysis.
Results
Treatment of diabetic mice with active peptide significantly decreased urine albumin to creatinine ratio by up to 50%, reduced renal weight, glomerular and tubulointerstitial damage, and restored podocyte numbers. Kidneys from treated mice exhibited lower inflammatory infiltrate, proinflammatory gene expression and STAT activation. Concomitantly, active peptide administration modulated redox balance markers and reduced lipid peroxidation and cholesterol transporter gene expression in diabetic kidneys.
Conclusion
Targeting SOCS proteins by mimetic peptides to control JAK/STAT signaling pathway ameliorates albuminuria, morphological renal lesions, inflammation, oxidative stress and lipotoxicity, and could be a therapeutic approach to T2D kidney disease.
Keywords: albuminuria, inflammation and oxidative stress, type 2 diabetes, lipotoxicity
Significance of this study.
What is already known about this subject?
Overactivation of Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway plays a role in chronic complications of diabetes.
Therapeutic benefit from the negative JAK/STAT regulators suppressors of cytokine signaling (SOCS) has already been established in preclinical models of type 1 diabetes.
What are the new findings?
In the BTBR (black and tan brachyuric) ob/ob (obese/obese) mouse model of type 2 diabetes (T2D) and obesity that recapitulates renal lesions observed in humans, administration of a cell-permeable peptide mimicking SOCS1 significantly improves albuminuria (>50% reduction on average) and reduces morphological kidney lesions.
This beneficial effect is mediated by changes in STAT activation, inflammatory gene expression, redox balance and lipotoxicity.
How might these results change the focus of research or clinical practice?
Currently, strict metabolic control is the main renoprotective action in the progression of diabetic nephropathy.
Our study proposes that targeting SOCS proteins by mimetic peptides to control JAK/STAT signaling pathway ameliorates albuminuria, morphological renal lesions, inflammation, oxidative stress and lipotoxicity, and could be a therapeutic approach to T2D kidney disease, independent of glycemic management.
Introduction
Among the tissue-specific manifestations of type 2 diabetes (T2D), diabetic nephropathy (DN) is a global public health problem, being the main cause of end-stage renal disease, with rising economic and social costs.1 Although currently available treatments slow the evolution of the disease, it is necessary to establish new therapeutic strategies in different stages of the disease in order to promote renoprotection and delay renal replacement therapies.2 3
Although genome association studies have strongly associated an inherited burden on the development of the disease,4–6 the progression of diabetic kidney disease is mainly due to the presence of a renal inflammatory milieu and oxidative stress due to the metabolic and hemodynamic effects of hyperglycemia and hypertension that activate signaling pathways responsible for the remodeling of renal architecture.7 8 Several studies have implicated the Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway in chronic complications of diabetes.9–12 The JAK/STAT pathway is negatively regulated by the suppressors of cytokine signaling (SOCS) proteins. In this regard, the delivery of SOCS1 gene or peptide therapies in mice with type 1 diabetes (T1D) did mitigate the clinical and pathological features of DN, contributing significantly to the modulation of kidney inflammatory and oxidative stress.13 14 These early experimental data, along with recent clinical studies, support the hypothesis that interfering the JAK/STAT pathway by either SOCS proteins or small-molecule inhibitors of JAK (JAKinibs) could be clinically relevant for therapeutic intervention in chronic inflammatory diseases, including diabetic complications.15 16
The use of transcriptional inhibitory peptides has gained great notoriety as a therapeutic tool, mainly due to their high specificity, good tolerance and safety.17 For this reason, the present work proposes a cell-permeable peptide mimicking SOCS1 (MiS1) as potential therapy for DN in a mouse model of T2D and obesity with great translational power to humans, as is the black and tan brachyuric (BTBR) obese/obese (ob/ob) mouse.18 19 Due in large part to the fact that metabolic, hemodynamic and inflammatory changes occur prematurely in this murine model of T2D and obesity, the efficacy of the MiS1 peptide in modulating the inflammatory and oxidative microenvironment of renal damage was also evaluated.
Research design and methods
Ethical considerations
For this study, male BTBR ob/ob diabetic mice (BTBR.Cg-Lepob/WiscJ; RRID:IMSR_JAX:004824, RRID:IMSR_JAX:004824) and BTBR wild type (WT) non-diabetic controls were used. Breeding pairs BTBR heterozygotes (BTBR ob+/−) were purchased from Jackson Laboratories (Bar Harbor, Maine) and housed at a density of four animals per cage in a temperature-controlled room (20°C–22°C) with 12-hour light–dark cycles. Standard food and water were available ad libitum.
For the realization of animal sacrifice, the mice were anesthetized with 2% 2,2,2-tribromoethanol (Sigma-Aldrich) dissolved in 2-methyl-2-butanol (Sigma-Aldrich). After the corresponding anesthetic evaluation, a blood sample was taken for serum collection and both kidneys were removed and decapsulated, and a sagittal section was made in the perihilar area in order to obtain two halves of each kidney. Half of each kidney (right and left) was fixed in 4% formaldehyde. A small portion of the renal cortex was embedded in 2% glutaraldehyde for the study by transmission electron microscopy. The remaining portion was stored immediately in liquid nitrogen and processed for RNA extraction.
Characterization of kidney early changes in BTBR ob/ob model
BTBR ob/ob and BTBR WT mice were sacrificed every 2 weeks, starting from week 4 through week 12 of age (n=5–6 mice/group). The measurement of glycemia and body weight was made every week using a glucometer Accu-Chek Performa (Roche) and digital balance, respectively. Serum and urine creatinine levels were measured by Jaffé reaction (LiquiColor, HUMAN Diagnostics, Germany). Urine spot samples were collected once a week and albuminuria analyzed by ELISA (Mouse Albumin, ALPCO, USA).
Peptidomimetic SOCS1 (MiS1) synthesis and treatment
Palmitoylated peptides derived from mouse SOCS1 kinase inhibitory region sequence residues 53–68 and mutant inactive (F→A) were synthesized by Proteogenix (Schiltigheim, France), then dissolved in 100% dimethyl sulfoxide (DMSO) in saline solution (NaCl 0.9%). Male BTBR ob/ob mice at 6 weeks of age (hyperglycemia onset) were randomized to receive three intraperitoneal injections per week of (1) active peptidomimetic SOCS1 (MiS1 group) at two different doses of 2 µg/g and 4 µg/g (n=7 for each group); (2) inactive mutated peptidomimetic SOCS1 (Mut group) at a dose of 4 µg/g (n=7); and (3) vehicle (Veh group) at a dose of DMSO <0.2% in saline solution (n=6). After 7 weeks of intervention, all groups were analyzed and euthanized.
