Abstract
Background:
Fabry disease (FD) is an X-linked lysosomal storage disorder engendered by a deficiency of the enzyme α-galactosidase A, leading to systemic accumulation of glycolipids. Studies have reported that the cardiac subtype of FD has a later onset and minimal extracardiac involvement. However, whether the severity of cardiac involvement differs between the classic and cardiac subtypes of FD remains unclear.
Methods:
We enrolled consecutive patients with classic FD (n = 22; median age [25th–75th percentile], 47.0 [32.75–56.25] years; men, 72.7%) as well as age- and sex-matched patients with a later-onset cardiac subtype of FD who were selected from our cohort of patients with IVS4 919G>A mutation. FD was diagnosed on the basis of clinical symptoms/signs and pedigree screening of index case, plasma α-galactosidase activity, and molecular analysis. Data on clinical manifestations, laboratory findings, and echocardiogram findings were collected before enzyme replacement treatment. Disease severity was evaluated using the Mainz Severity Score Index score.
Results:
All female patients demonstrated heterozygous mutations, with five, one, and four of them showing normal α-galactosidase activity, classic FD, and cardiac subtype of FD, respectively. The distributions of left ventricular performance indices and comorbidities, including hypertension, diabetes mellitus, and dyslipidemia, were similar between the two groups. Moreover, MSSI cardiovascular scores did not differ significantly between the groups (classic vs cardiac subtype, 10.0 [2.0–12.5] vs 10.5 [9.0–15.25]; p = 0.277).
Conclusion:
Cardiac manifestations are similar between patients with classic and cardiac subtype of FD.
Keywords: Anderson–Fabry disease, Cardiac variant, Transthoracic echocardiography
1. INTRODUCTION
Fabry disease (FD), a genetic disease initially described in 1898, is an X-linked lysosomal storage disorder induced by a deficiency of the enzyme α-galactosidase A (α-GLA); a deficiency of α-GLA results in systemic accumulation of glycolipids, particularly globotriaosylsphingosine (Gb3), in multiple organs, including the cardiac, neural, and renal systems.1,2 The estimated incidence of FD ranges from 1 in 40,000 to 1 in 117,000 worldwide.3 Patients with classic FD show <1% α-galactosidase activity and typically present with symptoms such as neuropathic pain, cornea verticillata, and angiokeratoma right from their childhood or adolescence. Manifestations including hypertrophic cardiomyopathy, cardiac rhythm disturbance, progressive renal failure, and stroke constitute the long-term indications of FD.4 In Taiwan, a high incidence of a cardiac subtype of FD with a special mutation (IVS4 919G>A) was discovered after newborn screening.5 Its manifestations include concentric left ventricular hypertrophy (LVH), valvular involvement, and arrhythmias in the fifth to eighth decades of life, with minimal extracardiac involvement. However, the question as to whether the severity of cardiac involvement differs between these two types of FD remains unclear. Accordingly, we conducted this study to investigate this question by using echocardiographic findings, amino-terminal pro-brain natriuretic peptide (NT-pro-BNP) levels, and the Mainz Severity Score Index (MSSI). MSSI is a clinical scoring system that is used to analyze the severity of FD and monitor its clinical course in response to enzyme replacement therapy (ERT).6
2. METHODS
We performed the study in accordance with the guidelines of the Declaration of Helsinki. The protocols were approved by an institutional review board and written informed consent was obtained from patients before participation.
We enrolled consecutive patients with classic FD (n = 22; median age, 47.0 [32.75–56.25] years; men, 72.7%); we also selected age- and sex-matched patients with the cardiac subtype of FD from our cohort of patients with the IVS4 + 919G>A mutation (n = 22; median age, 49.5 [33.75–57.0] years; men, 72.7%). FD was diagnosed on the basis of clinical symptoms/signs and pedigree screening of index case, plasma α-galactosidase activity, and molecular analysis of GLA. All female patients demonstrated heterozygous mutations, with five, one, and four of them showing normal range of α-galactosidase activity, classic FD, and cardiac subtype of FD, respectively.
We recorded data on clinical characteristics, including signs and symptoms, biochemical tests, and echocardiography findings, before the patients underwent ERT. Because the most common presentation, LVH, is usually symptomless initially, the disease duration is not precise, particularly in the cardiac subtype. Therefore, we used age-matched patients in this study to ensure the same duration of enzyme deficiency in both groups and thus minimize potential bias.
