Abstract
Independent studies over the last decade have characterized the properties of non-circulating CD8+ ‘resident’ memory T cells (TRM), which offer barrier protective immunity in non-lymphoid tissues and CD4+ follicular helper T cells (TFH), which mediate B-cell help in lymphoid sites. Despite their very different biological roles in the immune system, intriguing parallels have been noted between the trafficking properties and differentiation cues of these populations, parallels which have only sharpened with recent findings. In this review, we explore the features that underlie these similarities and discuss whether these indicate meaningful homologies in the development of CD8+ TRM and CD4+ TFH or reflect resemblances which are only ‘skin-deep’.
Keywords: KLF2, ICOS, P2RX7, S1PR1
Introduction
The differentiation of memory T cells has been a highly active area of research in immunology for decades. Diverse memory subsets are formed following a typical immune response and there is much interest in their properties and the cues that guide their differentiation. A major division between different memory T-cell subsets reflects their trafficking patterns: although the best-studied memory T-cell populations are found in the circulation and are abundant in lymphoid tissues, a distinct subset called ‘resident’ memory T cells (TRM) are thought not to access the circulation during normal homeostasis but are maintained in non-lymphoid tissues (and constitute a small population of the cells in lymphoid tissues) (1–3).
Tissue localization equips TRM to mediate immediate responses when foreign antigens (such as those encoded by pathogens) access these sites, which include barrier tissues such as the skin, lung and gut, providing a first line of defense. Recirculating cells, on the other hand, would be more flexible and able to initiate responses once they encounter presented antigen that has reached lymphoid tissues. Studies in mice indicate that both CD4+ and CD8+ T cells form TRM populations (1, 2), and such cells appear to account for the majority of T cells in non-lymphoid tissues of humans (3–5).
A distinct population of T cells also plays a role that requires them to be retained in tissues for extended periods of time. CD4+ follicular helper T cells (TFH) found in active germinal centers (GC-TFH) are key for B-cell help in the generation of class-switched, high-affinity antibody responses (6). GC-TFH are believed to be a more differentiated and specialized form of TFH and their ability to efficiently sustain ongoing B-cell responses in antigen-draining lymph nodes requires that TFH be retained in these lymphoid tissues.
However, there was considerable debate about whether GC-TFH and TFH are present only during the period of antigen exposure (and hence are what might be considered as an ‘effector’ cell population) or whether long-lived ‘memory’ TFH also exist [reviewed in refs (7, 8)]. This was apparently resolved by findings that cells with some TFH phenotypic characteristics (including expression of CXCR5, PD-1 and Bcl-6, although at reduced levels compared with recently activated TFH and GC-TFH) could be found at memory time points and even in the circulation (7, 8). Still, uncertainty remained, since some studies suggested that these memory phase cells had a heightened predilection to produce TFH in a recall response (9), but others suggested that these populations were essentially ‘central memory’ T cells (TCM) with the capacity to differentiate into various populations upon re-stimulation (10).
This topic will need substantial re-evaluation based on very recent studies from King et al., showing that cells with phenotypic, transcriptional and functional characteristics of TFH persist long after antigen clearance but are typically lost during standard tissue-processing procedures, leading to an underestimate of ‘long-lived TFH’ (11). Importantly, the long-lived TFH defined by King et al. (11) were maintained in the absence of antigen, responded efficiently to antigen re-exposure and displayed plasticity in their differentiation following recall stimulation—all of which are key characteristics of memory T cells. However, these cells were distinct from previously described CD4+ ‘CXCR5+ TCM’ (10) or ‘memory TFH’ (9), highlighting potential ambiguity in identification of the ‘true’ memory TFH population(s). This also colors the conclusions of other studies, such as a careful analysis of mouse CD4+ TRM, which suggested that the transcriptional profile of that population was quite distinct from memory TFH (12)—with the validity of that interpretation depending on how the ‘memory TFH’ pool (9) analyzed in that report relates to the more recently defined ‘long-lived TFH’ (11). To be clear, in this review, we focus on long-lived TFH (as defined in (11)) as the basis for comparison to CD8+ TRM.
The functions of CD4+ TFH and CD8+ TRM are, of course, quite distinct—but studies on CD4+ TFH and on CD8+ TRM suggest that these populations share some key features related to their migration, differentiation and maintenance (Fig. 1), as will be discussed here. It is unclear, however, whether these parallels are purely superficial or whether they suggest parallel mechanisms of induction and/or homeostasis of these T-cell subsets. The goal of this short review is to scrutinize characteristics of TFH and TRM, in order to evaluate whether their similarities are merely ‘skin-deep’ or reflect a more meaningful relationship.