Histological analysis and immunohistochemistry
The kidneys were fixed in 4% formaldehyde, embedded in paraffin and cut in 4 µm tissue sections for histochemical stain (periodic acid Schiff/Masson’s trichrome) and immunohistochemistry. The glomerular and tubulointerstitial lesions were classified according to a semiquantitative histopathological score damage, giving a score of 0–4 as previously described.20 The primary antibodies for immunodetection were sourced as follows: phosphorylated (p-) STAT3 serine 727 (Cell Signaling Technology Cat# 9134, RRID:AB_331589, dilution 1:100), p-STAT1 tyrosine 701 (Cell Signaling Technology Cat# 7649, RRID:AB_10950970, dilution 1:50), p-p65 subunit of nuclear factor-κB (NF-κB) serine 536 (Santa Cruz Biotechnology Cat# sc-33020, RRID:AB_2179018, dilution 1:100), p-nuclear factor erythroid 2-related factor 2 (NRF2) serine 40 (Abcam Cat# ab76026, RRID:AB_1524049, dilution 1:2000), SOCS1 (Abcam Cat# ab62584, RRID:AB_956316, dilution 1:1000), SOCS3 (Abcam Cat# ab16030, RRID:AB_443287, dilution 1:200), F4/80 monocytes/macrophages (Bio-Rad Cat# MCA497, RRID:AB_2098196, dilution 1:70), CD3 T lymphocytes (Agilent Cat# M7254, RRID:AB_2631163, dilution 1:100), Wilms tumor protein-1 (WT-1; Agilent Cat# M3561, RRID:AB_2304486, dilution 1:100), perilipin-1 (sc-390169, dilution 1:50, Santa Cruz Biotechnology, USA) and 4-hydroxy-2-nonenal (4-HNE; Abcam Cat# ab46545, RRID:AB_722490, dilution 1:200). All primary antibodies were assessed by indirect immunoperoxidase, except for WT-1 and p-NRF2, which were incubated with the M.O.M. Immunodetection Kit (Vector Laboratories Cat# BMK-2202, RRID:AB_2336833) and Vectastain Elite ABC HRP Kit RTU (Vector Laboratories Cat# PK-7100, RRID:AB_2336827), respectively. Sections were revealed with ImmPACT DAB Peroxidase Substrate (Vector Laboratories Cat# SK-4105, RRID:AB_2336520) and counterstained with Carazzi’s hematoxylin for later evaluation by optical microscopy. Intracellular superoxide anion in paraffin renal sections was visualized using the oxidation-sensitive fluorescent probe dihydroethidium (DHE; 2 μmol/L; Life Technologies, USA) followed by DAPI (4′,6-diamidino-2-phenylindole) nuclear counterstain. The samples were visualized by fluorescence microscopy (λEXC=488 nm and λEM=585 nm), mounted in aqueous medium (FluorSave Reagent, Millipore) and examined by a Leica TCS SP5 confocal microscope. Positive staining was quantified using Image-Pro Plus software and expressed as percentage of the total area and number of positive cells (per glomerular cross section or tubular field).
For analysis by electron microscopy, the kidney tissue was fixed in 2% glutaraldehyde (Merck, Germany), postfixed with 1% osmium tetroxide (Ted Pella, USA) and observed under a Philips Tecnai 12 electron microscope (Philips Eindhoven, The Netherlands) operated at 80 kV.
mRNA expression
Total RNA from renal tissue was isolated with TRIzol reagent (Ambion, USA). Complementary DNA (cDNA) was synthesized by a High Capacity cDNA Archive Kit (Applied Biosystems) using 2 µg total RNA primed with random primers. For the analysis of gene expression, commercial primers were used from the Applied Biosystems database and non-commercial probes designed through Primer-BLAST software and synthesized by Thermo Fisher Scientific (online supplementary table 1). Quantitative gene expression analysis was performed by real-time PCR 7500 Applied Biosystems, using 7500 System SDS software V.1.2b1c3. The expression of target genes was analyzed in duplicate and normalized to housekeeping 18s transcripts.
bmjdrc-2020-001242supp001.pdf (3.6MB, pdf)
Statistical analysis
The data are presented as scatter dot plots with mean±SD of the total number of animals. Graphs and corresponding statistical tests were carried out with the GraphPad Prism V.6 software. Statistical analyses were performed using non-parametric Mann-Whitney U test for comparison between two groups and one-way analysis of variance with Bonferroni post-hoc for multiple comparisons, considering differences to be statistically significant at p<0.05.
Results
Treatment with MiS1 significantly reduces albuminuria and kidney lesions in the BTBR ob/ob mouse model
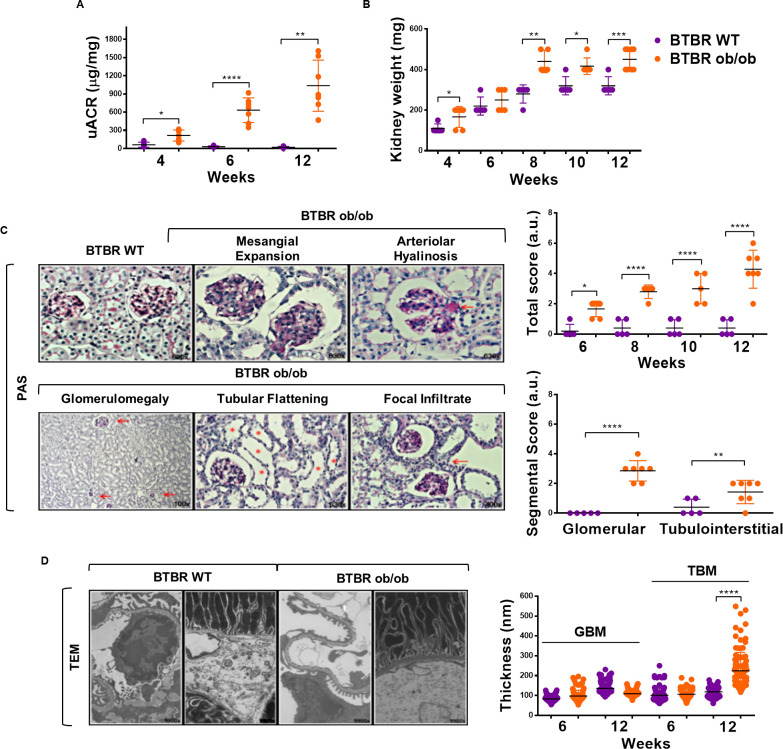
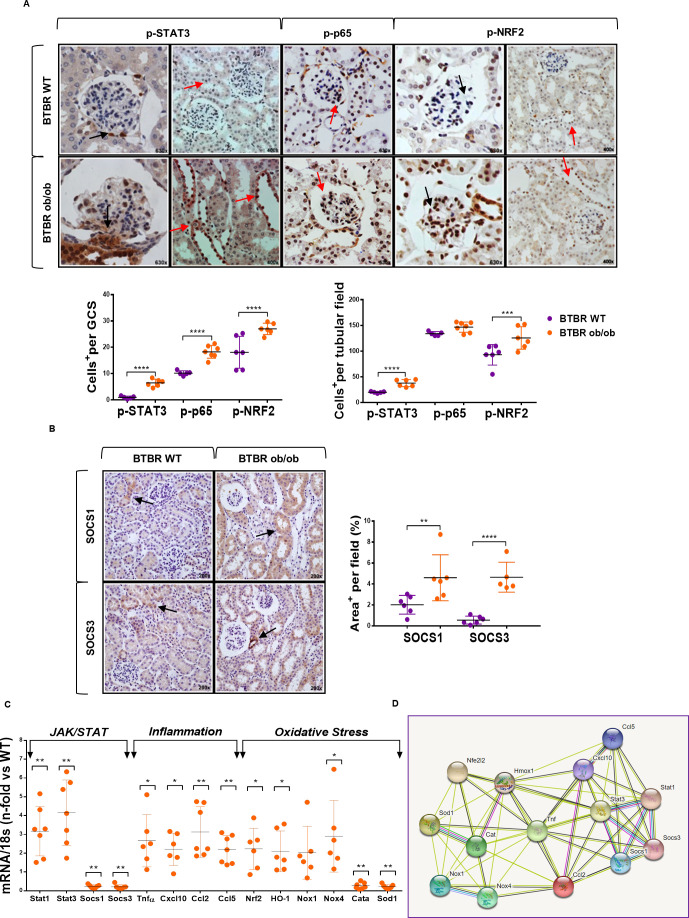
Our characterization of BTBR ob/ob mice showed early and progressive development of obesity, hyperglycemia, renomegalia, albuminuria, podocytopenia, inflammatory infiltrate and histopathological changes of renal damage (figure 1A–D, online supplementary figure 1 and online supplementary table 2), which is in line with previous studies.18 21 Interestingly, and compared with BTBR WT mice, kidneys from BTBR ob/ob mice showed a marked overactivation (phosphorylation) of three key transcription factors involved in inflammation and oxidative stress, namely p-STAT3, p-p65 NF-κB and p-NRF2 (figure 2A). Furthermore, SOCS1 and SOCS3 protein expression was substantially increased in tubular cells of diabetic BTBR ob/ob mice, with a similar pattern as p-STAT3 and p-NRF2 staining (figure 2B).