The MSSI cardiovascular score was used to assess disease severity. MSSI comprises four components that cover the general, neurological, cardiovascular, and renal signs and symptoms of FD. Different specialists, including a dermatologist, neurologist, ophthalmologist, and cardiologist, recorded a detailed medical history of and performed clinical investigations on the patients. The maximum MSSI score for the general and renal components is 18, and that for the neurological and cardiovascular components is 20. The sum of the scores for these individual components constitutes the total MSSI score. The severity of FD can be divided into three categories according to the total MSSI score: mild, <20; moderate, 20–40; and severe, >40.6
According to the recommendations of the American Society of Echocardiography, we measured left ventricular parameters, including left ventricular mass (LVM), diastolic interventricular septal thickness, systolic and diastolic left ventricular internal diameter, and diastolic left ventricular posterior wall thickness, by using serial two-dimensional guided M-mode echocardiography.7,8 LVM was calculated using the American Society of Echocardiography simplified cubed equation.
All results are reported as median (25th–75th percentile) and numbers (%). Variables were compared using the Wilcoxon rank-sum test or Pearson’s χ2 test, as appropriate. Statistical analyses were performed using SPSS version 19.0 (SPSS, Chicago, IL, USA). Statistical significance was set at p < 0.05.
3. RESULTS
Table 1 presents the baseline characteristics of the patients in the two groups. The patients were adequately matched for age and sex. Comorbidities, including hypertension, diabetes mellitus, and dyslipidemia, showed similar distributions between the classic FD and cardiac subtype groups; however, the frequency of smoking was higher in the cardiac subtype group (classic type vs cardiac subtype: 4.5% vs 18.2%; p = 0.039). Moreover, blood pressure levels were similar between the two groups; nevertheless, the resting heart rate was higher in the cardiac subtype group (classic type vs cardiac subtype: median, 59.6 [25th–75th percentile: 55.8–62.6] vs 64.9 [25th–75th percentile: 58.7–75.2]; p = 0.025). No significant differences existed between the two groups in terms of renal function parameters, lipid profile, or NT-pro-BNP levels. Table 2 lists cardiac structure and function indices investigated using echocardiography, including the left ventricular dimension in the end diastolic and systolic phases, left ventricular septal and posterior wall thickness, ejection fraction, LVM, left atrial dimension, pulmonary arterial systolic pressure, and tricuspid annular plane systolic excursion; we noted that these indices were comparable between the two groups. One patient in each group underwent permanent pacemaker implantation.
Table 1.
Baseline characteristics of patients with classical type vs cardiac subtype
| Demographic data | Classical subjects N = 22 | Cardiac subjects N = 22 | p |
|---|---|---|---|
| N (%) | N (%) | ||
| Age (y) | 47.0 (32.75–56.25) | 49.5 (33.75–57.0) | 0.655 |
| Gender | |||
| Male | 16 (72.7) | 16 (72.7) | 1.000 |
| Female | 6 (27.3) | 6 (27.3) | |
| Laboratory data | |||
| Systolic BP (mmHg) | 114.0 (105.5–128.25) | 120.5 (106.0–146.75) | 0.291 |
| Diastolic BP (mmHg) | 68.5 (64.125–71.625) | 74.5 (63.5–86.0) | 0.093 |
| Heart rate | 59.6 (55.8–62.6) | 64.9 (58.7–75.2) | 0.025 |
| Blood urea nitrogen | 12.0 (11.0–16.25) | 15.0 (13.0–16.0) | 0.247 |
| Creatinine | 0.81 (0.65–0.9475) | 0.81 (0.68–0.98) | 0.979 |
| eGFR (mL/min/1.73 m2) | 83.6 (59.8–112.2) | 86.1 (66.4–102.3) | 0.937 |
| Cholesterol (mg/dL) | 184.0 (140.0–244.0) | 187.0 (155.5–198.0) | 0.490 |
| Triglyceride (mg/dL) | 79 (53.0–152.0) | 127.5 (74.25–226.0) | 0.117 |
| HDL-cholesterol (mg/dL) | 55.5 (52.75–59.0) | 50.0 (44.5–60.5) | 0.163 |
| LDL-cholesterol (mg/dl) | 113.5 (82.0–149.5) | 104.0 (88.5–127.0) | 0.546 |
| NT-proBNP (pg/mL) | 145.8 (24.71–1341) | 42.7 (19.2–156.4) | 0.316 |
| Comorbidities | |||
| Hypertension | 9 (40.9) | 10 (45.5) | 0.223 |
| Diabetes mellitus | 1 (4.5) | 2 (9.1) | 0.554 |
| Dyslipidemia | 5 (22.7) | 1 (4.5) | 0.082 |
| Smoking | 1 (4.5) | 4 (18.2) | 0.039 |
The data were presented as median (25th percentile–75th percentile).
