Abstract
Background:
Abnormal serum sodium levels in various diseases increase mortality; however, hyperglycemia depresses serum sodium concentration significantly. This study aimed to evaluate the clinical impact of measured serum sodium levels and corrected sodium levels among patients with severe hyperglycemia.
Methods:
Patients with blood glucose levels ≥500 mg/dL visiting the emergency department between July 2008 and September 2010 were enrolled retrospectively. The participants were divided into five groups for measured sodium levels and five groups for corrected sodium levels according to blood glucose levels. Multivariate Cox regression was used. The primary outcome was all-cause 90-day mortality. Secondary outcomes included rate of intensive care unit hospitalization, respiratory failure, and renal failure.
Results:
A total of 755 patients with severe hyperglycemia were enrolled, and the 90-day mortality was 17.2%. Compared with the reference group, the 90-day mortality was higher in the patients with measured hypernatremia (adjusted hazard ratio [HR], 2.86; 95% confidence interval [CI], 1.39–5.87), corrected hyponatremia (adjusted HR, 3.56; 95% CI, 1.44–8.80), and severe corrected hypernatremia (adjusted HR, 2.68; 95% CI, 1.28–5.62). However, patients with severe measured hyponatremia did not show increased mortality (adjusted HR, 1.67; 95% CI, 0.84–3.32).
Conclusion:
Among patients with severe hyperglycemia, corrected sodium level is a better indicator of clinical outcomes compared with measured sodium levels, especially in this population with measured hyponatremia.
Keywords: Corrected sodium levels, Hyperglycemia, Mortality
1. INTRODUCTION
Abnormalities in serum sodium concentration in the form of hypernatremia and hyponatremia are common electrolyte disorders.1 The serum sodium concentration is regulated by water homeostasis, which is mediated by thirst, arginine vasopressin, and the kidneys.2 Hyperglycemia is associated with a decrease in serum sodium concentration. Water moves from the intracellular space to the extracellular space along the osmotic gradient, subsequently causing a reduction in the serum sodium level. Therefore, hyperglycemic patients are mostly mildly hyponatremic.3 However, patients with normal or even elevated serum sodium levels are seen sometimes when they develop osmotic diuresis without adequate fluid replacement, especially in elderly individuals with impaired thirst mechanism or diminished access to fluids.4
Hyponatremia and hypernatremia have been linked to increased mortality in hospitalized patients.5,6 Hyponatremia also indicates poor prognosis in a variety of subgroups, including patients with chronic kidney disease, heart failure, liver disease, or intracerebral hemorrhage.7–11 Previous studies have demonstrated that the prognostic factors for mortality among severe hyperglycemic patients include altered mental status on admission, pneumonia, older age, stroke, myocardial infarction, sepsis, renal impairment, hyperkalemia, and lower blood pressure.12–14
Because hyperglycemia can depress sodium concentration, patients with hyponatremia might be overlooked during severe hyperglycemia. We hypothesized that the corrected serum sodium level for severe hyperglycemia should be a prognostic factor to predict clinical outcomes in severe hyperglycemic patients. Therefore, we conducted this study to evaluate the clinical impact of measured serum sodium and corrected sodium levels among severe hyperglycemic patients in the emergency department (ED).
2. METHODS
2.1. Patient selection
Patients with blood glucose levels ≥500 mg/dL who visited the ED of Taipei Veterans General Hospital from July 1, 2008 to September 30, 2010 were enrolled retrospectively in this study. Patients aged <30 years or >99 years were excluded. Data were extracted from the electronic medical records and included vital signs, complete blood count, and serum biochemical analysis on arrival. The study was approved by the institutional review board of Taipei Veterans General Hospital.
2.2. Laboratory measurements
Data from charts included age, sex, date of arrival at the ED, blood glucose levels, vital signs, white blood cell (WBC) count, hemoglobin (Hb), platelet count, C-reactive protein (CRP), blood urea nitrogen (BUN), creatinine, sodium, potassium, and alanine aminotransferase (ALT). Effective serum osmolality was defined as 2 [measured Na+ (mmol/L)] + [glucose (mg/dL)]/18.15 Calculated osmolality was defined as 2 [measured Na+ (mmol/L)] + [glucose (mg/dL)]/18 + [BUN (mg/dL)]/2.8.16 The data of intensive care unit (ICU) hospitalization rates, respiratory failure rates, and renal failure rates were also obtained.
