Abstract
Background:
Evidences that support the use of targeted temperature management (TTM) for in-hospital cardiac arrest (IHCA) are lacking. We aimed to investigate the hypothesis that TTM benefits for patients with IHCA are similar to those with out-of-hospital cardiac arrest (OHCA) and to determine the independent predictors of resuscitation outcomes in patients with cardiac arrest receiving subsequent TTM.
Methods:
This is a retrospective, matched, case-control study (ratio 1:1) including 93 patients with IHCA treated with TTM after the return of spontaneous circulation, who were admitted to Partners HealthCare system in Boston from January 2011 to December 2018. Controls were defined as the same number of patients with OHCA, matched for age, Charlson score, and sex. Survival and neurological outcomes upon discharge were the primary outcome measures.
Results:
Patients with IHCA were more likely to have experienced a witnessed arrest and receive bystander cardiopulmonary resuscitation, a larger total dosage of epinephrine, and extracorporeal membrane oxygenation. The time duration for ROSC was shorter in patients with IHCA than in those with OHCA. The IHCA group was more likely associated with mild thrombocytopenia during TTM than the OHCA group. Survival after discharge and favorable neurological outcomes did not differ between the two groups. Among all patients who had cardiac arrest treated with TTM, the initial shockable rhythm, time to ROSC, and medical history of heart failure were independent outcome predictors for survival to hospital discharge. The only factor to predict favorable neurological outcomes at discharge was initial shockable rhythm.
Conclusion:
The beneficial effects of TTM in eligible patients with IHCA were similar with those with OHCA. Initial shockable rhythm was the only independent predictor of both survival and favorable neurological outcomes at discharge in all cardiac arrest survivors receiving TTM.
Keywords: Cardiac arrest, Cardiopulmonary resuscitation, Neurological outcome, Targeted temperature management, Therapeutic hypothermia
1. INTRODUCTION
Owing to the increasing growth of the population contributing to increased cardiovascular deaths worldwide, sudden cardiac arrest has become an increasing event and a global health issue.1,2 Many patients who survive from the cardiac arrest event have postcardiac arrest syndrome, a condition characterized by ischemia-reperfusion injury leading to oxidative stress, neurological injury, myocardial dysfunction, and systemic inflammatory response.3 Targeted temperature management (TTM) has been associated with decreasing mortality and improving neurological function in patients with out-of-hospital cardiac arrest (OHCA) since the two original landmark studies in 2002.4,5 Advances in postcardiac arrest care incorporating TTM have emerged as the standard of care for OHCA in comatose adults to improve functional outcomes.4–7
As TTM benefits for patients with OHCA have been well described, those for patients with in-hospital cardiac arrest (IHCA) have been rarely reported. The varieties of physiology, comorbidities, and polypharmacy in patients with IHCA make intensive care substantially complex.8 However, majority of previous studies that focused on TTM paid paltry attention on the outcomes in patients with IHCA. Evidences that support the use of TTM for patients with IHCA are lacking, and evidences also showed that it might not improve the outcomes in this population.9,10 Therefore, clinicians should possess a thorough understanding of the factors affecting the outcomes of in-patient resuscitation and the disparate features between patients with IHCA and OHCA who received TTM.
The benefits of TTM remain increasingly debatable as recent studies have shown attenuated improvement in favorable neurological survival as TTM carried risks of bleeding, arrhythmias, and electrolyte derangements.11,12 This leads to the question of whether therapeutic hypothermia is appropriate as a “one-size-fits-all” therapy in the postresuscitation care for all patients with cardiac arrest. At present, which patients with cardiac arrest may benefit the most from TTM remains uncertain. Patients who experienced IHCA may be different from those who experienced OHCA13,14 Therefore, this study was conducted to examine the hypothesis that TTM benefits for patients with IHCA are similar to those for patients with OHCA and to determine the independent predictors of resuscitation outcomes in patients with cardiac arrest receiving subsequent TTM.
2. METHODS
2.1. Study design and setting
The electronic health record data from two large U.S. academic provider networks were linked with Medicare claims data.15 The first network consisted of 1 tertiary hospital (Massachusetts General Hospital), 2 community hospitals, and 17 primary care centers. The second network included 1 tertiary hospital (Brigham and Women’s Hospital), 1 community hospital, and 16 primary care centers. This retrospective matched case-control study (ratio 1:1) assessed all adult patients (aged ≥18 years) who were admitted from January 2011 to December 2018 and experienced a cardiac arrest event. Cardiac arrest was defined as the absence of a palpable central pulse, apnea, and unresponsiveness. Patients who survived the initial cardiac arrest and underwent therapeutic hypothermia were selected and classified into two groups: the case group including patients who experienced IHCA and the control group including those who experienced OHCA, with 1:1 ratio matched for age (within 3 years), Charlson score (within 3 points), and sex manually. The exclusion criteria included early mortality before the TTM was completed, missing data, or patients with “do not resuscitate” orders. Clinical, epidemiological, and resuscitation characteristics; possible TTM-related complications; and outcomes in cases and controls were compared. The local Ethical Committee of Partners HealthCare Institutional Review Board (Protocol Number: 2019P000111) approved the study and waived the requirement for informed consent.