BP = blood pressure; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; LDL = low-density lipoprotein; NT-proBNP = N-terminal pro-Brain Natriuretic Peptide.
Table 2.
Echocardiographic findings of patients with classical type vs cardiac subtype
| Demographic data | Classical subjects N = 22 | Cardiac subjects N = 22 | p |
|---|---|---|---|
| Echocardiographic findings | |||
| Aortic root (mm) | 26.5 (26.0–30.0) | 29.0 (26.0–30.25) | 0.171 |
| IVSTd (mm) | 11.0 (9.0–14.0) | 10.0 (8.0–13.25) | 0.645 |
| PWTd (mm) | 11.5 (10.0–13.0) | 10.5 (8.0–13.75) | 0.554 |
| LVIDd (mm) | 45.0 (41.0–49.25) | 45.5 (41.25–50.25) | 0.503 |
| LVIDs (mm) | 26.0 (23.0–32.0) | 27.5 (25.0–32.0) | 0.823 |
| LVEDV (mL) | 90.0 (74.0–116.0) | 96.0 (77.5–120.0) | 0.883 |
| LVESV (mL) | 25.0 (18.0–44.0) | 30.0 (22.5–39.0) | 0.376 |
| LVEF (%) | 70.0 (66.75–75.25) | 68.0 (60.5–73.8) | 0.496 |
| LV mass (g) | 191.5 (130.5–246.75) | 154.0 (121.5–225.0) | 0.882 |
| LV mass index (g/m2) | 115.8 (81.5–138.5) | 90.1 (66.9–133.9) | 0.918 |
| Left atrium (mm) | 34.0 (30.75–36.5) | 32.8 (27.0–36.5) | 0.417 |
| PASP (mmHg) | 24.0 (21.0–29.0) | 22.0 (13.0–25.0) | 0.053 |
| TAPSE (mm) | 2.0 (2.0–3.0) | 2.0 (2.0–2.0) | 0.221 |
IVSTd = interventricular septum thickness in end-diastole; PWTd = posterior wall thickness in enddiastole; LVIDd = left ventricular internal dimension in end-diastole; LVIDs = left ventricular internal dimension in end-systole; LVEDV = left ventricular end-diastole volume; LVESV = left ventricular endsystole volume; LVEF = left ventricular ejection fraction; PASP = pulmonary arterial systolic pressure; TAPSE = tricuspid annular plane systolic excursion.
The total MSSI score was significantly higher for the classic FD group compared with the cardiac subtype group. This was because the classic FD group had higher scores for the general (classic type vs cardiac subtype; median, 1.0 [25th–75th percentile: 0.0–3.0] vs 0.0 [25th–75th percentile: 0.0–1.0]; p = 0.001), neurological (classic type vs cardiac subtype: median, 5.0 [25th–75th percentile: 0.0–6.0] vs 0.0 [25th–75th percentile: 0.0–1.5]; p = 0.002), and renal (classic type vs cardiac subtype: median, 4.0 [25th–75th percentile: 0.0–4.0] vs 0.0 [25th–75th percentile: 0.0–0.0]; p = 0.007) components. The scores for the cardiovascular component did not differ significantly between the two groups (classic type vs cardiac subtype: median, 10.0 [25th–75th percentile: 2.0–12.5] vs 10.5 [25th–75th percentile: 9.0–15.25]; p = 0.277; Table 3 and Figure 1).
Table 3.