2.3. Grouping according to measured or corrected sodium levels
The sodium level was corrected according to the glucose level, with a correction factor of a 2.4 mmol/L decrease in sodium concentration per 100 mg/dL increase in glucose concentration.3 The participants were categorized into five groups based on measured sodium levels, and defined as severe measured hyponatremia (Na ≤ 125 mmol/L), moderate measured hyponatremia (Na = 126–130 mmol/L), mild measured hyponatremia (Na = 131–134 mmol/L), measured normonatremia (Na = 135–145 mmol/L), and measured hypernatremia (Na ≥ 146 mmol/L). The participants were also categorized into five groups according to corrected sodium levels, and defined as corrected hyponatremia (corrected Na < 135 mmol/L), low corrected normonatremia (corrected Na = 135–139.9 mmol/L), high corrected normonatremia (corrected Na = 140–144.9 mmol/L), mild corrected hypernatremia (corrected Na = 145–149.9 mmol/L), and severe corrected hypernatremia (corrected Na ≥ 150 mmol/L).
2.4. Study outcomes
The primary outcome for this analysis was all-cause 90-day mortality. The secondary outcomes included ICU admission rate, respiratory failure rate, which was defined as the dependence on a mechanical ventilator, and renal failure rate, which was defined if the patients commenced hemodialysis after admission. Information on date of death was obtained from the Department of Health, Executive Yuan, ROC (Taiwan).
2.5. Statistical analysis
Results were presented as mean ± standard deviation (SD) for continuous variables with a normal distribution and median (interquartile range) for continuous variables with a nonnormal distribution. We used ANOVA to define p values for baseline characteristics across the different groups of corrected serum sodium levels. The reference quartile was defined as the one with lowest mortality. The Cox regression model was used to calculate the risk of 90-day mortality, rate of ICU admission, respiratory failure, and renal failure according to the measured sodium levels and the corrected sodium levels. The results were expressed as hazard ratios (HRs) with 95% CIs. The reference group was defined as the one with lowest mortality. Variables with p values < 0.05 in the univariate analysis were considered for inclusion in the multivariate Cox regression model. In addition, the following models were used to evaluate the impact of sodium level on mortality: (1) unadjusted; (2) adjusted for sex and age; and (3) adjusted for age, sex, glucose level, respiratory rate (RR), systolic blood pressure (SBP), pulse rate (PR), WBC, Hb, CRP, BUN, and creatinine. Survival curves between different groups were established with the Kaplan–Meier method, and log-rank tests were used for comparison. Analyses were performed using SPSS for Windows, version 19.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was considered as p < 0.05.
3. RESULTS
3.1. Study participants and baseline characteristics
A total of 781 patients presented at the ED with blood glucose levels ≥500 mg/dL, of whom 25 patients were excluded due to age, and one patient was excluded because the sodium level was not obtained on arrival. The remaining 755 patients were analyzed (Supplemental Figure 1, http://links.lww.com/JCMA/A57), of whom 510 (67.5%) were men. The mean age at baseline was 68.7 ± 15.4 years, mean blood glucose level was 691.8 ± 204.5 mg/dL, CRP level was 7.0 ± 9.6 mg/dL, BUN level was 47.1 ± 35.1 mg/dL, creatinine level was 2.6 ± 2.1 mg/dL, sodium level was 132.6 ± 8.8 mmol/L, and potassium level was 4.6 ± 1.0 mmol/L.
Baseline characteristics according to corrected sodium levels are presented in Table 1. The mean measured sodium level was 119.4 ± 4.0 mmol/L in patients with corrected hyponatremia, 125.2 ± 3.2 mmol/L in low corrected normonatremia, 129.7 ± 2.9 mmol/L in high corrected normonatremia, 132.9 ± 5.0 mmol/L in mild corrected hypernatremia, and 142.9 ± 9.1 mmol/L in severe corrected hypernatremia. Compared with patients with low corrected normonatremia, participants with severe corrected hypernatremia were older and had higher glucose levels, RR, PR, WBC counts, Hb levels, BUN, serum creatinine, calculated osmolality, effective osmolality, and serum sodium levels; however, they had lower SBP. Participants with corrected hyponatremia had lower calculated osmolality, effective osmolality, and serum sodium levels when compared with patients with low corrected normonatremia.