2.2. TTM protocols
TTM protocols may vary from hospital to hospital but are accepted by the national academic societies and adhere to international guidelines. TTM refers to strict temperature control to target the core body temperature between 33°C and 36°C following the cardiac arrest. Patients who fulfilled the following criteria were eligible for the initiation of TTM: 1) time within 6–12 h following the return of spontaneous circulation (ROSC) after the cardiac arrest regardless of initial rhythm; 2) ability to maintain a mean arterial pressure (MAP) of ≥65 mmHg with or without inotropes, or circulatory support devices; 3) comatose state, defined as lack of a meaningful response to verbal commands. No absolute contraindications to TTM existed. The ultimate decision to begin TTM was based on an assessment of potential risks and benefits of hypothermia in each individual patient considering the complete clinical situation and possible comorbidities. Patients highly at risk of adverse events with hypothermia due to recent head trauma, active bleeding, major surgery within the last 14 days, severe sepsis, pregnancy, or refractory hypotension received TTM targeted specifically toward reaching 36°C rather than the range of 33–36°C.
The cooling treatment was immediately initiated, and the protocol allowed up to 4 h to reach the target temperature. Patients were then maintained at the designated target temperature of 24 h and subsequently rewarmed to 37°C at a rate of 0.25°C per hour (not >0.5°C per hour) during a period of 12–16 h. Sedation and low-dose analgesia were mandatory throughout the cooling and rewarming phases to attain a Bedside Shivering Assessment Scale score of 0.16 A neuromuscular blockade agent was administered as necessary. Due to the pragmatic study design, no fixed standardized treatment regimens were applied. After TTM, active intervention to prevent fever was recommended for another 48 h.
2.3. Data collection and outcomes
Routine patient demographic data, event date, and time, medical history as recorded by the International Statistical Classification of Diseases and Related Health Problems (ICD-10) diagnostic code, physiologic measures, blood analysis data, and patient outcomes were retrospectively extracted and collected from the registry database and hospital records. These data were subsequently verified by two different attending physicians. In patients with multiple ensuing IHCAs in the same patient within 48 h, only data from the first episode were included to prevent confounding effects between events. The time to ROSC was defined as the time from collapse or alarm call until ROSC was established. The sequential organ failure assessment (SOFA) score within the first 24 h after admission was used to evaluate the severity of multiple organ dysfunction.17 Neurological outcome and survival at hospital discharge were the primary outcome measures. Subsequent hospital length of stay (LOS) after the cardiac arrest event was the secondary outcome. The neurological outcome was assessed using the Glasgow–Pittsburgh Cerebral Performance Categories (CPC) scale at discharge and recorded as CPC 1 (good performance), CPC 2 (moderate disability), CPC 3 (severe disability), CPC 4 (vegetative state), or CPC 5 (brain death or death).18 The neurological outcome at discharge was dichotomized by the CPC to good (1–2) or poor (3–5).
2.4. Statistical analysis
Results are expressed as n (%) for categorical variables. Descriptive statistics were reported as mean ± SD or median interquartile range for continuous variables. The groups were compared using the Student’s t-test for numerical data and Pearson’s chi-squared test or Fisher’s exact test for categorical data, as appropriate. Logistic regression models were used to explore independent risk factors for in-hospital mortality and patients’ neurological outcomes. Univariate analyses were performed separately for each risk factor to ascertain the odds ratio and 95% CI. All biologically plausible variables with p value of <0.10 in the univariate analysis were considered for inclusion in the logistic regression model during the multivariate analysis. A backward selection process was used. All analyses were processed using the IBM SPSS Statistics software (version 20.0; IBM Corp., Armonk, NY USA). Differences were considered to have reached the significance level with a two-tailed p < 0.05.