Mainz Severity Score Index of patients with classical type vs cardiac subtype
| Demographic data | Classical subjects N = 22 | Cardiac subjects N = 22 | p |
|---|---|---|---|
| General score | 1.0 (0.0–3.0) | 0.0 (0.0–1.0) | 0.001 |
| Characteristic facial | 0 (0) | 0 (0) | 1.000 |
| Angiokeratoma | 2 (9.1) | 0 (0) | 0.148 |
| Edema | 2 (9.1) | 0 (0) | 0.148 |
| Musculoskeletal | 4 (18.2) | 0 (0) | 0.036 |
| Cornea verticillata | 7 (31.8) | 0 (0) | 0.004 |
| Diaphoresis | 0.060 | ||
| Hypo/hyperhidrosis | 1 (4.5) | 0 (0) | |
| Anhidrosis | 4 (18.2) | 0 (0) | |
| Abdominal pain | 1 (4.5) | 0 (0) | 0.312 |
| Diarrhea/constipation | 1 (4.5) | 0 (0) | 0.312 |
| Hemorrhoids | 0 (0) | 0 (0) | 1.000 |
| Pulmonary | 2 (9.1) | 1 (4.5) | 0.550 |
| NYHA | 0.769 | ||
| Class I | 4 (18.2) | 6 (27.3) | |
| Class II | 1 (4.5) | 1 (4.5) | |
| Class III | 0 (0) | 0 (0) | |
| Class IV | 0 (0) | 0 (0) | |
| Appearance | |||
| Neurologic score | 5.0 (0.0–6.0) | 0.0 (0.0–1.5) | 0.002 |
| Tinnitus | 0 (0) | 1 (4.5) | 0.312 |
| Vertigo | 2 (9.1) | 1 (4.5) | 0.550 |
| Acroparesthesia | |||
| Occasional | 6 (27.3) | 3 (13.6) | <0.001 |
| Chronic | 9 (40.9) | 0 (0) | |
| Fever pain crisis | 4 (18.2) | 0 (0) | 0.036 |
| Cerebrovascular | 0 (0) | 2 (9.1) | 0.148 |
| Psychiatric | |||
| Depression | 0 (0) | 0 (0) | 1.000 |
| Fatigue | 1 (4.5) | 1 (4.5) | 1.000 |
| Reduced activity level | 0 (0) | 2 (9.1) | 0.148 |
| Cardiovascular score | 10.0 (2.0–12.5) | 10.5 (9.0–15.25) | 0.277 |
| Left ventricle hypertrophy | |||
| Thickening of wall | 1 (4.5) | 0 (0) | 0.197 |
| LVH seen in ECG | 3 (13.6) | 0 (0) | |
| Cardiomyopathy (<15 mm) | 9 (40.9) | 11 (50) | |
| Severe cardiomyopathy (>15 mm) | 4 (18.2) | 8 (36.4) | |
| ECG abnormalities | 13 (59.1) | 11 (50) | 0.545 |
| Hypertension | 9 (40.9) | 13 (59.1) | 0.228 |
| Valve insufficiency | 20 (90.9) | 19 (86.4) | 0.635 |
| Pacemaker | 1 (4.5) | 1 (4.5) | 1.000 |
| Renal score | 4.0 (0.0–4.0) | 0.0 (0.0–0.0) | 0.007 |
| Proteinuria | 10 (45.5) | 3 (13.6) | 0.021 |
| Low GFR | 2 (9.1) | 1 (4.5) | 0.550 |
| Creatinine > 3.5 mg/dL | 0 (0) | 0 (0) | 1.000 |
| Dialysis | 1 (4.5) | 0 (0) | 0.312 |
| Total score | 17.5 (13.25–23.0) | 14.5 (9.75–18.0) | 0.051 |
We expressed the individual parameter with number (percentage) for categorical variables and median (25th percentile–75th percentile) for numerical variables.
GFR = glomerular filtration rate; LVH = left ventricular hypertrophy.
Fig. 1.
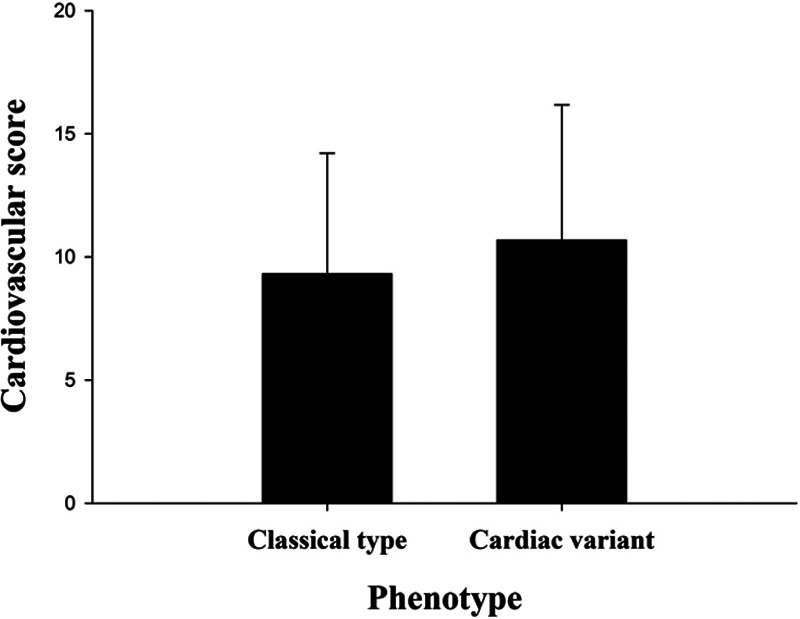
Cardiovascular subscore of the Mainz Severity Score Index (MSSI) in patients with classic and cardiac subtypes of Fabry disease. The error bars represent standard deviation.