Table 1.
Baseline characteristics of patients according to corrected sodium levels
| Corrected hyponatremia (<135 mmol/L) | Low corrected normonatremia (135–139.9 mmol/L) | High corrected normonatremia (140–144.9 mmol/L) | Mild corrected hypernatremia (145–149.9 mmol/L) | Severe corrected hypernatremia (≥150 mmol/L) | p | |
|---|---|---|---|---|---|---|
| Numbers (%) | 34 (4.5) | 120 (15.9) | 251 (33.2) | 157 (20.8) | 193 (25.6) | |
| Age (years)a | 68.2 ± 13.7 | 63.9 ± 15.5 | 67.9 ± 15.3 | 68.0 ± 16.4 | 73.4 ± 13.8d | <0.001d |
| Male sex, n (%) | 17 (50.0) | 73 (60.8) | 174 (69.3) | 111 (70.7) | 135 (69.9) | 0.078 |
| Blood glucose (mg/dL)a | 628.5 ± 106.4 | 631.1 ± 121.9 | 633.5 ± 114.8 | 694.3 ± 207.2 | 828.7 ± 285.3d | <0.001d |
| Body temperature (°C)a | 37.0 ± 1.1 | 36.8 ± 1.0 | 36.6 ± 0.9 | 36.7 ± 1.0 | 36.8 ± 3.1 | 0.487 |
| RR (breath per minute)a | 19.5 ± 4.8 | 19.8 ± 2.7 | 20.1 ± 4.2 | 20.0 ± 4.4 | 22.3 ± 6.0d | <0.001d |
| SBP (mm Hg)a | 132.0 ± 31.2 | 140.7 ± 39.3 | 145.9 ± 32.9 | 145.2 ± 36.8 | 123.7 ± 37.2d | <0.001d |
| PR (beats per minute)a | 90.5 ± 31.0 | 97.4 ± 22.9 | 94.3 ± 21.8 | 95.6 ± 24.9 | 105.6 ± 28.5d | <0.001d |
| WBC count (/cumm)b | 13,800 (9200–21,300) | 10,650 (7600–15,100) | 9100 (6900–12,500) | 9700 (7300–14,200) | 11,900 (8550–16,000) | <0.001 |
| Hb (g/dL)a | 11.3 ± 3.1 | 11.5 ± 2.6 | 12.6 ± 2.6 | 12.8 ± 2.5 | 12.7 ± 3.0d | <0.001d |
| PLT (/cumm)b | 218K (145K–323K) | 245K (175K–304K) | 218K (164K–274K) | 225K (171K–275K) | 228K (156K–288K) | 0.344 |
| CRP (mg/dL)b | 10.4 (2.21–16.09) | 2.80 (0.55–13.39) | 1.34 (0.31–7.84) | 1.29 (0.30–8.50) | 3.61 (0.86–10.94) | 0.009 |
| BUN (mg/dL)b | 38.0 (27.0–75.0) | 36.5 (25.0–62.0) | 30.0 (19.0–51.0) | 29.0 (18.0–44.5) | 57.0 (34.5–87.0)d | <0.001d |
| Creatinine (mg/dL)a | 2.75 ± 1.78 | 2.92 ± 3.06 | 2.35 ± 1.93 | 2.34 ± 1.94 | 2.99 ± 1.76 | 0.004 |
| Calculated osmolalitya | 291.1 ± 10.0c | 302.6 ± 12.9 | 308.3 ± 9.8 | 317.4 ± 11.4 | 355.5 ±28.1d | <0.001c,d |
| Effective osmolalitya | 273.7 ± 5.9c | 285.5 ± 2.8 | 294.6 ± 3.0 | 304.3 ± 3.3 | 331.8 ± 19.4d | <0.001c,d |
| Na (mmol/L)a | 119.4 ± 4.0c | 125.2 ± 3.2 | 129.7 ± 2.9 | 132.9 ± 5.0 | 142.9 ± 9.1d | <0.001c,d |
| K (mmol/L)a | 5.0 ± 1.5 | 4.5 ± 0.9 | 4.6 ± 0.9 | 4.4 ± 0.9 | 4.7 ± 1.2 | 0.054 |
| ALT (U/L)a | 22 (18–34) | 20 (14–38) | 23 (16–35) | 22 (16–35) | 27 (17–43) | 0.951 |
aMean (standard deviation).
bMedian (interquatile range).
cp value < 0.05 between corrected hyponatremia and low corrected normonatremia.
dp value <0.05 between severe corrected hypernatremia and low corrected normonatremia.