Figure 1.
Kidney damage markers in BTBR ob/ob model. BTBR ob/ob diabetic mice (orange dots) and their respective control, BTBR WT non-diabetic mice (purple dots), were studied from 4 to 12 weeks old. (A) Progression of urinary albumin to creatinine ratio (UACR) from 6 weeks old. (B) Progression of kidney weight in BTBR WT and BTBR ob/ob mice at 12 weeks. (C) Evolution of kidney damage in BTBR ob/ob was measured starting from week 6 to week 12 by histopathological kidney score. Shown are representative images of histopathological features observed in kidney tissue sections with periodic acid Schiff (PAS) staining in 12-week-old mice. Magnification ×100, ×400 and ×630. (D) Representative images of thickening of the glomerular and tubular basal membrane by transmission electron microscopy (TEM) and quantified by 100 measurements in each representative animal of renal damage, BTBR WT and BTBR ob/ob at 6 and 12 weeks old. Magnification ×9900. Data are shown as scatter dot plots and mean±SD of each group (n=5–7 mice/group); *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 versus BTBR WT control. a.u., arbitrary units; BTBR, black and tan brachyuric; GBM, glomerular basal membrane; ob/ob, obese/obese; TBM, tubular basal membrane; WT, wild type.
Figure 2.
JAK/STAT, inflammatory and oxidative stress pathways in kidney tissue of BTBR ob/ob model. (A) Representative images of phosphorylated (p-)STAT3, p-p65 NF-κB and p-NRF2, and quantification of positive cells in glomerular and tubular fields of BTBR WT and ob/ob mice. Magnification ×400 and ×630. Arrows indicate positive stained cells. (B) Representative images of SOCS1/SOCS3 proteins and quantification of positive stained area per tubular field. Magnification ×200. (C) Real-time PCR analysis of JAK/STAT, inflammatory and oxidative stress genes. Values normalized by endogenous control gene 18s are expressed as n-fold of the average value from BTBR WT. Data are shown as scatter dot plots and mean±SD of each group (n=5–7 mice/group); *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 versus BTBR WT control. (D) Schematic protein–protein interaction prediction of JAK/STAT, inflammatory and oxidative stress markers in Mus musculus according to STRING software. More information can be found at https://string-db.org/. a.u., arbitrary units; BTBR, black and tan brachyuric; GCS, glomerular cross section; JAK/STAT, Janus kinase/signal transducers and activators of transcription; NF-κB, nuclear factor-κB; NRF2, nuclear factor erythroid 2-related factor 2; ob/ob, obese/obese; SOCS, suppressor of cytokine signaling; WT, wild type.
Real-time PCR analysis of genes associated with JAK/STAT, inflammatory and redox balance pathways revealed significant increases in STAT members (Stat1 and Stat3), cytokines (Tnfα), chemokines (Cxcl10, Ccl2 and Ccl5) and oxidative stress-activated molecules (Nrf2, HO-1, Nox1 and Nox4), and downregulation of SOCS genes (Socs1 and Socs3) and antioxidant enzymes (Catalase and Sod1) in BTBR ob/ob mice (figure 2C). Schematic protein–protein interaction prediction of these markers in Mus musculus was performed with STRING software (figure 2D).
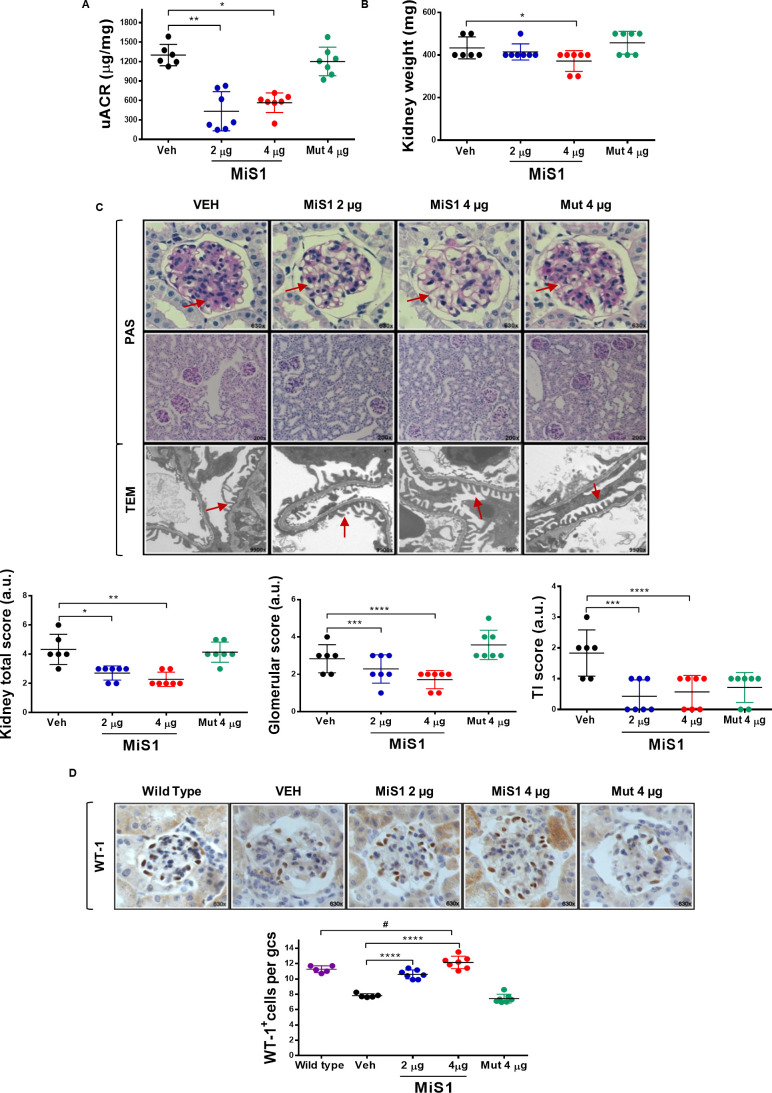
After establishing that the expression/activation profile of JAK/STAT/SOCS axis is altered in BTBR ob/ob mice, we further explored the therapeutic potential of its targeting in the context of T2D. Administration of MiS1 peptide to BTBR ob/ob mice significantly decreased albuminuria by 57%–67% (figure 3A) and kidney weight (figure 3B) relative to vehicle control group. Histological and ultrastructural analyses of diabetic kidneys revealed that MiS1 treatment ameliorated glomerular and tubulointerstitial lesions, including glomerulomegaly, mesangial expansion, arteriolar hyalinosis, focal inflammatory infiltrate and tubular flattening, and also prevented pedicelar effacement (figure 3C). Additionally, total podocyte count was significantly increased following MiS1 administration, reaching values similar to those of non-diabetic mice (figure 3D), as assessed by WT-1 immunostaining. These functional and structural modifications at the renal level were not associated with changes in metabolic and biochemical parameters such as glycemia, body weight and serum creatinine (online supplementary table 2).