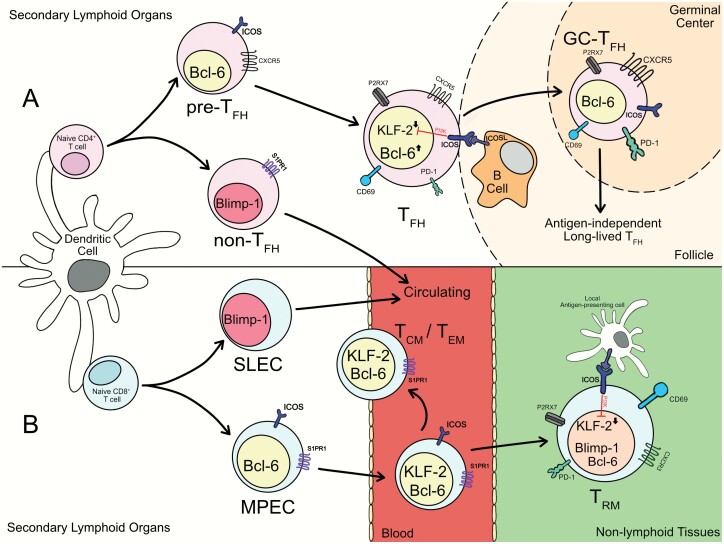
Fig. 1.
Parallels in the development of CD4+ TFH and CD8+ TRM. (A). When naive CD4+ T cells get activated, those receiving TFH-inducing signals up-regulate Bcl-6 to become pre-TFH. Bcl-6+ pre-TFH expressing CXCR5 and ICOS migrate to the T–B border of the follicle and contact B cells. CD4+ T cells receiving ICOS co-stimulation from interactions with B cells down-regulate KLF2 in a PI3K-dependent manner. ICOS engagement and additional signals drive the maturation of TFH cells. With the formation of the germinal center, TFH cells further differentiate into GC-TFH and assist in the production of high-affinity antibodies. After the germinal center has resolved, long-lived TFH can persist in the absence of antigen. (B) Activated CD8+ T cells can develop into either short-lived effector cells (SLECs) or memory precursor effector cells (MPECs) in a Blimp-1/Bcl-6-dependent manner. Both SLECs and MPECs re-express KLF2 and rejoin the circulation. Some MPECs mature into circulating memory cells including TCM and TEM, which continuously traffic around the body. Others enter non-lymphoid tissues and receive additional signals, including ICOS stimulation, within the local environment which drive establishment of the residency programming (including down-regulation of KLF2 and S1PR1), generating the TRM population.
T-cell trafficking
The quintessential defining feature of TRM is their long-term residence in tissues (1–3). In contrast to the well-studied recirculating pools [which include TCM and ‘effector’ memory T cells (TEM)], TRM are usually rare in lymphoid tissues and typically absent in the lymph or blood. Established TRM are certainly capable of migrating to draining lymph nodes when activated (13), and reports indicate that some CD4+ TRM-phenotype cells may access the circulation in some circumstances (14), but numerous studies indicate that, during normal homeostasis, maintenance of the TRM pool is independent of circulating memory cells (1–3).
Numerous mechanisms are thought to enforce TRM residency in tissues. One of these relates to lower expression of the ‘egress’ factor sphingosine-1-phosphate receptor 1 (S1pr1) by TRM compared with recirculating T cells (1–3). S1pr1, through recognition of S1P in the blood and lymph, is long recognized as being essential for recirculation of naive T cells through the lymphoid sites and the vasculature (15). The most trusted hallmark feature of TRM is their expression of CD69; while typically considered an activation marker, CD69 is expressed at lower levels even in resting T cells, but protein expression at the cell surface is limited by mutual competition with S1pr1 (16, 17). Transcription of the S1pr1 gene in T cells is driven by Kruppel-like factor 2 (Klf2) (18) and studies indicate that TRM express markedly lower levels of both Klf2 and S1pr1 compared with their recirculating counterparts in mice (19) and humans (20).