RR = respiratory rate; SBP = systolic blood pressure; PR = pulse rate; WBC = white blood cell; Hb = hemoglobin; PLT = platelet; CRP = C-reactive protein; BUN = blood urea nitrogen; Na = sodium; K = potassium; ALT = alanine aminotransferase.
3.2. Primary outcomes
Among the 755 patients with severe hyperglycemia, the 90-day mortality was 17.2% (n = 130). The causes of death are presented in Supplemental Table 1, http://links.lww.com/JCMA/A57. The baseline characteristics of the individuals who were deceased and those who were alive within 90 days of visiting the ED are presented in Supplemental Table 2, http://links.lww.com/JCMA/A57. According to the measured sodium level, patients with moderate measured hyponatremia (Na = 126–130 mmol/L) had the lowest 90-day mortality (10.8%) and was defined as the reference group (Table 2). Patients with measured normonatremia had a 2.2-fold risk of 90-day mortality (unadjusted HR, 2.20; 95% CI, 1.32–3.66) compared with that in the reference group. Patients with measured hypernatremia also had a 4.5-fold risk of 90-day mortality (unadjusted HR, 4.56; 95% CI, 2.56–8.14). To adjust for the possible confounders, we used multivariate analysis, adjusting for age and sex in Model 2; age, sex, glucose, RR, SBP, PR, WBC count, Hb, CRP, BUN, and creatinine in Model 3. Patients with measured normonatremia and measured hypernatremia remained as independent risk factors for 90-day mortality in Model 3. However, patients with severe measured hyponatremia did not show increased risk of 90-day mortality in any models.
Table 2.
Hazard ratios for 90-day mortality according to the measured sodium level
| Measured sodium | N | Mortality (%) | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|---|
| Total | 755 | 130 (17.2) | ||||
| Severe hyponatremia | ≤ 125 | 128 | 22 (17.2) | 1.66 (0.93–2.98) | 1.69 (0.94–3.04) | 1.67 (0.84–3.32) |
| Moderate hyponatremia | 126–130 | 212 | 23 (10.8) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Mild hyponatremia | 131–134 | 166 | 21 (12.7) | 1.19 (0.66–2.16) | 1.18 (0.65–2.13) | 1.40 (0.72–2.72) |
| Normonatremia | 135–145 | 191 | 41 (21.5) | 2.20 (1.32–3.66) | 2.04 (1.22–3.41) | 2.10 (1.16–3.81) |
| Hypernatremia | ≥ 146 | 58 | 23 (39.7) | 4.56 (2.56–8.14) | 4.00 (2.22–7.18) | 2.86 (1.39–5.87) |
We further analyzed the corrected sodium levels according to blood glucose levels (Table 3). Patients with low corrected normonatremia (135–139.9 mmol/L) had the lowest 90-day mortality (9.2%) and was defined as the reference group. Patients with corrected hyponatremia and severe corrected hypernatremia had significantly higher 90-day mortality compared with that in the reference group, with unadjusted HR of 4.87 (95% CI, 2.18–10.87) and 3.38 (95% CI, 1.76–6.47), respectively. After adjustment for possible confounders, patients with low corrected sodium levels and high corrected sodium levels both remained as independent factors for 90-day mortality. Figure 1A, B shows the Kaplan–Meier survival curves for patient groups with different measured sodium levels and corrected sodium levels, respectively. The p values for the log-rank test of equality over the strata were both < 0.001.
Table 3.
Hazard ratios for 90-day mortality according to the corrected sodium level
| Corrected sodium | N | Mortality (%) | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|---|
| Total | 755 | 130 (17.2) | ||||
| Hyponatremia | <135 | 34 | 13 (38.2) | 4.87 (2.18–10.87) | 4.45 (1.99–9.95) | 3.56 (1.44–8.80) |
| Low normonatremia | 135–139.9 | 120 | 11 (9.2) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| High normonatremia | 140–144.9 | 251 | 31 (12.4) | 1.37 (0.69–2.72) | 1.32 (0.66–2.64) | 1.31 (0.63–2.69) |
| Mild hypernatremia | 145–149.9 | 157 | 23 (14.6) | 1.68 (0.82–3.44) | 1.63 (0.79–3.35) | 2.40 (1.11–5.15) |
| Severe hypernatremia | ≥ 150 | 193 | 52 (26.9) | 3.38 (1.76–6.47) | 3.02 (1.56–5.84) | 2.68 (1.28–5.62) |
Model 1: unadjusted; Model 2: adjusted for age/sex; Model 3: Adjusted for age, sex, glucose, respiratory rate, systolic blood pressure, pulse rate, white blood cell, hemoglobin, C-reactive protein, blood urea nitrogen, creatinine.