Regarding the general component, the classic FD group exhibited a higher prevalence of cornea verticillata (classic type vs cardiac subtype, 7 [31.8] vs 0 [0]; p = 0.004) and musculoskeletal disorders (classic type vs cardiac subtype, 4 [18.2] vs 0 [0]; p = 0.036). Furthermore, concerning the neurological component, the classic FD group had a higher prevalence of acroparesthesia (classic type vs cardiac subtype, 15 [68.2] vs 3 [13.6]; p < 0.001) and fever/pain crisis (classic type vs cardiac subtype, 4 [18.2] vs 0 [0]; p = 0.036). Regarding the renal component, the classic FD group exhibited more severe proteinuria (classic type vs cardiac subtype, 10 [45.5] vs 3 [13.6]; p = 0.021).
4. DISCUSSION
Our study’s most noteworthy finding is that cardiovascular manifestations were similar between the classic and cardiac subtype of FD according to echocardiographic findings, NT-pro-BNP levels, and MSSI scores.
FD is an X-linked lysosomal storage disorder resulting from a deficiency of the enzyme α-GLA, which leads to a systemic accumulation of glycolipids in major organs, particularly in the vascular endothelium and smooth muscle cells of the renal and cardiovascular systems.9,10 Classic symptoms observed in hemizygous male patients include acroparesthesia, angiokeratoma, hypohidrosis, corneal opacities, and dysfunction of the kidney, brain, and heart. The cardiac manifestations of FD include LVH,11 valvular involvement,12 and arrhythmias,13 which usually present in the fifth to eighth decades of life. Atypical variants of FD that occur because of residual low levels of α-GLA activity, resulting in a “milder” and later-onset phenotype,14 are being increasingly recognized. We demonstrated that the degrees of severity of cardiac manifestations were similar between the classic FD and cardiac subtype groups, as indicated by echocardiographic findings and MSSI scores. A previous study revealed that the MSSI score is a highly specific tool for distinguishing FD from other severe afflictions and that it could be used to evaluate disease severity.6 The severity of FD (MSSI) is significantly correlated with age.15,16
The increasing frequency of later-onset mutations detected through neonatal screening may render FD a broad cardiovascular problem that extends beyond childhood. In general, cardiac manifestations of FD, such as arrhythmia, angina, and LVH, are considered to result from Gb3 accumulation in the sinoatrial node, conduction system, vascular endothelium, and cardiomyocytes. Moreover, the more severe the α-Gal A defect is, the more severe the clinical manifestations are and the earlier the disease onset becomes. Our findings suggest that Gb3 accumulation is not the only factor determining the clinical symptoms of FD.17 A previous study revealed that glycolipid accumulation in the myocardium constitutes only 1%–3% of the total mass in a hypertrophic heart.18 Other pathogenic factors such as inflammatory cytokines19–22and oxidative stress may also contribute to clinical manifestations.19,23–25
With the availability of ERT as a possible treatment, cardiac disease has become the leading cause of death in patients with FD.26,27 Improvement in renal care and early initiation of ERT can reduce the impact of renal disease as a cause of death in patients with FD.28,29 Previous research reported that early ERT also led to favorable outcomes in patients with Fabry cardiomyopathy.30 Moreover, a study indicated that late diagnosis was a factor for early death in patients with FD.27 Although the level of cardiac involvement in the cardiac subtype of FD is similar to that in classic FD, the cardiac subtype of FD is usually diagnosed late because of minimal extracardiac involvement.31 Therefore, early diagnosis, careful evaluation of disease progression, and early initiation of therapy might be crucial to improve the outcomes of patients with FD of the cardiac subtype.