3. RESULTS
During the study period, 400 eligible patients received TTM after the cardiac arrest (Fig. 1). Among them, 93 patients with IHCA were selected as the case group. The control group was identified by obtaining the same number of patients with OHCA, matched for age, Charlson score, and sex. Demographic data, underlying diseases, and resuscitation variables are displayed in Tables 1 and 2. No significant differences were detected in the body mass index, ethnicity, smoking status, coexisting conditions, initial rhythm, presumed cardiac cause, and coronary reperfusion with percutaneous coronary intervention. Patients with IHCA were more likely to have experienced a witnessed arrest (93.5% vs. 36.6%; p < 0.001), receive by-stander cardiopulmonary resuscitation (CPR) (93.5% vs. 36.6%; p < 0.001), larger total dosage of epinephrine (3.4 mg vs. 2.4 mg; p < 0.045), and extracorporeal membrane oxygenation (6.5% vs. 0%; p = 0.013). The time duration for ROSC was shorter in patents with IHCA (mean difference, 19.5–16.6 minutes = 174 seconds) than that in those with OHCA.
Fig. 1.
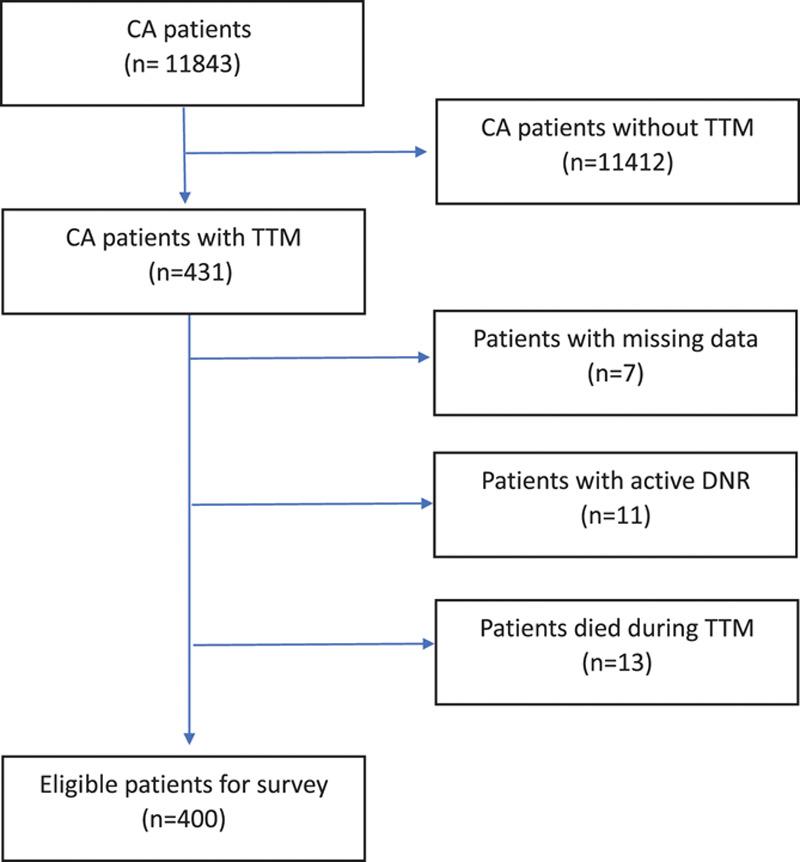
A schematic diagram showing the selection process of patients for analysis (from 2011 to 2018). CA = cardiac arrest; TTM = targeted temperature management; DNR = do not resuscitate.
Table 1.