In this way, the expression of the canonical TRM marker CD69 can be seen as corresponding to low expression of S1pr1—mediated through either heightened expression of CD69 preventing S1pr1 protein expression, or as a passive marker reflecting low expression of Klf2 and S1pr1 mRNA that permits basal levels of CD69 to reach the cell surface. Some studies suggest both mechanisms operate, sequentially (21), although other reports suggest modest functional significance of CD69 expression in dictating the generation of TRM (22). The lack of S1pr1 expression was found to be mechanistically important, since enforced S1pr1 (or Klf2) expression impaired induction of CD8+ TRM (19). Other characteristics of at least some TRM include expression of CD103, the αE integrin chain (which pairs with β7 integrin to permit adhesion to E-cadherin) (1, 3). This can be seen as an ‘anchor’ to limit migration, whereas reduced S1pr1 expression could be viewed as lack of an ‘engine’ for tissue exit.
In a distinct way, TFH—especially GC-TFH—could be considered as resident cells in lymphoid tissues and possess some of the same features as TRM in this regard. We and others found that TFH had markedly reduced expression of Klf2 and S1pr1 compared with non-TFH populations [including T helper 1 cells (Th1) and developing TCM] and expressed CD69 (23, 24). Since TCR activation can also drive the loss of Klf2 and S1pr1, and promote CD69 expression, it was difficult to disentangle the potential role for persistent TCR stimulation (as would be expected for TFH interacting with antigen-specific GC B cells). However, analysis of long-lived TFH revealed that their survival is not dependent on continued antigen exposure and this population displays sustained cell surface CD69 and low S1pr1 and Klf2 gene expression (11) (Carolyn King, personal communication). Similar to the effect on CD8+ TRM, enforced expression of either S1pr1 or Klf2 was sufficient to limit the generation of TFH (23).
At the same time, some skepticism about the parallels between the trafficking patterns of TFH and TRM is in order since cells with phenotypic characteristics of TFH have been identified in the blood of both mice and humans (6). Some studies suggest these are distinct populations—TFH-like cells in the antigen-draining lymph node being of higher TCR affinity and functionally distinct compared with their circulating counterparts, suggesting that they may represent phenotypically similar but distinct subsets (25); however, it is currently unclear whether any populations of long-lived TFH have the stringent residency characteristics of CD8+ TRM defined in non-lymphoid tissues. Careful parabiosis studies may be needed to shed light on this.
Although residing in lymphoid organs, long-lived CD4+ TFH cells do not express the well-defined lymph node homing chemokine receptor CCR7 (11), a feature they share with TRM. However, the expression of other chemokine receptors does not closely align between CD8+ TRM and CD4+ TFH, perhaps reflective of their distinct localization within tissues—CD8+ TRM typically express CXCR3 and may require CXCR6 to establish residency in some sites (20, 26, 27), whereas CXCR5 expression is a key functional characteristics of TFH (6). Further characterization of long-lived TFH may be needed to define their core trafficking characteristics and the presence of these cells in blood is unclear. These findings do not deny the potential significance of shared regulation of S1pr1 by TRM and TFH but highlight that distinct trafficking cues may be involved in establishing or retaining these cells in tissues.
Roles of ICOS and Bcl-6
Potentially related to similarities in their expression of molecules regulating egress, TFH and CD8+ TRM share a few other gene expression similarities (11), which might indicate parallels in the differentiation program. Indeed, it has previously been reported that both human and mouse CD8+ TRM express the inducible co-stimulatory molecule ‘ICOS’ (20, 28). ICOS has a well-defined and indispensable role in TFH generation and GC maintenance, arising from ICOS–ICOS ligand interactions between activated CD4+ T cells and both cognate and non-cognate B cells (6). ICOS does not affect the early commitment of TFH, particularly Bcl-6 expression, but is essential for the maintenance of the TFH phenotype (24). The study from King et al. also reported that ICOS engagement is required for long-lived TFH identity, maintenance and optimal humoral immunity (11).
ICOS signals through multiple pathways (29), but its induction of phosphoinositide 3-kinase (PI3K) is thought to play a key role in inducing down-regulation of KLF2 and FOXO-1 (which, among many targets, drives KLF2 expression) (24, 30). The induced loss of KLF2 in CD8+ TRM is also thought to entail PI3K activity (19) and previous studies indicated that various tissue cytokines can cooperate to suppress the expression of KLF2 in a PI3K-dependent manner (19). However, whether ICOS plays some role in regulation of CD8+ TRM has not been reported.