Fig. 1.
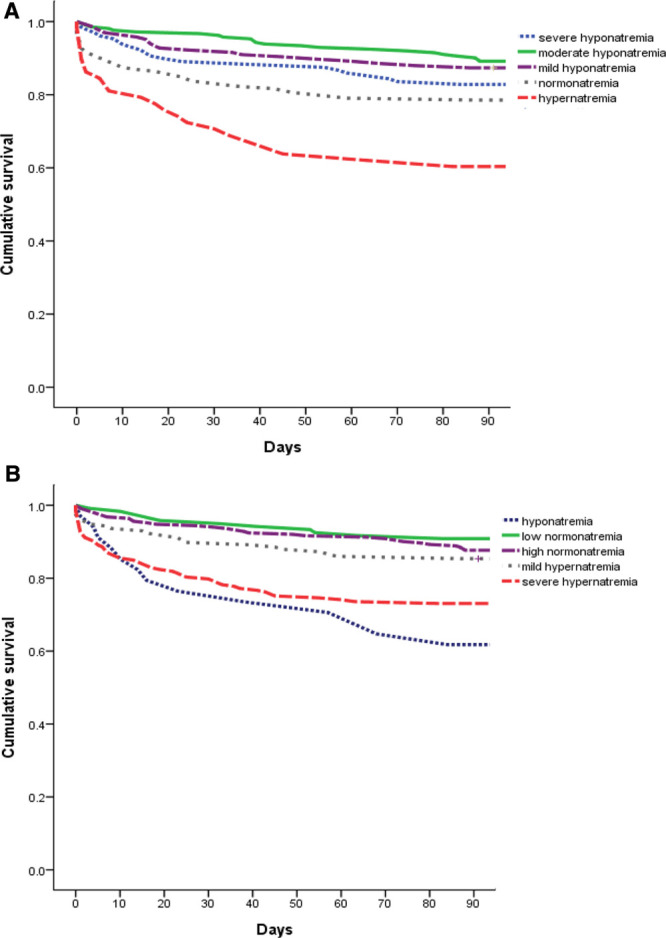
A, Kaplan–Meier curves of unadjusted cumulative survival according to the measured sodium level. The participants were categorized into five groups for measured sodium levels, and defined as severe measured hyponatremia (Na ≤ 125 mmol/L), moderate measured hyponatremia (Na = 126–130 mmol/L), mild measured hyponatremia (Na = 131–134 mmol/L), measured normonatremia (Na = 135–145 mmol/L), and measured hypernatremia (Na ≥ 146 mmol/L). There was a significant trend toward decreasing survival from the moderate measured hyponatremia to the measured hypernatremia (log-rank test for trend p < 0.001). B, Kaplan–Meier curves of unadjusted cumulative survival according to the corrected sodium level. The participants were categorized into five groups for corrected sodium levels, and defined as corrected hyponatremia (corrected Na <135 mmol/L), low corrected normonatremia (corrected Na = 135–139.9 mmol/L), high corrected normonatremia (corrected Na = 140–144.9 mmol/L), mild corrected hypernatremia (corrected Na = 145–149.9 mmol/L), and severe corrected hypernatremia (corrected Na ≥150 mmol/L). There was a significant trend toward decreasing survival from the low corrected normonatremia to the severe corrected hypernatremia (log-rank test for trend p < 0.001). Furthermore, corrected hyponatremia had higher risk of 90-day mortality compared with low corrected normonatremia (log-rank test for p < 0.001).