Demographic data of patients with IHCA and OHCA receiving TTM
| IHCA (N = 93) | OHCA (N = 93) | p | |
|---|---|---|---|
| Sex | |||
| Male, N (%) | 61 (65.6) | 61 (65.6) | 1.000 |
| Female, N (%) | 32 (34.4) | 32 (34.4) | |
| Age, mean (SD) | 65.5 (13.7) | 65.5 (13.8) | 0.977 |
| BMI, mean (SD) | 28.1 (5.7) | 28.9 (6.7) | 0.982 |
| Ethnicity, N (%) | 0.693 | ||
| White | 63 (67.6) | 68 (73.0) | |
| Black | 17 (18.3) | 11 (11.8) | |
| Hispanic | 8 (8.6) | 6 (6.5) | |
| Asian | 1 (1.1) | 1 (1.1) | |
| Mixed | 2 (2.2) | 2 (2.2) | |
| Not recorded | 2 (2.2) | 5 (5.4) | |
| Smoking status, N (%) | |||
| Former smoker | 27 (29.0) | 27 (29.0) | 1.000 |
| Current smoker | 17 (18.3) | 24 (25.8) | 0.216 |
| Nonsomker | 31 (33.3) | 22 (23.7) | 0.131 |
| Unknown | 18 (19.4) | 20 (21.5) | 0.716 |
| Coexisting conditions, N (%) | |||
| Hypertension | 57 (61.3) | 57 (61.3) | 1.000 |
| Diabetes mellitus | 47 (50.5) | 36 (38.7) | 0.105 |
| Chronic lung disease | 27 (29.0) | 26 (28.0) | 0.871 |
| Coronary artery disease | 39 (41.9) | 46 (49.5) | 0.303 |
| Heart failure | 34 (36.6) | 25 (26.9) | 0.156 |
| Arrhythmia | 33 (35.5) | 43 (46.2) | 0.136 |
| Peptic ulcer disease | 19 (20.4) | 18 (19.4) | 0.854 |
| Hepatic insufficiency | 7 (7.5) | 6 (6.5) | 0.774 |
| Renal insufficiency | 39 (41.9) | 35 (37.6) | 0.549 |
| Uremia under regular hemodialysis | 13 (14.0) | 6 (6.5) | 0.090 |
| Cerebrovascular disease | 13 (14.0) | 17 (18.3) | 0.425 |
| Dementia | 1 (1.1) | 4 (4.3) | 0.368 |
| Connective tissue disease | 6 (6.5) | 3 (3.2) | 0.305 |
| Solid tumor | 18 (19.4) | 23 (24.7) | 0.376 |
| Leukemia/lymphoma | 7 (7.5) | 1 (1.1) | 0.064 |
| Charlson score, mean (SD) | 6.0 (2.9) | 5.5 (2.5) | 0.192 |
| 0–2 | 12 (12.9) | 11 (11.8) | 0.643 |
| 3 or 4 | 15 (16.1) | 20 (21.5) | |
| ≥5 | 66 (71.0) | 62 (66.7) | |
IHCA = in-hospital cardiac arrest; OHCA = out-of-hospital cardiac arrest; TTM = targeted temperature management; Age (in years); BMI = body mass index.
Table 2.
Event-associated parameters of IHCA and OHCA patients receiving TTM
| IHCA (N = 93) | OHCA (N = 93) | p | |
|---|---|---|---|
| Initial rhythm, N (%) | |||
| Shockable | 24 (25.8) | 36 (38.7) | 0.060 |
| Nonshockable | 69 (74.2) | 57 (61.3) | |
| Witnessed, N (%) | 87 (93.5) | 34 (36.6) | <0.001* |
| Bystander CPR, N (%) | 87 (93.5) | 34 (36.6) | <0.001* |
| Being transferred, N (%) | 34 (36.6) | 33 (35.5) | 0.879 |
| Presumed cardiac cause, N (%) | 30 (32.3) | 32 (34.4) | 0.756 |
| PCI, N (%) | 27 (29.0) | 31 (33.3) | 0.527 |
| Time to return for spontaneous circulation (min) | |||
| Mean (SD) | 16.6 (16.3) | 19.5 (15.5) | 0.034* |
| Median (IQR) | 12 (7–23) | 15 (10–25) | |
| <10 | 35 (37.6) | 16 (17.2) | 0.018* |
| 10~19 | 29 (31.2) | 40 (43.0) | |
| 20~29 | 10 (10.8) | 18 (19.4) | |
| 30~59 | 17 (18.3) | 15 (16.1) | |
| >60 | 2 (2.2) | 4 (4.3) | |
| Cumulative dosage of epinephrine, N (%) | |||
| 0 | 15 (16.1) | 29 (31.0) | |
| 1 | 10 (10.8) | 11 (11.8) | |
| 2 | 27 (28.9) | 14 (15.1) | |
| 3 | 12 (12.9) | 17 (18.3) | |
| 4–6 | 12 (12.9) | 17 (18.3) | |
| 7–9 | 11 (11.9) | 3 (3.3) | |
| ≥10 | 6 (6.5) | 2 (2.2) | |
| Total dosage of epinephrine, mean (SD) | 3.4 (3.7) | 2.4 (2.7) | 0.046* |
| ECMO use, N (%) | 6 (6.5) | 0 (0.0) | 0.013* |
IHCA = in-hospital cardiac arrest; OHCA = out-of-hospital cardiac arrest; TTM = targeted temperature management; CPR = cardiopulmonary resuscitation; PCI = percutaneous coronary intervention IQR = interquartile range; ECMO = extracorporeal membrane oxygenation.
*p < 0.05.