Figure 3.
MiS1 treatment reduces kidney damage markers in BTBR ob/ob model. Graphs and images represent the changes observed in diabetic mice treated with active MiS1 (2 µg and 4 µg) and inactive mutated peptide (Mut 4 µg) compared with vehicle controls (Veh). (A) Urinary albumin creatinine ratio (UACR). (B) Kidney weight. (C) Representative images of light microscopy of glomerular and tubular fields stained with PAS and the quantification of histopathological total score. Magnification ×200 and ×630. Additionally, TEM of glomerular filtration barrier was observed. Magnification ×9900. Arrows indicate areas of mesangial expansion (PAS images) and pedicelar effacement (TEM images). (D) Immunohistochemistry against WT-1 protein, used as podocyte marker. Graphs represent the average number of WT-1+ cells per glomerular cross section (GCS) in BTBR WT and ob/ob (vehicle and treatments). Magnification ×630. Data are shown as scatter dot plots and mean±SD of each group (n=5–7 mice/group); #p<0.05 versus BTBR WT; *p<0.05, **p<0.01, ****p<0.0001 versus diabetic vehicle control. a.u., arbitrary units; BTBR, black and tan brachyuric; ob/ob, obese/obese; PAS, periodic acid Schiff; TI, tubulointerstitial; TEM, transmission electron microscopy; WT-1, Wilms tumor protein-1.
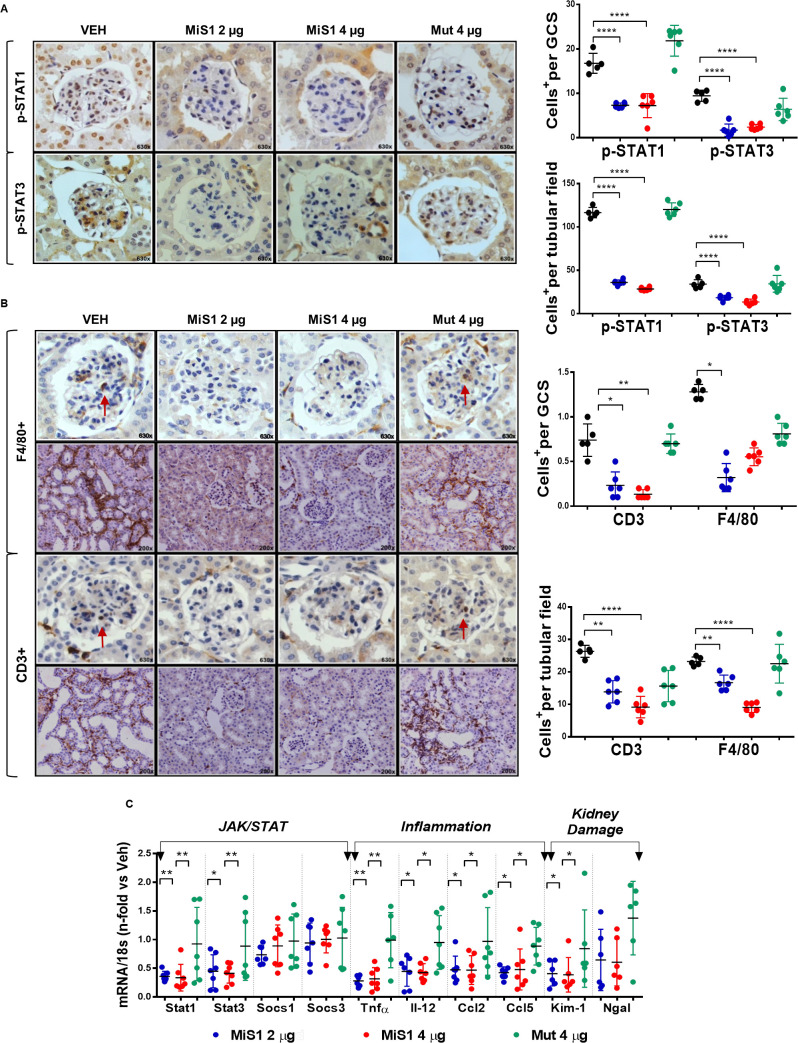
MiS1 therapy inhibits JAK/STAT pathway and reduces markers of inflammation, oxidative stress and kidney damage in diabetic mice
Administration of MiS1 peptide caused a potent inhibition of the nuclear translocation of transcription factors p-STAT1 and p-STAT3, at both glomerular and tubulointerstitial levels (figure 4A), which is in agreement with our previous studies in T1D models.13 14 In addition, a reduction in gene expression of both transcription factors was observed, explaining the marked downregulation of STAT activity (figure 4C).
Figure 4.
MiS1 treatment inhibits kidney JAK/STAT activation and renal microinflammatory milieu in the BTBR ob/ob model. (A) Graphs and images represent the changes observed in JAK/STAT activation (p-STATs) in diabetic mice treated with active MiS1 (2 µg and 4 µg) and inactive mutated peptide (Mut 4 µg) compared with vehicle controls (Veh), quantified per number of positive cells p-STAT1+ and p-STAT3+, both at the glomerular and tubular fields. Magnification ×630. (B) Representative images of immunohistochemistry against F4/80 and CD3. Magnification ×200 and ×630. Graphs represent the quantification of average number of monocytes/macrophages F4/80+ and CD3+ T lymphocytes, both at the glomerular and interstitial fields. Arrows indicate positively stained cells. (C) Gene expression analysis of mRNA related with JAK/STAT pathway (Stat1, Stat3, Socs1 and Socs3), inflammatory cytokines (Tnfα and Il-12) and chemokines (Mcp-1 and Rantes), and kidney damage markers (Kim-1 and Ngal) were evaluated by real-time PCR, being normalized in each sample by endogenous control gene 18s and expressed as n-fold the average value obtained in the vehicle group (Veh). Data are shown as scatter dot plots and mean±SD of each group (n=6–7 mice/group); *p<0.05, **p<0.01, ****p<0.0001 versus diabetic vehicle control. BTBR, black and tan brachyuric; GCS, glomerular cross section; JAK/STAT, Janus kinase/signal transducers and activators of transcription; ob/ob, obese/obese.
In order to study the cellular and molecular mechanisms underlying the beneficial therapeutic effects of MiS1 on albuminuria and renal lesions, we focused mainly on inflammation and oxidative stress, two phenomena closely linked to chronic hyperglycemia. Immunohistochemical analysis of renal infiltrating cells showed that treatment with MiS1 peptide at both doses reduced the number of F4/80+ monocytes/macrophages and CD3+ T lymphocytes in both glomerular and tubulointerstitial compartments (figure 4B). Furthermore, kidneys from MiS1-treated mice exhibited a decrease in the gene expression of inflammatory cytokines (Tnfα, Il-12), chemokines (Ccl2, Ccl5) and renal damage markers (Kim-1, Ngal) (figure 4C). These data suggest that the reduction of early renal damage observed in this model is due, at least in part, to the reduction of inflammation.