Tantalizing clues come from the work of Liu et al., which showed that ICOS overexpression leads activated CD8+ T cells to preferentially accumulate in non-lymphoid tissues (31). Furthermore, in our own unpublished studies, we found that ICOS deficiency does not compromise CD8+ T-cell activation or the generation of circulating memory cells but leads to a substantial defect in establishment of TRM (C. Peng et al., manuscript in preparation). At early stages of the immune response, ICOS-deficient CD8+ T cells are present in non-lymphoid sites, but these cells show impaired down-regulation of KLF2 (C. Peng et al., manuscript in preparation), suggesting that ICOS-mediated down-regulation of KLF2 may be a component in generation of CD8+ TRM, paralleling the proposed pathway that occurs in developing TFH (24).
The key role of the transcription factor Bcl-6 in development of CD4+ TFH cells was defined over a decade ago (32–34). Bcl-6 is mutually antagonistic with the transcriptional repressor Blimp-1 (35) and, in CD4+ T cells, strong Blimp-1 expression is associated with the generation of effector Th populations, whereas Bcl-6 expression is necessary and sufficient to drive TFH (6, 35). Bcl-6 and Blimp-1 appear to play parallel roles in CD8+ T-cell differentiation, with Blimp-1 driving generation of effector cells (typically short-lived) and Bcl-6 promoting generation of memory [discussed in ref. (35)].
As has been discussed previously, the participation of Bcl-6 and Blimp-1 should not be considered as digital choices, rather the relative contribution of each factor may fine-tune T-cell differentiation (35). At present, there is limited information on whether, as for TFH, Bcl-6 plays a more critical role in differentiation or maintenance of CD8+ TRM than for other CD8+ memory populations. Enforced expression of BCL-6 in human CD4+ T cells activated in vitro strongly inhibits expression of KLF2 (36), potentially supporting that model. However, seemingly in contrast to this hypothesis, Blimp-1 and the closely related factor Hobit are required for the generation of most CD8+ TRM populations (37).
Confusingly, in some situations, such as for skin CD8+ TRM generated following herpes simplex virus (HSV) infection, deficiency of Blimp-1 alone leads to increased representation of TRM, whereas Hobit deficiency (and especially loss of both Hobit and Blimp-1) causes a reduction in skin TRM (37)—but in other models, such as for lung CD8+ TRM induced following influenza infection, Blimp-1 but not Hobit is critical (38). Interpretation of these findings is further complicated by the unclear relationship between Bcl-6 and Hobit and the overlap between genes regulated by Blimp-1 and Hobit [which have very similar DNA-binding domains (37)], and the role of Bcl-6 in maintenance of TRM has not been explicitly tested [although there is published evidence that Bcl-6 is not essential for maintenance of established circulating memory CD8+ T cells (39)].
Role of P2RX7 and ARTC2
Another shared feature of mouse CD8+ (and CD4+) TRM and CD4+ TFH is their relatively high expression of the ATP sensor P2RX7 and the NAD+-dependent ADP ribosyltransferase ARTC2.2 (12, 40–42). Strong stimulation of P2RX7 by extracellular ATP can promote cell death, and this function is also induced by ribosylation of P2RX7 by ARTC2.2 (43, 44). These pathways can inadvertently be induced during tissue isolation, which can lead to a substantial underestimate of both TRM and long-lived TFH (11, 42, 45). Indeed, acute blockade of this pathway during tissue harvesting was critical to the ‘rescue’ of long-lived TFH for experimental analysis (11) and there is evidence that the poor recovery of CD8+ TRM from non-lymphoid tissues for flow cytometric analysis (46) reflects, at least in part, activation of the ARTC2.2–P2RX7 axis (45).
Interestingly, the physiological functions of P2RX7 on CD8+ TRM and CD4+ TFH appear to be polar opposites, with compelling evidence that P2RX7 is required for the generation of CD8+ TRM (40, 45) but limits the maintenance of CD4+ TFH (11, 41, 47), for reasons that are not yet clear. Similar functions of mouse and human P2RX7 have been reported, although there is not a human equivalent of ARTC2.2, arguing that this pathway may be more of an issue for studies in murine models (43, 48). The fact that typical tissue isolation methods and in vitro staining techniques can inadvertently stimulate these pathways presents a substantial hurdle to characterization of both TFH and TRM populations and may contribute to numerous discrepancies in the literature. Implementation of improved methods to isolate and characterize cells vulnerable to these cell death pathways (11, 42, 44, 45, 49) may need to be standardized to improve consistency of analysis.