Patient characteristics after ROSC, TTM effects on coagulation and electrolyte parameters, and resuscitation outcomes at discharge are shown in Table 3. Overall, after ROSC, patients with IHCA had higher SOFA scores (9.5 vs. 8.4; p = 0.004), lower blood glucose levels (119.1 mg/dL vs. 259.6 mg/dL; p = 0.002), lower MAP (73.1 mmHg vs. 85.5 mmHg; p < 0.001), and longer subsequent LOS (19.7 days vs. 12.7 days; p = 0.025) than that in those with OHCA. The IHCA group was more likely to be associated with mild thrombocytopenia (platelet count, <150 K/μL) during TTM; however, neither the standard coagulation nor the electrolyte parameters showed any differences between the two groups. Finally, survival to discharge and favorable neurological outcomes did not differ between the IHCA and OHCA groups.
Table 3.
Characteristics after return of spontaneous circulation and outcome of patients receiving TTM
| IHCA (N = 93) | OHCA (N = 93) | p | |
|---|---|---|---|
| SOFA within 24 hours after event | |||
| Mean (SD) | 9.5 (2.4) | 8.4 (2.7) | 0.004* |
| Median (IQR) | 9 (8-11) | 8 (7-11) | |
| Glucose (mg/dL), mean (SD) | 199.1 (101.9) | 259.6 (193.1) | 0.002* |
| MAP (mmHg), mean (SD) | 73.1 (16.1) | 85.5 (24.0) | <0.001* |
| pH, mean (SD) | 7.28 (0.17) | 7.27 (0.16) | 0.896 |
| Lactate (mmol/L), mean (SD) | 5.6 (5.3) | 5.8 (3.9) | 0.226 |
| Creatinine (mg/dL), mean (SD) | 2.3 (2.2) | 1.8 (1.3) | 0.413 |
| Coagulopathy after TTM, N (%) | |||
| Thrombocytopenia <150 K/μL | 48 (51.6) | 29 (31.2) | 0.004* |
| Thrombocytopenia <50 K/μL | 7 (7.5) | 1 (1.1) | 0.064 |
| Thrombocytopenia <20 K/μL | 0 (0.0) | 0 (0.0) | … |
| Prolonged PT >14.4 s | 62 (66.7) | 57 (61.3) | 0.276 |
| Prolonged aPTT >36,6 s | 36 (38.7) | 31 (33.3) | 0.549 |
| Electrolyte imbalance after TTM, N (%) | |||
| Hypernatremia, sodium >145mmol/L | 7 (7.5) | 2 (2.2) | 0.090 |
| Hyponatremia, sodium <136 mmol/L | 26 (28.0) | 24 (25.8) | 0.774 |
| Hyperkalemia, potassium >5.0 mmol/L | 9 (9.7) | 13 (14.0) | 0.344 |
| Hypokalemia, potassium <3.4 mmol/L | 4 (4.3) | 6 (6.5) | 0.502 |
| Outcome | |||
| Subsequent LOS, mean (SD) | 19.7 (28.4) | 12.7 (11.7) | 0.025* |
| Acute kidney injury, N (%) | 28 (30.1) | 27 (29.0) | 0.830 |
| Survival to discharge, N (%) | 50 (53.8) | 42 (45.2) | 0.241 |
| Favorable CPC scale at discharge, N (%) | 24 (25.8) | 26 (28.0) | 0.182 |
TTM = targeted temperature management; IHCA = in-hospital cardiac arrest; OHCA = out-of-hospital cardiac arrest; SOFA = sequential organ failure assessment; IQR= interquartile range; MAP = mean arterial pressure; PT = prothrombin time; aPTT = activated partial thromboplastin time; LOS = length of hospital stay; CPC scale = cerebral performance category scale.
*p < 0.05.
The univariate analysis showed that initial shockable rhythm, time to ROSC, total epinephrine dosage, presumed cardiac cause, coronary artery disease, heart failure, and SOFA within 24 hours after the event are predictors of survival at discharge among patients receiving TTM (Table 4). In the multivariate regression analysis, the initial shockable rhythm, time to ROSC, and past medical history of heart failure were independent outcome predictors for survival to hospital discharge. Factors that significantly predicted the favorable neurological outcome at discharge in the logistic regression analysis are shown in Table 5. The univariate analysis of predictors was an initial shockable rhythm, total epinephrine dosage, presumed cardiac cause, and SOFA within 24 hours after the event. Multivariate analysis revealed that the only factor to predict favorable neurological outcomes at discharge was the initial shockable rhythm. IHCA was not a pivotal factor affecting the resuscitation outcomes of patients after the cardiac arrest who received TTM.
Table 4.