3.3. Secondary clinical outcomes
Figure 2 shows the HRs of secondary outcomes according to the measured and corrected sodium levels. Patients with severe measured hyponatremia were associated with increased risk of ICU admission, with HR of 2.18 (95% CI, 1.45–3.29). Patients with corrected hyponatremia were associated with increased risk of ICU admission (HR, 3.49; 95% CI, 1.99–6.12), respiratory failure (HR, 3.98; 95% CI, 1.57–10.09), and renal failure (HR, 3.56; 95% CI, 1.19–10.62). Patients with measured hypernatremia were associated with increased risk of ICU admission (HR, 5.60; 95% CI, 3.60–8.71) and respiratory failure (HR, 5.96; 95% CI, 3.25–10.95). Patients with severe corrected hypernatremia were associated with increased risk of ICU admission (HR, 3.67; 95% CI, 2.63–5.13) and respiratory failure (HR, 4.05; 95% CI, 2.05–7.99).
Fig. 2.
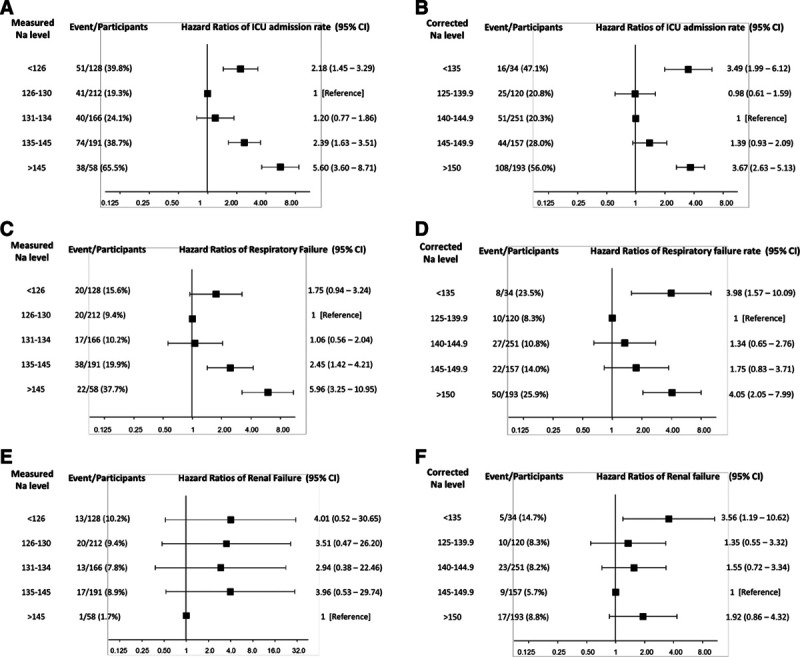
The hazard ratios (HRs) of secondary outcomes according to the measured and corrected sodium level. HRs for ICU admission rate according to the (A) measured and (B) corrected sodium level. HRs for respiratory failure rate according to the (C) measured and (D) corrected sodium level. HRs for renal failure rate according to the (E) measured and (F) corrected sodium level. The reference group was defined as the one with lowest events rate. The HRs with 95% confidence intervals are presented without adjustment.
4. DISCUSSION
This study demonstrated the relationship between measured/corrected sodium levels and clinical outcomes in extremely hyperglycemic patients. Measured hypernatremia, corrected hyponatremia, and corrected hypernatremia were independently associated with higher 90-day mortality. However, the patients with severe measured hyponatremia did not show increased risk of 90-day mortality in any models. To our knowledge, this is the first study to describe the relationship between low corrected sodium levels for hyperglycemia and clinical outcomes in patients with severe hyperglycemia. If we only use measured sodium level to predict clinical outcomes, we may overlook the clinical impact of true hyponatremia.