Conclusions
Although there are striking parallels between CD4+ TFH and CD8+ TRM in terms of their trafficking (including the importance of KLF2 and S1PR1 down-regulation) and differentiation (including the role of ICOS), it is premature to determine whether these constitute more than occasional points of similarity between the populations. This uncertainty comes about in no small part from the realization that these populations are vulnerable to cell death during standard isolation procedures because of another shared feature—strong expression of P2RX7 (and, in mice, ARTC2.2)—which, ironically, may obscure a better characterization of their relatedness. Further investigation will, therefore, be needed to discern whether shared traits of CD4+ TFH and CD8+ TRM are merely superficial or reflect a more substantial kinship.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (AI38903).
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Masopust, D. and Soerens, A. G. 2019. Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol. 37:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mueller, S. N. and Mackay, L. K. 2016. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16:79. [DOI] [PubMed] [Google Scholar]
- 3. Szabo, P. A., Miron, M. and Farber, D. L. 2019. Location, location, location: tissue resident memory T cells in mice and humans. Sci. Immunol. 4:eaas9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sathaliyawala, T., Kubota, M., Yudanin, N.et al. 2013. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thome, J. J., Yudanin, N., Ohmura, Y.et al. 2014. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crotty, S. 2019. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50:1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crotty, S. 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity 41:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hale, J. S. and Ahmed, R. 2015. Memory T follicular helper CD4 T cells. Front. Immunol. 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hale, J. S., Youngblood, B., Latner, D. R.et al. 2013. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity 38:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pepper, M., Pagán, A. J., Igyártó, B. Z., Taylor, J. J. and Jenkins, M. K. 2011. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kunzli, M., Schreiner, D., Pereboom, T. C.et al. 2020. Long-lived T follicular helper cells retain plasticity and help sustain humoral immunity. Sci. Immunol. 5:eaay5552. [DOI] [PubMed] [Google Scholar]
- 12. Beura, L. K., Fares-Frederickson, N. J., Steinert, E. M.et al. 2019. CD4+ resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med. 216:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beura, L. K., Wijeyesinghe, S., Thompson, E. A.et al. 2018. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity 48:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klicznik, M. M., Morawski, P. A., Hollbacher, B.et al. 2019. Human CD4(+)CD103(+) cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci. Immunol. 4:eaav8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwab, S. R. and Cyster, J. G. 2007. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8:1295. [DOI] [PubMed] [Google Scholar]
- 16. Bankovich, A. J., Shiow, L. R. and Cyster, J. G. 2010. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J. Biol. Chem. 285:22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shiow, L. R., Rosen, D. B., Brdicková, N.et al. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440:540. [DOI] [PubMed] [Google Scholar]
- 18. Carlson, C. M., Endrizzi, B. T., Wu, J.et al. 2006. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442:299. [DOI] [PubMed] [Google Scholar]
- 19. Skon, C. N., Lee, J. Y., Anderson, K. G., Masopust, D., Hogquist, K. A. and Jameson, S. C. 2013. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 14:1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar, B. V., Ma, W., Miron, M.et al. 2017. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 20:2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mackay, L. K., Braun, A., Macleod, B. L.et al. 2015. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 194:2059. [DOI] [PubMed] [Google Scholar]
- 22. Walsh, D. A., Borges da Silva, H., Beura, L. K.et al. 2019. The functional requirement for CD69 in establishment of resident memory CD8+ T cells varies with tissue location. J. Immunol. 203:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee, J. Y., Skon, C. N., Lee, Y. J.et al. 2015. The transcription factor KLF2 restrains CD4⁺ T follicular helper cell differentiation. Immunity 42:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber, J. P., Fuhrmann, F., Feist, R. K.et al. 2015. ICOS maintains the T follicular helper cell phenotype by down-regulating Krüppel-like factor 2. J. Exp. Med. 212:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asrir, A., Aloulou, M., Gador, M., Pérals, C. and Fazilleau, N. 2017. Interconnected subsets of memory follicular helper T cells have different effector functions. Nat. Commun. 8:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zaid, A., Hor, J. L., Christo, S. N.et al. 2017. Chemokine receptor-dependent control of skin tissue-resident memory T cell formation. J. Immunol. 199:2451. [DOI] [PubMed] [Google Scholar]