Logistic regression analysis of predictors of survival at discharge among patients receiving TTM in this cohort
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| IHCA | 1.412 (0.793–2.514) | 0.241 | ||
| Age | 0.998 (0.978–1.020) | 0.877 | ||
| Sex, male | 1.419 (0.772–2.607) | 0.260 | ||
| Charlson score | 1.075 (0.966–1.196) | 0.185 | ||
| Initial shockable rhythm | 3.173 (1.657–6.078) | <0.001 | 3.876 (1.876–8.008) | <0.001* |
| Time to ROSC | 0.966 (0.943–0.989) | 0.004 | 0.966 (0.940–0.992) | 0.011* |
| Witnessed cardiac arrest | 1.687 (0.874–3.260) | 0.119 | ||
| Bystander CPR | 1.348 (0.736–2.470) | 0.333 | ||
| Total dosage of epinephrine | 0.855 (0.764–0.957) | 0.006 | ||
| Presumed cardiac cause | 1.857 (1.000–3.448) | 0.050 | ||
| Coronary artery disease | 2.195 (1.219–3.953) | 0.009 | ||
| Arrhythmia | 1.134 (0.632–2.035) | 0.674 | ||
| Heart failure | 2.202 (1.168–4.149) | 0.015 | 2.589 (1.269–5.283) | 0.009* |
| Cerebrovascular disease | 1.203 (0.550–2.632) | 0.644 | ||
| Thrombocytopenia (PLT < 150,000) | 1.073 (0.584–1.972) | 0.821 | ||
| Hypokalemia | 0.413 (0.103–1.653) | 0.211 | ||
| Glucose level after ROSC | 0.998 (0.995–1.000) | 0.114 | ||
| SOFA within 24 hours after event | 0.901 (0.801–1.014) | 0.083 | ||
| Mean arterial pressure | 1.003 (0.989–1.017) | 0.665 | ||
TTM = targeted temperature management; IHCA = in-hospital cardiac arrest; ROSC = return of spontaneous circulation; CPR = cardiopulmonary resuscitation; PLT = platelet; SOFA = sequential organ failure assessment.
*p < 0.05.
Table 5.
Logistic regression analysis of predictors of favorable neurological outcome among survivors after TTM
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| IHCA | 0.568 (0.247–1.308) | 0.184 | ||
| Age | 1.015 (0.983–1.049) | 0.364 | ||
| Sex, male | 1.581 (0.647–3.863) | 0.315 | ||
| Charlson score | 1.027 (0.879–1.201) | 0.734 | ||
| Initial shockable rhythm | 5.221 (2.099–12.986) | <0.001 | 6.570 (2.435–17.728) | <0.001* |
| Time to ROSC | 0971 (0.933–1.010) | 0.145 | ||
| Witnessed cardiac arrest | 1.616 (0.597–4.380) | 0.345 | ||
| Bystander CPR | 0.631 (0.257–1.549) | 0.315 | ||
| Total dosage of epinephrine | 0.749 (0.600–0.934) | 0.010 | ||
| Presumed cardiac cause | 3.757 (1.524–9.257) | 0.004 | ||
| Coronary artery disease | 1.795 (0.781–4.126) | 0.168 | ||
| Arrhythmia | 1.155 (0.503–2.654) | 0.733 | ||
| Heart failure | 0.681 (0.294–1.575) | 0.369 | ||
| Cerebrovascular disease | 0.810 (0.275–2.382) | 0.701 | ||
| Thrombocytopenia (PLT < 150,000) | 1.182 (0.497–2.810) | 0.706 | ||
| Hypokalemia | 0.409 (0.036–4.696) | 0.473 | ||
| Glucose level after ROSC | 0.999 (0.995–1.003) | 0.709 | ||
| SOFA within 24 hours after event | 0.821 (0.686–0.982) | 0.031 | ||
| Mean arterial pressure | 1.017 (0.995–1.039) | 0.130 | ||
TTM = targeted temperature management; IHCA = in-hospital cardiac arrest; ROSC = return of spontaneous circulation; CPR = cardiopulmonary resuscitation; PLT = platelet; SOFA = sequential organ failure assessment.
*p < 0.05.