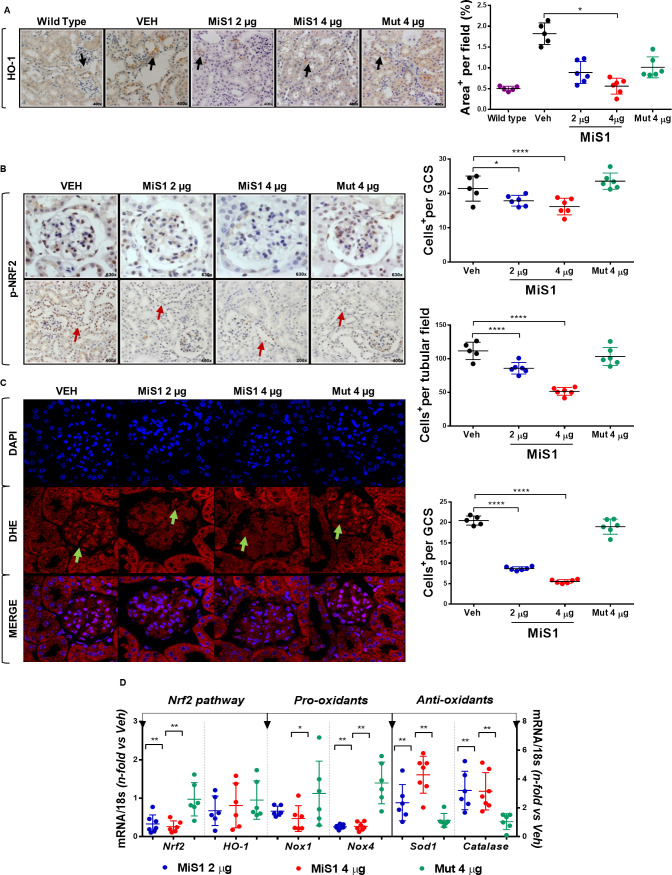
We next examined changes in NRF2 pathway, an essential endogenous antioxidant mechanism activated in response to stress signals, including oxidative damage in DN.22 23 Remarkably, MiS1 treatment caused a dose-dependent reduction of NRF2 and its target gene heme-oxygenase-1 (HO-1) in diabetic kidneys, as assessed by immunohistochemistry (figure 5A, B) and mRNA expression (figure 5D). Concomitantly, a reduction of genes encoding pro-oxidant enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox1 and Nox4 subunits) and increased antioxidant enzymes superoxide dismutase-1 (Sod1) and catalase were also observed in MiS1-treated mice (figure 5D). Confocal microscopy with DHE fluorogenic probe further confirmed a lower production of superoxide anion in MiS1-treated mice compared with control mice, particularly at the glomerular level (figure 5C). These results suggest that regulation of redox-sensitive signaling pathways could be responsible for the clinical and histopathological improvement by MiS1 therapy in this preclinical T2D model.
Figure 5.
MiS1 treatment modulates NRF2 activation, superoxide anion production and gene expression of redox balance markers in BTBR ob/ob model. (A) Graphs and images represent the changes observed in immunohistochemistry against heme-oxygenase-1 in BTBR WT, vehicle and each of the groups treated (2 µg, 4 µg and Mut 4 µg), quantified per analysis of percentage of the positive staining area per tubular field. Magnification ×400. (B) Graphs and images represent the changes observed in NRF2 activation (p-NRF2) in diabetic mice treated with active MiS1 (2 µg and 4 µg) and inactive mutated peptide (Mut 4 µg) compared with vehicle controls (Veh), quantified per number of positive cells p-NRF2+, both at the glomerular and tubular fields. Magnification ×200–×630. Arrows indicate positive staining. (C) Representative fluorescence images of superoxide anion (DHE, red), cell nuclei (DAPI, blue) and merge. Arrows show positive staining of superoxide anion at the glomerular level. Graph shows DHE-positive cells per glomerular field. Magnification ×630. (D) Gene expression analysis of mRNA related with Nrf2 pathway (Nrf2, HO-1), pro-oxidants enzymes (Nox1, Nox4) and antioxidants enzymes (Sod1, Catalase) was evaluated by real-time PCR. Values normalized by endogenous control gene 18s are expressed as n-fold of the average value obtained in the vehicle group (Veh). Data are shown as scatter dot plots and mean±SD of each group (n=6–7 mice/group); *p<0.05, **p<0.01, ****p<0.0001 versus diabetic vehicle control. BTBR, black and tan brachyuric; DAPI, 4′,6-diamidino-2-phenylindole; DHE, dihydroethidium; GCS, glomerular cross section; Mut 4 μg, inactive mutated peptide; NRF2, nuclear factor erythroid 2-related factor 2; ob/ob, obese/obese; WT, wild type.
MiS1 reduces tubular and vascular lipid peroxidation in kidneys of BTBR ob/ob mice
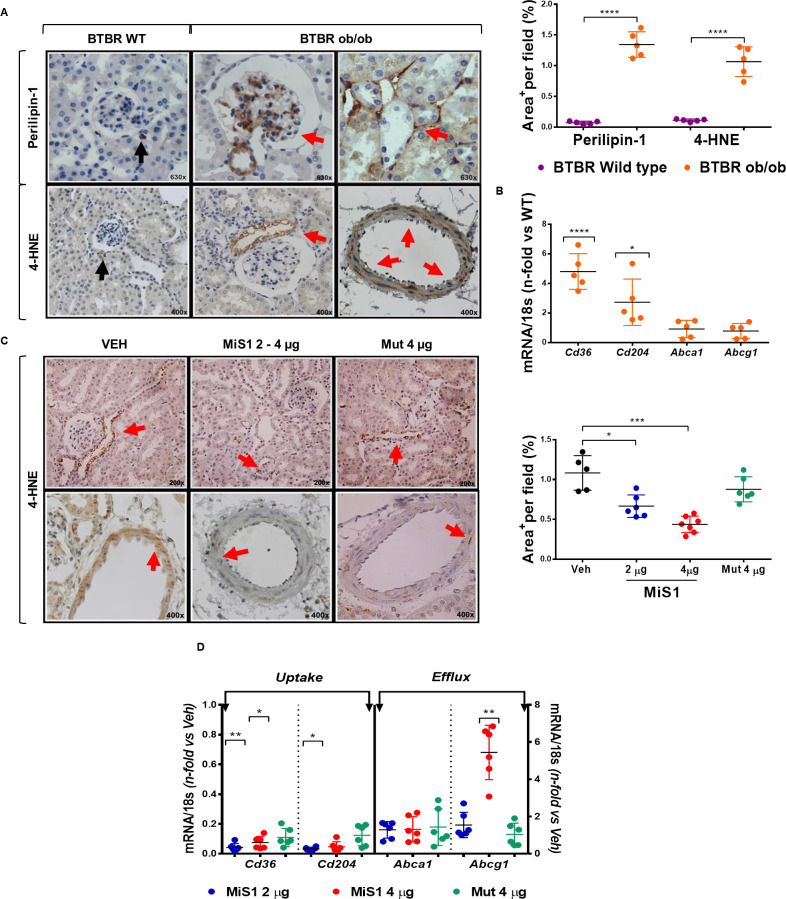
Recent evidence indicates that lipotoxicity is a mechanism of kidney damage in the context of obesity, with cytoplasmic accumulation of fatty acids being one of the most relevant findings.24 25 In line with this, a large amount of visceral fatty tissue was observed in BTBR ob/ob mice (online supplementary figure 2). At kidney level, positive staining for perilipin-1, a marker of mature lipid droplets, was observed in the mesangium, tubulointerstitium and infiltrated cells (figure 6A). The lipid peroxidation marker 4-HNE was also abundantly detected in the periglomerular tubular cells and in the middle layer of the arterial vessels (figure 6A). Furthermore, real-time PCR showed overexpression of scavenger receptors associated with the uptake of fatty acids (Cd36 and Cd204), but no significant changes in cholesterol efflux genes (ATP bind cassette transporter (ABC) A1 and G1) (figure 6B). Notably, and compared with diabetic control mice, MiS1-treated animals depicted a significant reduction in lipid peroxidation at both tubular and vascular cells (figure 6C). Moreover, MiS1 peptide downregulated the renal gene expression of scavenger receptors (Cd36, Cd204) and upregulated the reverse cholesterol transporter Abcg1 without changes in Abca1 transporter (figure 6D). These findings uncover the antilipotoxic effects of the JAK/STAT inhibition in diabetic kidneys, therefore adding another potential beneficial effect of SOCS mimetic peptide.