- 27. Wein, A. N., McMaster, S. R., Takamura, S.et al. 2019. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J. Exp. Med. 216:2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mackay, L. K., Rahimpour, A., Ma, J. Z.et al. 2013. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 14:1294. [DOI] [PubMed] [Google Scholar]
- 29. Pedros, C., Zhang, Y., Hu, J. K.et al. 2016. A TRAF-like motif of the inducible costimulator ICOS controls development of germinal center TFH cells via the kinase TBK1. Nat. Immunol. 17:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stone, E. L., Pepper, M., Katayama, C. D.et al. 2015. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity 42:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu, D., Burd, E. M., Coopersmith, C. M. and Ford, M. L. 2016. Retrogenic ICOS expression increases differentiation of KLRG-1hiCD127loCD8+ T cells during Listeria infection and diminishes recall responses. J. Immunol. 196:1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nurieva, R. I., Chung, Y., Martinez, G. J.et al. 2009. Bcl6 mediates the development of T follicular helper cells. Science 325:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnston, R. J., Poholek, A. C., DiToro, D.et al. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu, D., Rao, S., Tsai, L. M.et al. 2009. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31:457. [DOI] [PubMed] [Google Scholar]
- 35. Crotty, S., Johnston, R. J. and Schoenberger, S. P. 2010. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hatzi, K., Nance, J. P., Kroenke, M. A.et al. 2015. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J. Exp. Med. 212:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mackay, L. K., Minnich, M., Kragten, N. A.et al. 2016. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352:459. [DOI] [PubMed] [Google Scholar]
- 38. Behr, F. M., Kragten, N. A. M., Wesselink, T. H.et al. 2019. Blimp-1 rather than Hobit drives the formation of tissue-resident memory CD8+ T cells in the lungs. Front. Immunol. 10:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu, Z., Guo, Y., Tang, S.et al. 2019. Cutting edge: transcription factor BCL6 is required for the generation, but not maintenance, of memory CD8+ T cells in acute viral infection. J. Immunol. 203:323. [DOI] [PubMed] [Google Scholar]
- 40. Borges da Silva, H., Beura, L. K., Wang, H.et al. 2018. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8(+) T cells. Nature 559:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Proietti, M., Cornacchione, V., Rezzonico Jost, T.et al. 2014. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer’s patches to promote host-microbiota mutualism. Immunity 41:789. [DOI] [PubMed] [Google Scholar]
- 42. Georgiev, H., Ravens, I., Papadogianni, G., Malissen, B., Förster, R. and Bernhardt, G. 2018. Blocking the ART2.2/P2X7-system is essential to avoid a detrimental bias in functional CD4 T cell studies. Eur. J. Immunol. 48:1078. [DOI] [PubMed] [Google Scholar]
- 43. Di Virgilio, F., Dal Ben, D., Sarti, A. C., Giuliani, A. L. and Falzoni, S. 2017. The P2X7 receptor in infection and inflammation. Immunity 47:15. [DOI] [PubMed] [Google Scholar]
- 44. Rissiek, B., Haag, F., Boyer, O., Koch-Nolte, F. and Adriouch, S. 2015. P2X7 on mouse T cells: one channel, many functions. Front. Immunol. 6:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Borges da Silva, H., Wang, H., Qian, L. J., Hogquist, K. A. and Jameson, S. C. 2019. ARTC2.2/P2RX7 signaling during cell isolation distorts function and quantification of tissue-resident CD8(+) T cell and invariant NKT subsets. J. Immunol. 202:2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steinert, E. M., Schenkel, J. M., Fraser, K. A.et al. 2015. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Perruzza, L., Gargari, G., Proietti, M.et al. 2017. T follicular helper cells promote a beneficial gut ecosystem for host metabolic homeostasis by sensing microbiota-derived extracellular ATP. Cell Rep. 18:2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rissiek, B., Haag, F., Boyer, O., Koch-Nolte, F. and Adriouch, S. 2015. ADP-ribosylation of P2X7: a matter of life and death for regulatory T cells and natural killer T cells. Curr. Top. Microbiol. Immunol. 384:107. [DOI] [PubMed] [Google Scholar]
- 49. Danquah, W., Meyer-Schwesinger, C., Rissiek, B.et al. 2016. Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci. Transl. Med. 8:366ra162. [DOI] [PubMed] [Google Scholar]