This study has some limitations. First, our sample size was small because of the limited number of patients with classic FD. The rare nature of the disease contributed to the small sample size, although we included all consecutive cases of classic FD. Second, classic FD is usually diagnosed early because of extracardiac manifestations. However, we chose age- and sex-matched patients with cardiac subtype of FD to reduce potential confounding factors.
In conclusion, our results demonstrate no significant differences between the classic and cardiac subtypes of FD in terms of echocardiographic findings or MSSI cardiovascular scores. Therefore, we suggest that the levels of severity of cardiac involvement are similar in both subtypes of FD. These findings should be considered in the clinical evaluation and treatment of FD.
ACKNOWLEDGMENTS
This study was supported in part by intramural grants from the Taipei Veterans General Hospital (Grant No. V101C-187, V103C-166, and V104C-175) and grants in aid from the Research Foundation of Cardiovascular Medicine, (100-01-010), Taipei, Taiwan.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest related to the subject matter or materials discussed in this article.
REFERENCES
- 1.Eng CM, Niehaus DJ, Enriquez AL, Burgert TS, Ludman MD, Desnick RJ. Fabry disease: twenty-three mutations including sense and antisense CpG alterations and identification of a deletional hot-spot in the alpha-galactosidase A gene. Hum Mol Genet 1994;3:1795–9. [DOI] [PubMed] [Google Scholar]
- 2.Ferrans VJ, Hibbs RG, Burda CD. The heart in Fabry’s disease. A histochemical and electron microscopic study. Am J Cardiol 1969;24:95–110. [DOI] [PubMed] [Google Scholar]
- 3.Mehta A, Ricci R, Widmer U, Dehout F, Garcia de Lorenzo A, Kampmann C, et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest 2004;34:236–42. [DOI] [PubMed] [Google Scholar]
- 4.Arends M, Wanner C, Hughes D, Mehta A, Oder D, Watkinson OT, et al. Characterization of classical and nonclassical Fabry disease: a Multicenter Study. J Am Soc Nephrol 2017;28:1631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin HY, Chong KW, Hsu JH, Yu HC, Shih CC, Huang CH, et al. High incidence of the cardiac variant of Fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet 2009;2:450–6. [DOI] [PubMed] [Google Scholar]
- 6.Whybra C, Kampmann C, Krummenauer F, Ries M, Mengel E, Miebach E, et al. The Mainz Severity Score Index: a new instrument for quantifying the Anderson-Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet 2004;65:299–307. [DOI] [PubMed] [Google Scholar]
- 7.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450–8. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- 9.Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med 1967;276:1163–7. [DOI] [PubMed] [Google Scholar]
- 10.Desnick RJ, Allen KY, Desnick SJ, Raman MK, Bernlohr RW, Krivit W. Fabry’s disease: enzymatic diagnosis of hemizygotes and heterozygotes. Alpha-galactosidase activities in plasma, serum, urine, and leukocytes. J Lab Clin Med 1973;81:157–71. [PubMed] [Google Scholar]
- 11.Colucci WS, Lorell BH, Schoen FJ, Warhol MJ, Grossman W. Hypertrophic obstructive cardiomyopathy due to Fabry’s disease. N Engl J Med 1982;307:926–8. [DOI] [PubMed] [Google Scholar]
- 12.Desnick RJ, Blieden LC, Sharp HL, Hofschire PJ, Moller JH. Cardiac valvular anomalies in Fabry disease. Clinical, morphologic, and biochemical studies. Circulation 1976;54:818–25. [DOI] [PubMed] [Google Scholar]
- 13.Ikari Y, Kuwako K, Yamaguchi T. Fabry’s disease with complete atrioventricular block: histological evidence of involvement of the conduction system. Br Heart J 1992;68:323–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, et al. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med 2003;138:338–46. [DOI] [PubMed] [Google Scholar]
- 15.Franzen D, Gerard N, Bratton DJ, Wons A, Gaisl T, Sievi NA, et al. Prevalence and risk factors of sleep disordered breathing in Fabry disease: a prospective cohort study. Medicine (Baltimore) 2015;94:e2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ries M, Ramaswami U, Parini R, Lindblad B, Whybra C, Willers I, et al. The early clinical phenotype of Fabry disease: a study on 35 European children and adolescents. Eur J Pediatr 2003;162:767–72. [DOI] [PubMed] [Google Scholar]