Serum sodium concentration is measured routinely for the majority of medical patients. A Danish cohort study showed hyponatremia increased 30-day mortality in patients admitted to the Department of Internal Medicine, regardless of comorbidities, with adjusted risk ratio of 1.3–1.7.5 However, the sodium levels reported in the article were the measured levels on arrival, not adjusted according to the glucose levels. Previous studies also demonstrated that hyponatremia is strongly and independently associated with poor prognosis and in-hospital mortality in a variety of diseases such as chronic kidney disease, congestive heart failure, liver cirrhosis, acute ischemic stroke, and intracerebral hemorrhage.7–11,17–19 In congestive heart failure and cirrhosis, hyponatremia may reflect more severe activation of the renin–angiotensin–aldosterone system or the sympathetic nervous system and/or vasopressin release.8 However, the mechanism of relationship between hyponatremia and poorer outcomes remains unclear in patients with other conditions. Hyperglycemia causes hyperosmolality, and the water moves from intracellular space to extracellular space, which in turn produces a dilutional decrease in serum sodium level. Therefore, hyperglycemic patients are mostly mildly hyponatremic.4 A previous study showed that hyponatremia did not increase the mortality in patients with severe hyperglycemia.14 In the present study, patients with moderate measured hyponatremia had the lowest mortality rate, which further supported the notion that these patients might have the ability to compensate for the change in osmolality. However, parts of patients with measured hyponatremia had corrected hyponatremia after adjustment with blood glucose, which might increase risk of 90-day mortality, with HR 3.56 (95% CI, 1.44–8.80). This phenomenon might imply that the corrected hyponatremia was likely to be “true hyponatremia” in extremely hyperglycemic patients, associated with higher risk of 90-day mortality irrespective of underlying disease, as previously reported. To our knowledge, this is the first study to describe the relationship between low corrected sodium levels for hyperglycemia and clinical outcomes in patients with severe hyperglycemia. If we only use measured sodium level to predict clinical outcomes, we may overlook the clinical impact of true hyponatremia.
Another study recognized hypernatremia on admission as a predictor for mortality in hyperglycemic crisis.20 The possible reason is that hypernatremia indicates that the patient loses the ability to compensate for the osmolality and severe dehydration, especially in patients with osmotic diuresis and inadequate fluid replacement. Dehydration is a prominent feature of hyperglycemia–hyperosmolality states. Hyperglycemia induces osmotic diuresis, which results in intravascular volume depletion and then high serum osmolality. Volume status could be objectively assessed by blood pressure, PR, osmolality, and ratio of serum urea nitrogen to creatinine.21 Previous studies have shown that dehydration is a prognostic factor for mortality.20,22 In our study, patients with severe corrected hypernatremia were older and had higher PR, lower SBP, and higher osmolality level, which were compatible with the dehydration status. Patients with severe corrected hypernatremia had higher rates of respiratory failure and ICU admission. The ICU admission rate in this group was as high as 56.0%. The possible explanation is that dehydration might increase the viscosity of respiratory secretions and lead to respiratory compromise.22 In other words, dehydration is simply a marker for the severity of disease and the ability of overall homeostatic mechanisms to compensate for the severe hyperglycemic status.
It is notable that the sodium levels described in previous studies were not adjusted according to glucose levels. Our study is the first to discuss sodium correction for hyperglycemia. The mean measured sodium level in patients with severe corrected hypernatremia was 142.9 ± 9.1 mmol/L. This is a reminder to clinicians that even when the measured sodium level is within the normal limit, it is important to calculate the corrected sodium level according to the glucose level. Based on the observation in the present study, dysnatremia after correction could be directly interpreted as a poor prognostic factor.
Our study has some limitations. First, this is a retrospective study, and the analyses were mostly based on the data upon ED arrival. Whether treatments or changes to sodium levels during the hospital stay impacted outcomes is not known. Second, this study was conducted in a tertiary medical center; thus, the result may not be applicable universally. Third, we did not collect information of some possible confounding factors that may have influenced serum sodium levels or mortality rate, including underlying diseases, medications, and certain laboratory tests not conducted routinely, such as lactate and blood gas levels. These factors might have affected the results. Fourth, we did not analyze the cause of mortality between the groups due to scanty information on mortality events. The all-cause 90-day mortality rate was 17.2%, which was similar to that of previous reports.13,23 Finally, the correlation between corrected sodium level and 90-day mortality was U shape instead of linear shape. Therefore, area under the curve of receiver operating characteristic curve might not be appropriate to compare the diagnostic performance between serum glucose levels, measured sodium levels, and corrected sodium levels. Further multicenter studies with larger sample sizes and comprehensive clinical databases are needed to demonstrate the relationship between sodium levels and clinical outcomes.
In conclusion, dysnatremia after correction by serum glucose level is associated with worse clinical outcomes and higher 90-day mortality rate in hyperglycemic patients. Corrected sodium level is a better indicator of prognosis compared with measured sodium, as the former could be easily interpreted based on usual laboratory references. Further well-designed case–control studies are warranted to confirm the relationship.