Figure 6.
MiS1 treatment reduces tubular and vascular lipid peroxidation and modulates gene expression of scavenger receptors in BTBR ob/ob model. (A) Presence of intrarenal lipids was evidenced by immunohistochemistry against perilipin-1 and 4-HNE in BTBR WT (purple dots) and BTBR ob/ob (orange dots) mice. Isolated specific perilipin-1 and 4-HNE positive cells were observed in non-diabetic BTBR WT mice (black arrow). Mesangial, tubular and vascular staining was observed in diabetic BTBR ob/ob mice (red arrow). Magnification ×400–×630. Quantitative analysis of percentage of the positive staining area per tubular field was determined in 12-week-old non-diabetic control and diabetic mice. (B) Gene expression analyses of scavenger receptors associated with fatty acid uptake (SR-B/Cd36, SR-A/Cd204) and reverse cholesterol transport (Abca1, Abcg1) were evaluated by real-time PCR. Values normalized by endogenous control gene 18s are expressed as n-fold of the average value obtained in the BTBR WT. (C) Images represent the changes observed in immunohistochemistry against 4-HNE+ tubular and vascular area in vehicle and each of the groups treated (2 µg, 4 µg and Mut 4 µg), quantified per analysis of percentage of the positive staining area per tubular field. Magnification ×200 and ×400. (D) Gene expression analysis of indicated genes was evaluated by real-time PCR, and normalized values expressed as fold increase versus vehicle group (Veh). Data are shown as scatter dot plots and mean±SD of each group (n=5–7 mice/group); *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 versus BTBR WT or diabetic vehicle control. BTBR, black and tan brachyuric; 4-HNE, 4-hydroxy-2-nonenal; Mut 4 μg, inactive mutated peptide; ob/ob, obese/obese; WT, wild type.
Discussion
Our study demonstrates that targeting JAK/STAT/SOCS axis exerts a marked beneficial effect on albuminuria and renal lesions in experimental T2D. The BTBR ob/ob mouse model was chosen for its recapitulation of clinical and morphological renal lesions in patients with T2D.18 26 Indeed, our preclinical evaluation of MiS1, a cell-permeable peptide mimicking SOCS1, demonstrates a potent inhibition of renal inflammation, oxidative stress and lipotoxicity, underlying the mechanisms of its renoprotective actions in T2D. The reduction of proteinuria observed after MiS1 treatment could be attributed to its pleiotropic effect in improving the glomerular filtration barrier through several mechanisms: (1) modulation of the local JAK/STAT pathway; (2) reduction of inflammatory and oxidative state in resident and infiltrating kidney cells; (3) increase in podocyte number; and (4) reduction of kidney lipotoxicity (online supplemental figure 3).
Following the detailed characterization of renal lesions in the BTBR ob/ob mouse model of T2D by Alpers group,18 as well as the potential reversibility of glomerular damage after leptin administration, the BTBR ob/ob model has been widely recognized as an excellent preclinical model to evaluate novel therapies in the progression of DN.19 26–28 Moreover, treatment with therapeutic peptides to modify key transcriptional regulatory proteins involved in organ injury continues to be increasingly relevant, mainly due to its high specificity and safety, with reduced adverse or undesirable effects compared with the development of pharmacological active compounds.17 29 A good example about the therapeutic utility of mimetic peptides in the field of diabetes is the subcutaneous glucagon-like peptide-1 receptor agonists largely used in clinical practice to treat patients with diabetes with and without renal disease.30 31
The participation of JAK/STAT signaling in the development and progression of DN has been validated as one of the main ways of eliciting the production of cytokines, chemokines, interferons, transcription and growth factors by kidney cells, and therefore responsible for maintaining the local proinflammatory state in the diabetic kidney.32–34 Transcriptomic analysis in human DN revealed a direct relationship between tubulointerstitial JAK1, JAK2 and JAK3 (mainly JAK2), STAT1-3 gene expression, and the progression of kidney failure.35 Our findings suggest an upregulation and compartmental activation of STAT1/3 in BTBR ob/ob diabetic kidneys, and a compensatory increase of SOCS1/3 proteins, which is in line with our previous findings in kidney biopsies of patients with T2D and experimental models.13 However, gene expression analysis showed downregulated expression of SOCS genes. This discrepancy between gene and protein expression could be attributed to several factors such as RNA binding proteins and microRNAs targeting SOCS mRNA, or post-translational modifications (eg, phosphorylation by Pim kinases) increasing the stability of SOCS protein.36
Recently, an experimental study conducted in podocyte JAK2-overexpressing Akita mice with angiotensin II infusion demonstrated that the JAK1/2 inhibitors, tyrphostin and baricitinib, reduced proteinuria and glomerular kidney damage.37 In addition, a phase II trial on baricitinib has shown promise for high-risk patients with diabetic kidney disease.38 Our early studies have shown effective reduction of renal damage when using different SOCS delivery systems (adenovirus and cell-permeable peptide) in animal models of kidney disease, including T1D.13 14 So far, this is the first study in the context of T2D and obesity for successful improvement of proteinuria (>50% reduction on average) and renal damage by SOCS1 mimetics. Carefully designed clinical studies will determine the viability of such strategy in humans.
Podocytopenia and pedicelar effacement are classically observed findings in DN.39 The reversibility of podocyte damage observed in the BTBR ob/ob model may be mainly due to the restoration of the intraglomerular inflammatory and oxidative microenvironment, mobilization of renal progenitor cells (CD133+ CD24+ cells) and JAK/STAT pathway inhibition.26 40 41
Our previous studies have established the pivotal role of JAK/STAT in regulating the inflammatory and oxidative microenvironment in experimental models of atherosclerosis and T1D kidney disease.42 43 The present data highlight the anti-inflammatory and antioxidant effect in the MiS1 peptide in T2D diabetic kidneys, as evidenced by the reduction of infiltrating cells (T lymphocytes and macrophages), cytokine/chemokine expression, and superoxide anion levels. In vitro, SOCS1 has been reported to reduce cell migration and proliferation, and to modulate the functional polarization of kidney macrophages from a proinflammatory state (M1) to an anti-inflammatory phenotype (M2).13 14 Recently, our group demonstrated a reduction in the activation of STAT3 with a selective interleukin-17A (IL17A) antibody in BTBR ob/ob mouse model,21 potentiating possible additional effects of the JAK/STAT pathway inhibition in a subset of T cells, as it has been described in other inflammatory-based diseases.44 This result is reminiscent of that noted in a mouse model of encephalomyelitis, in which the treatment with an SOCS1-derived peptide suppressed IL17A production, prevented infiltration of lymphocytes into the brain, and reversed the ongoing pathology.45
In the BTBR ob/ob mouse, treatment with MiS1 peptide generates a reduction of the NRF2 phosphorylation at both glomerular and tubulointerstitial level. In turn, a modulation of the redox balance was also modified, with a reduction in superoxide anion and NADPH oxidase (Nox1/Nox4), as well as an increase in the antioxidant enzymes Sod1 and catalase. Although the antioxidant role of the NRF2/HO-1 pathway is significantly described, growing evidence demonstrates possible new effects of NRF2 signaling. Under physiological conditions NRF2 prevents oxidative damage; however, the overactivation or maintenance of constitutive activity of NRF2 could enhance the mechanisms of damage progression, as it has been observed in preclinical models and human renal cell carcinoma.46–48 Therefore, it is necessary to continue studying the role of NRF2 in the progression of the DN.