4. DISCUSSION
Despite substantial improvements in resuscitation efforts over the last few decades, outcomes of OHCA and IHCA remain poor.19–22 In clinical scenarios, significant disparity was observed in the utilization of TTM. In contrast to OHCA patients with ROSC of whom at least 50% qualify for TTM,23,24 <7% of patients with IHCA qualify for therapeutic hypothermia.9,25 However, differences in TTM influence between IHCA and OHCA should be investigated because cardiac arrest still affects approximately 200,000 hospitalized individuals annually in the USA.26
The use of TTM remains controversial in patients with IHCA, and the International Liaison Committee on Resuscitation makes a much weaker recommendation for TTM utilization in patients with IHCA than those with OHCA.27 Contradictory to previous findings,9,10,14,28 the beneficial effects of TTM in IHCA regarding survival and neuroprotection were found to be similar to those in OHCA. The majority of patients with IHCA experienced a witnessed cardiac arrest and received bystander CPR. However, the IHCA group had higher SOFA scores and lower initial MAPs, which are associated with early in-hospital mortality.29 The detrimental effect might be compensated by significantly shorter time duration to ROSC in the IHCA group. Time to ROSC possibly acts as a mortality marker and brain injury severity among cardiac arrest survivors and is inversely associated with the likelihood of neurological recovery.30–32 Effects of TTM may not be consistent over the full spectrum of the time to ROSC. Theoretically, time to ROSC may be further dichotomized into “no-flow time” and “low-flow time.” In 1987, Bulkley postulated a bimodal model of injury after a prolonged ischemia followed by reperfusion.33,34 Postresuscitation hypothermia treatment imparts beneficial effects by diminishing early excitotoxic and intermediate inflammatory reactions and later apoptotic cell death.35–37 The duration of resuscitation efforts, corresponding to the “low-flow time,” influences the TTM effectiveness.38 A prolonged period of “low-flow time” with subphysiological perfusion worsens the reperfusion injury and therefore treating these processes, even with the TTM application, may no longer be possible. In this study, the exact “no-flow time” and “low-flow time” of each patient were not measured because of insufficient medical records. It should be pointed out that some heterogeneity on the actual exposure to “no-flow time” must be expected, and thus, the time correlation to ROSC and the actual magnitude of the reperfusion injury may not be perfect. Nevertheless, a significantly shorter time to ROSC did play a major role in advantageously influencing the outcomes.
Aside from describing the comparisons of IHCA and OHCA, we sought to assess the prognostic implication of pertinent factors for patients with cardiac arrest who received TTM. With regard to outcome analysis, significant predictors of survival at discharge were the initial shockable rhythm and shorter time to ROSC. Interestingly, another significant predictor was a past medical history of heart failure. We assumed that these patients may have a certain degree of protective effect conferred upon them from the routine use of heart failure medications. The actual mechanism of this effect requires further holistic investigation.
Hypothermia itself can inhibit the cellular immune response; cause cardiac arrhythmias, decrease cardiac, renal, gastrointestinal, and cerebral function; and induce coagulopathy.39 With the paucity of complete documentation, only platelet counts, prothrombin time, and activated partial thromboplastin time were presented, instead of actual bleeding or thrombosis complications, such as bleeding in critical organs or in-stent thrombosis after the percutaneous coronary intervention. The probability of hypothermia-related complications such as severe coagulopathy and renal dysfunction was not significantly different between the IHCA and OHCA groups.
In our study, detailed information regarding the targeted temperature and cooling method for each patient is shown. However, a previous trial published in 2013 showed that hypothermia at a target temperature of 33°C was not beneficial compared with a target temperature of 36°C.12 In addition, a nationwide multicenter analysis revealed no significant difference in the neurological recovery regarding the cooling methods for TTM.40 Thus, we recruited all patients receiving TTM instead of categorizing with regard to the targeted temperature or cooling methods. TTM is largely attributed to intracellular shifts and increased potassium excretion from cold diuresis,41,42 although no tendency was observed toward developing hypokalemia among the study population. Previous studies have demonstrated that extracorporeal cardiopulmonary resuscitation has favorable outcomes for the treatment of cardiac arrest, particularly with IHCA.43–45 However, pertinent data in our study were too limited to exhaustively assess its efficacy on patients receiving TTM.