- 17.Vedder AC, Linthorst GE, van Breemen MJ, Groener JE, Bemelman FJ, Strijland A, et al. The Dutch Fabry cohort: diversity of clinical manifestations and Gb3 levels. J Inherit Metab Dis 2007;30:68–78. [DOI] [PubMed] [Google Scholar]
- 18.Elleder M, Bradová V, Smíd F, Buděsínský M, Harzer K, Kustermann-Kuhn B, et al. Cardiocyte storage and hypertrophy as a sole manifestation of Fabry’s disease. Report on a case simulating hypertrophic non-obstructive cardiomyopathy. Virchows Arch A Pathol Anat Histopathol 1990;417:449–55. [DOI] [PubMed] [Google Scholar]
- 19.Biancini GB, Vanzin CS, Rodrigues DB, Deon M, Ribas GS, Barschak AG, et al. Globotriaosylceramide is correlated with oxidative stress and inflammation in Fabry patients treated with enzyme replacement therapy. Biochim Biophys Acta 2012;1822:226–32. [DOI] [PubMed] [Google Scholar]
- 20.De Francesco PN, Mucci JM, Ceci R, Fossati CA, Rozenfeld PA. Fabry disease peripheral blood immune cells release inflammatory cytokines: role of globotriaosylceramide. Mol Genet Metab 2013;109:93–9. [DOI] [PubMed] [Google Scholar]
- 21.Chien Y, Chien CS, Chiang HC, Huang WL, Chou SJ, Chang WC, et al. Interleukin-18 deteriorates Fabry cardiomyopathy and contributes to the development of left ventricular hypertrophy in Fabry patients with GLA IVS4 + 919 G>A mutation. Oncotarget 2016;7:87161–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen KH, Chien Y, Wang KL, Leu HB, Hsiao CY, Lai YH, et al. evaluation of proinflammatory prognostic biomarkers for Fabry cardiomyopathy with enzyme replacement therapy. Can J Cardiol 2016;32:1221.e1–9. [DOI] [PubMed] [Google Scholar]
- 23.Biancini GB, Moura DJ, Manini PR, Faverzani JL, Netto CB, Deon M, et al. DNA damage in Fabry patients: an investigation of oxidative damage and repair. Mutat Res Genet Toxicol Environ Mutagen 2015;784-785:31–6. [DOI] [PubMed] [Google Scholar]
- 24.Chen KH, Chou YC, Hsiao CY, Chien Y, Wang KL, Lai YH, et al. Amelioration of serum 8-OHdG level by enzyme replacement therapy in patients with Fabry cardiomyopathy. Biochem Biophys Res Commun 2017;486:293–9. [DOI] [PubMed] [Google Scholar]
- 25.Tseng WL, Chou SJ, Chiang HC, Wang ML, Chien CS, Chen KH, et al. Imbalanced production of reactive oxygen species and mitochondrial antioxidant SOD2 in Fabry disease-specific human induced pluripotent stem cell-differentiated vascular endothelial cells. Cell Transplant 2017;26:513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta A, Clarke JT, Giugliani R, Elliott P, Linhart A, Beck M, et al. Natural course of Fabry disease: changing pattern of causes of death in FOS – Fabry outcome survey. J Med Genet 2009;46:548–52. [DOI] [PubMed] [Google Scholar]
- 27.Waldek S, Patel MR, Banikazemi M, Lemay R, Lee P. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry Registry. Genet Med 2009;11:790–6. [DOI] [PubMed] [Google Scholar]
- 28.West M, Nicholls K, Mehta A, Clarke JT, Steiner R, Beck M, et al. Agalsidase alfa and kidney dysfunction in Fabry disease. J Am Soc Nephrol 2009;20:1132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Germain DP, Waldek S, Banikazemi M, Bushinsky DA, Charrow J, Desnick RJ, et al. Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol 2007;18:1547–57. [DOI] [PubMed] [Google Scholar]
- 30.Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, Störk S, et al. Long-term effects of enzyme replacement therapy on fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation 2009;119:524–9. [DOI] [PubMed] [Google Scholar]
- 31.Liu HC, Perrin A, Hsu TR, Yang CF, Lin HY, Yu WC, et al. Age at First Cardiac Symptoms in Fabry Disease: Association with a Chinese Hotspot Fabry Mutation (IVS4 + 919G>A), Classical Fabry Mutations, and Sex in a Taiwanese Population from the Fabry Outcome Survey (FOS). JIMD Rep 2015;22:107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