The cytoplasmic accumulation of fatty acids in ectopic tissues such as the muscle, heart, liver and kidney has been described as part of physiological processes such as intracellular signaling, vesicular transport, energy metabolism and structural functions.49 50 However, renal lipotoxicity is described as a toxic and dysfunctional finding by the generation of reactive oxygen/nitrogen species, mitochondrial dysfunction, alterations in intracellular signaling pathways, release of proinflammatory and profibrotic factors and lipid-mediated apoptosis (lipoapoptosis).51 Although the molecular mechanisms of lipotoxicity in DN remain unclear, the presence of lipids at glomerular and tubulointerstitial levels has been described as a factor promoting kidney damage.52 53 Conversely, the reduction of renal lipotoxicity could potentiate the protective effects in DN. In the present work, treatment with MiS1 caused a reduction of lipid peroxidation and also altered the gene expression of scavenger receptors associated with uptake of fatty acids (Cd36 and Cd204) and efflux of cholesterol (Abcg1). Therefore, modulation of JAK/STAT activity by SOCS-derived peptide provides a new mechanism of action to improve lipotoxicity and lipid metabolism dysregulation in diabetic kidney damage associated with T2D and obesity.
In conclusion, in an experimental mouse model that recapitulates the lesions observed in patients with T2D patients with DN, the treatment with MiS1 peptide markedly reduces albuminuria, morphological renal lesions, inflammation, oxidative stress and kidney lipotoxicity. Targeting SOCS proteins to control JAK/STAT signaling pathway could be a therapeutic approach to DN in humans.
Acknowledgments
We thank Maria Eugenia Burgos and Graciela Valderrama (Division of Nephrology, Universidad Austral de Chile) for their professional support in the development of this research.
Footnotes
JE, CG-G and SM contributed equally.
Contributors: LO-R contributed to the conception, design and performance of the experiments, acquisition, analysis and interpretation of all data, and drafting of the manuscript. YSM contributed to performance of the experiments and acquisition and analysis of data. RRR-D contributed to performance of the experiments and acquisition of data. DC contributed to analysis and interpretation of data. AD contributed to design and performance of the experiments. JE contributed to interpretation of data, drafting and critical review of the manuscript, and in securing financial support for the study. CG-G contributed to conception, design and performance of the experiments, analysis and interpretation of data, drafting of the manuscript, and in securing financial support for the study. SM contributed to interpretation of data, critical review of the manuscript and in securing financial support for the study. All authors reviewed the manuscript and approved the final version to be published. SM is the guarantor of this work and as such had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by grants Fondecyt Project N° 1160465 (to SM) and PhD Grant N° 21150768 (to LO-R), Division of Nephrology, Universidad Austral de Chile, the Spanish Ministry of Economy and Competitiveness (MINECO/FEDER; SAF2015-63696-R, to CG-G), the Ministry of Science, Innovation and Universities (MICINN/FEDER; RTI2018-098788-B-I00, to CG-G), and Instituto de Salud Carlos III (FIS/FEDER; PI17/01495, DTS-2017/00203 and DTS19/00093, to JE).
Competing interests: JE and CG-G are inventors on a patent application regarding clinical utility of SOCS peptide.
Patient consent for publication: Not required.
Ethics approval: The experimental protocol was reviewed and approved by the Ethics Committee for Animal Experiments of the University Austral of Chile (Permit N° 222–2015) according to the National Institutes of Health guidelines.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. The data sets and resources generated during the current study are available from the corresponding author upon reasonable request.
References
- 1.Yang W, Dall TM, Beronjia K, et al. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–28. 10.2337/dci18-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gnudi L, Coward RJM, Long DA. Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol Metab 2016;27:820–30. 10.1016/j.tem.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 3.MacIsaac RJ, Jerums G, Ekinci EI. Effects of glycaemic management on diabetic kidney disease. World J Diabetes 2017;8:172. 10.4239/wjd.v8.i5.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y-C, Chang EY-C, Chuang L-M. Recent progress in the genetics of diabetic microvascular complications. World J Diabetes 2015;6:715. 10.4239/wjd.v6.i5.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028–40. 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyengar SK, Sedor JR, Freedman BI, et al. Genome-wide association and trans-ethnic meta-analysis for advanced diabetic kidney disease: family investigation of nephropathy and diabetes (find). PLoS Genet 2015;11:e1005352–19. 10.1371/journal.pgen.1005352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anders H-J, Huber TB, Isermann B, et al. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 2018;14:361–77. 10.1038/s41581-018-0001-y [DOI] [PubMed] [Google Scholar]
- 8.Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers 2015;1:1–20. 10.1038/nrdp.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee S, Biehl A, Gadina M, et al. JAK–STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 2017;77:521–46. 10.1007/s40265-017-0701-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med 2015;66:311–28. 10.1146/annurev-med-051113-024537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang PY, He JC. Jak/Stat signaling in renal diseases. Kidney Int 2010;78:231–4. 10.1038/ki.2010.158 [DOI] [PubMed] [Google Scholar]
- 12.Zitman-Gal T, Einbinder Y, Ohana M, et al. Effect of liraglutide on the Janus kinase/signal transducer and transcription activator (JAK/STAT) pathway in diabetic kidney disease in db/db mice and in cultured endothelial cells. J Diabetes 2019;11:656–64. 10.1111/1753-0407.12891 [DOI] [PubMed] [Google Scholar]
- 13.Ortiz-Muñoz G, Lopez-Parra V, Lopez-Franco O, et al. Suppressors of cytokine signaling abrogate diabetic nephropathy. J Am Soc Nephrol 2010;21:763–72. 10.1681/ASN.2009060625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Recio C, Lazaro I, Oguiza A, et al. Suppressor of cytokine signaling-1 peptidomimetic limits progression of diabetic nephropathy. J Am Soc Nephrol 2017;28:575–85. 10.1681/ASN.2016020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed CMI, Larkin J, Johnson HM. SOCS1 mimetics and antagonists: a complementary approach to positive and negative regulation of immune function. Front Immunol 2015;6:183. 10.3389/fimmu.2015.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez GAM, Reinhardt A, Ramsey S, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest 2018;128:3041–52. 10.1172/JCI98814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today 2015;20:122–8. 10.1016/j.drudis.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 18.Hudkins KL, Pichaiwong W, Wietecha T, et al. BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J Am Soc Nephrol 2010;21:1533–42. 10.1681/ASN.2009121290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka S, Sugiura Y, Saito H, et al. Sodium–glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int 2018;94:1–14. 10.1016/j.kint.2018.04.025 [DOI] [PubMed] [Google Scholar]