ACKNOWLEDGMENTS
The study was sponsored in part by Taipei Veterans General Hospital (V102C-043 and V105C-161)
APPENDIX A. SUPPLEMENTARY DATA
Supplementary data related to this article can be found at http://doi.org/10.1097/JCMA.0000000000000264.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest related to the subject matter or materials discussed in this article.
References
- 1.Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med 2006;1197 Suppl 1S30–5. [DOI] [PubMed] [Google Scholar]
- 2.Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med 2000;342:1493–9. [DOI] [PubMed] [Google Scholar]
- 3.Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med 1999;106:399–403. [DOI] [PubMed] [Google Scholar]
- 4.Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases 2014;2:488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland-Bill L, Christiansen CF, Heide-Jørgensen U, Ulrichsen SP, Ring T, Jørgensen JO, et al. Hyponatremia and mortality risk: a Danish cohort study of 279 508 acutely hospitalized patients. Eur J Endocrinol 2015;173:71–81. [DOI] [PubMed] [Google Scholar]
- 6.Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 2010;170:294–302. [DOI] [PubMed] [Google Scholar]
- 7.Angeli P, Wong F, Watson H, Ginès P; CAPPS Investigators Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology 2006;44:1535–42. [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O’Connor CM, et al. ; OPTIMIZE-HF Investigators and Coordinators Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J 2007;28:980–8. [DOI] [PubMed] [Google Scholar]
- 9.Huang H, Jolly SE, Airy M, Arrigain S, Schold JD, Nally JV, et al. Associations of dysnatremias with mortality in chronic kidney disease. Nephrol Dial Transplant 2017;32:1204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008;359:1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuramatsu JB, Bobinger T, Volbers B, Staykov D, Lücking H, Kloska SP, et al. Hyponatremia is an independent predictor of in-hospital mortality in spontaneous intracerebral hemorrhage. Stroke 2014;45:1285–91. [DOI] [PubMed] [Google Scholar]
- 12.Chen HF, Wang CY, Lee HY, See TT, Chen MH, Jiang JY, et al. Short-term case fatality rate and associated factors among inpatients with diabetic ketoacidosis and hyperglycemic hyperosmolar state: a hospital-based analysis over a 15-year period. Intern Med 2010;49:729–37. [DOI] [PubMed] [Google Scholar]
- 13.Chung ST, Perue GG, Johnson A, Younger N, Hoo CS, Pascoe RW, et al. Predictors of hyperglycaemic crises and their associated mortality in Jamaica. Diabetes Res Clin Pract 2006;73:184–90. [DOI] [PubMed] [Google Scholar]
- 14.Guo YW, Wu TE, Chen HS. Prognostic factors of mortality among patients with severe hyperglycemia. Am J Manag Care 2015;21:e9–e22. [PubMed] [Google Scholar]
- 15.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32:1335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purssell RA, Pudek M, Brubacher J, Abu-Laban RB. Derivation and validation of a formula to calculate the contribution of ethanol to the osmolal gap. Ann Emerg Med 2001;38:653–9. [DOI] [PubMed] [Google Scholar]
- 17.Chiu DY, Kalra PA, Sinha S, Green D. Association of serum sodium levels with all-cause and cardiovascular mortality in chronic kidney disease: results from a prospective observational study. Nephrology (Carlton) 2016;21:476–82. [DOI] [PubMed] [Google Scholar]
- 18.Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation 2012;125:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues B, Staff I, Fortunato G, McCullough LD. Hyponatremia in the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis 2014;23:850–4. [DOI] [PubMed] [Google Scholar]
- 20.Anthanont P, Khawcharoenporn T, Tharavanij T. Incidences and outcomes of hyperglycemic crises: a 5-year study in a tertiary care center in Thailand. J Med Assoc Thai 2012;95:995–1002. [PubMed] [Google Scholar]
- 21.Schrock JW, Glasenapp M, Drogell K. Elevated blood urea nitrogen/creatinine ratio is associated with poor outcome in patients with ischemic stroke. Clin Neurol Neurosurg 2012;114:881–4. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson T, Bennett K, Silke B. Serum osmolarity as an outcome predictor in hospital emergency medical admissions. Eur J Intern Med 2012;23:e39–43. [DOI] [PubMed] [Google Scholar]
- 23.Nyenwe EA, Kitabchi AE. Evidence-based management of hyperglycemic emergencies in diabetes mellitus. Diabetes Res Clin Pract 2011;94:340–51. [DOI] [PubMed] [Google Scholar]