- 20.Zoja C, Corna D, Camozzi D, et al. How to fully protect the kidney in a severe model of progressive nephropathy: a multidrug approach. J Am Soc Nephrol 2002;13:2898–908. 10.1097/01.ASN.0000034912.55186.EC [DOI] [PubMed] [Google Scholar]
- 21.Lavoz C, Matus YS, Orejudo M, et al. Interleukin-17A blockade reduces albuminuria and kidney injury in an accelerated model of diabetic nephropathy. Kidney Int 2019;95:1418–32. 10.1016/j.kint.2018.12.031 [DOI] [PubMed] [Google Scholar]
- 22.Zoja C, Benigni A, Remuzzi G. The Nrf2 pathway in the progression of renal disease. Nephrol Dial Transplant 2014;29:i19–24. 10.1093/ndt/gft224 [DOI] [PubMed] [Google Scholar]
- 23.Cui W, Min X, Xu X, et al. Role of nuclear factor erythroid 2-related factor 2 in diabetic nephropathy. J Diabetes Res 2017;2017:1–14. 10.1155/2017/3797802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opazo-Ríos L, Mas S, Marín-Royo G, et al. Lipotoxicity and diabetic nephropathy: novel mechanistic insights and therapeutic opportunities. Int J Mol Sci 2020;21. 10.3390/ijms21072632. [Epub ahead of print: 10 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murea M, Freedman BI, Parks JS, et al. Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin J Am Soc Nephrol 2010;5:2373–9. 10.2215/CJN.08160910 [DOI] [PubMed] [Google Scholar]
- 26.Pichaiwong W, Hudkins KL, Wietecha T, et al. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol 2013;24:1088–102. 10.1681/ASN.2012050445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gembardt F, Bartaun C, Jarzebska N, et al. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol 2014;307:F317–25. 10.1152/ajprenal.00145.2014 [DOI] [PubMed] [Google Scholar]
- 28.Chittka D, Banas B, Lennartz L, et al. Long-term expression of glomerular genes in diabetic nephropathy. Nephrol Dial Transplant 2018;33:1533–44. 10.1093/ndt/gfx359 [DOI] [PubMed] [Google Scholar]
- 29.Pennington MW, Czerwinski A, Norton RS. Peptide therapeutics from venom: current status and potential. Bioorg Med Chem 2018;26:2738–58. 10.1016/j.bmc.2017.09.029 [DOI] [PubMed] [Google Scholar]
- 30.Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (pioneer 5): a placebo-controlled, randomised, phase 3A trial. Lancet Diabetes Endocrinol 2019;7:515–27. 10.1016/S2213-8587(19)30192-5 [DOI] [PubMed] [Google Scholar]
- 31.Muskiet MHA, Tonneijck L, Smits MM, et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 2017;13:605–28. 10.1038/nrneph.2017.123 [DOI] [PubMed] [Google Scholar]
- 32.Wada J, Makino H, Hirofumi M. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol 2016;12:13–26. 10.1038/nrneph.2015.175 [DOI] [PubMed] [Google Scholar]
- 33.Moreno JA, Gomez-Guerrero C, Mas S, et al. Targeting inflammation in diabetic nephropathy: a tale of hope. Expert Opin Investig Drugs 2018;27:917–30. 10.1080/13543784.2018.1538352 [DOI] [PubMed] [Google Scholar]
- 34.Rayego-Mateos S, Morgado-Pascual JL, Opazo-Ríos L, et al. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int J Mol Sci 2020;21. 10.3390/ijms21113798. [Epub ahead of print: 27 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berthier CC, Zhang H, Schin M, et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 2009;58:469–77. 10.2337/db08-1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen XP, Losman JA, Cowan S, et al. Pim serine/threonine kinases regulate the stability of SOCS-1 protein. Proc Natl Acad Sci U S A 2002;99:2175–80. 10.1073/pnas.042035699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dieter BP, Meek RL, Anderberg RJ, et al. Serum amyloid A and Janus kinase 2 in a mouse model of diabetic kidney disease. PLoS One 2019;14:e0211555–17. 10.1371/journal.pone.0211555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuttle KR, Brosius FC, Adler SG, et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a phase 2 randomized controlled clinical trial. Nephrol Dial Transplant 2018;33:1950–9. 10.1093/ndt/gfx377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Najafian B, Fogo AB, Lusco MA, et al. AJKD atlas of renal pathology: diabetic nephropathy. Am J Kidney Dis 2015;66:e37–8. 10.1053/j.ajkd.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 40.Poulsom R, Little MH. Parietal epithelial cells regenerate podocytes. J Am Soc Nephrol 2009;20:231–3. 10.1681/ASN.2008121279 [DOI] [PubMed] [Google Scholar]
- 41.Shankland SJ, Freedman BS, Pippin JW. Can podocytes be regenerated in adults? Curr Opin Nephrol Hypertens 2017;26:154–64. 10.1097/MNH.0000000000000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Sanz L, Bernal S, Recio C, et al. SOCS1-targeted therapy ameliorates renal and vascular oxidative stress in diabetes via STAT1 and PI3K inhibition. Lab Invest 2018;98:1276–90. 10.1038/s41374-018-0043-6 [DOI] [PubMed] [Google Scholar]
- 43.Recio C, Oguiza A, Lazaro I, et al. Suppressor of cytokine signaling 1-derived peptide inhibits Janus kinase/signal transducers and activators of transcription pathway and improves inflammation and atherosclerosis in diabetic mice. Arterioscler Thromb Vasc Biol 2014;34:1953–60. 10.1161/ATVBAHA.114.304144 [DOI] [PubMed] [Google Scholar]
- 44.Yan Z, Yang W, Parkitny L, et al. Deficiency of SOCS3 leads to brain-targeted EAE via enhanced neutrophil activation and ROS production. JCI Insight 2019;5:e126520. 10.1172/jci.insight.126520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jager LD, Dabelic R, Waiboci LW, et al. The kinase inhibitory region of SOCS-1 is sufficient to inhibit T-helper 17 and other immune functions in experimental allergic encephalomyelitis. J Neuroimmunol 2011;232:108–18. 10.1016/j.jneuroim.2010.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubben N, Zhang W, Wang L, et al. Repression of the antioxidant NRF2 pathway in premature aging. Cell 2016;165:1361–74. 10.1016/j.cell.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao S, Ghosh A, Lo C-S, et al. Nrf2 deficiency upregulates intrarenal angiotensin-converting enzyme-2 and angiotensin 1-7 receptor expression and attenuates hypertension and nephropathy in diabetic mice. Endocrinology 2018;159:836–52. 10.1210/en.2017-00752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuki H, Kamai T, Murakami S, et al. Increased Nrf2 expression by renal cell carcinoma is associated with postoperative chronic kidney disease and an unfavorable prognosis. Oncotarget 2018;9:28351–63. 10.18632/oncotarget.25322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimmel AR, Sztalryd C. The perilipins: major cytosolic lipid droplet-associated proteins and their roles in cellular lipid storage, mobilization, and systemic homeostasis. Annu Rev Nutr 2016;36:471–509. 10.1146/annurev-nutr-071813-105410 [DOI] [PubMed] [Google Scholar]
- 50.Bobulescu IA. Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens 2010;19:393–402. 10.1097/MNH.0b013e32833aa4ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaishy B, Abel ED. Lipids, lysosomes, and autophagy. J Lipid Res 2016;57:1619–35. 10.1194/jlr.R067520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izquierdo-Lahuerta A, Martínez-García C, Medina-Gómez G. Lipotoxicity as a trigger factor of renal disease. J Nephrol 2016;29:603–10. 10.1007/s40620-016-0278-5 [DOI] [PubMed] [Google Scholar]
- 53.Herman-Edelstein M, Scherzer P, Tobar A, et al. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res 2014;55:561–72. 10.1194/jlr.P040501 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2020-001242supp001.pdf (3.6MB, pdf)