Our study has the unique advantage by involving a large cohort of diverse population that included patients with IHCA and OHCA from multiple centers, making our findings fairly generalizable. However, our study has limitations that should be addressed. First, this study was conducted using the registry data and was thus subject to limitations of any retrospective analysis, given that the causal relationship between variables could not be established although several associations were demonstrated. Second, a selection bias might have occurred, because the final decision to manage a patient was left by the clinical physician’s discretion. Therefore, a higher proportion of patients with expected favorable outcomes would possibly receive TTM to optimize the prognosis. In addition, we do not have data on why patients did not receive TTM or the quality of TTM itself. Third, our study measured end points only at the time of hospital discharge, despite the knowledge that the neurological function can ameliorate for at least 6 months after resuscitation from cardiac arrest. Namely, long-term functional outcomes may be a more robust measurement of any treatment effect on patients recovering from cardiac arrest.46 Good neurological outcome was defined as a CPC score of 1 or 2, which may be considered a rather crude measurement of neurological outcome. However, subtle differences in cognitive outcomes might not be recognized via CPC score, and a more detailed, thorough assessment of milder cognitive impairment is necessary in evaluating the potentially subtle treatment effects. Fourth, information regarding the usage and selection of sedation regimens was not collected, which are also considered to have an impact on outcomes in the postcardiac arrest care.47 Furthermore, no record regarding the vasopressor use was observed among our study population; however, relevant complications, such as arrhythmia and decreased peripheral perfusion, could potentially have been unavoidably precipitated by a high dosage of vasopressors.48 Rapid rewarming and longer preinduction time (the time from ROSC to TTM initiation) have been reported to have an adverse effect on outcomes;49,50 however, no pertinent data were found for that assessment. Fifth, clinical outcomes were only compared, not for medical costs or hospital resource utilization such as the workload and LOS in the intensive care unit. Finally, our observational period is 8 years and the intensive care treatment, especially the postresuscitation care standards, might have changed over the period.
In conclusion, similar beneficial effects of TTM were found in eligible patients with IHCA compared with those with OHCA in this matched cohort study. Initial shockable rhythm was the only predictor of both survival and favorable neurological outcomes at discharge in all cardiac arrest survivors receiving subsequent TTM.
ACKNOWLEDGMENTS
This work was conducted with the support from the Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The authors were solely responsible of the contents and do not necessarily represent the official views of the Harvard Catalyst, Harvard University, its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest related to the subject matter or materials discussed in this article.
References
- 1.Arrich J, Holzer M, Havel C, Müllner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2016;2:CD004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015;372:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008;118:2452–83. [DOI] [PubMed] [Google Scholar]
- 4.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–63. [DOI] [PubMed] [Google Scholar]
- 5.Hypothermia after Cardiac Arrest Study G Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–56. [DOI] [PubMed] [Google Scholar]
- 6.Gaieski DF, Band RA, Abella BS, Neumar RW, Fuchs BD, Kolansky DM, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation 2009;80:418–24. [DOI] [PubMed] [Google Scholar]
- 7.Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015;95:202–22. [DOI] [PubMed] [Google Scholar]
- 8.Wang CH, Huang CH, Chang WT, Tsai MS, Yu PH, Wu YW, et al. Associations among gender, marital status, and outcomes of adult in-hospital cardiac arrest: a retrospective cohort study. Resuscitation 2016;107:1–6. [DOI] [PubMed] [Google Scholar]
- 9.Arrich J; European Resuscitation Council Hypothermia After Cardiac Arrest Registry Study Group Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med 2007;35:1041–7. [DOI] [PubMed] [Google Scholar]
- 10.Kory P, Fukunaga M, Mathew JP, Singh B, Szainwald L, Mosak J, et al. Outcomes of mild therapeutic hypothermia after in-hospital cardiac arrest. Neurocrit Care 2012;16:406–12. [DOI] [PubMed] [Google Scholar]
- 11.Mahmoud A, Elgendy IY, Bavry AA. Use of targeted temperature management after out-of-hospital cardiac arrest: a meta-analysis of randomized controlled trials. Am J Med 2016;129:522–7.e2. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013;369:2197–206. [DOI] [PubMed] [Google Scholar]
- 13.Caltekin I, Savrun A, Gokcen E, Atik D, Demirtas E, Demir B, et al. Comparison of the factors affecting neurological outcome in out-of vs in-hospital cardiac arrest cases. J Pak Med Assoc 2016;66:1412–7. [PubMed] [Google Scholar]
- 14.Nichol G, Huszti E, Kim F, Fly D, Parnia S, Donnino M, et al. Does induction of hypothermia improve outcomes after in-hospital cardiac arrest? Resuscitation 2013;84:620–5. [DOI] [PubMed] [Google Scholar]
- 15.Hennessy S. Use of health care databases in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006;98:311–3. [DOI] [PubMed] [Google Scholar]
- 16.Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke 2008;39:3242–7. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998;26:1793–800. [DOI] [PubMed] [Google Scholar]
- 18.Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA 2004;291:870–9. [DOI] [PubMed] [Google Scholar]
- 19.Bergum D, Nordseth T, Mjølstad OC, Skogvoll E, Haugen BO. Causes of in-hospital cardiac arrest—incidences and rate of recognition. Resuscitation 2015;87:63–8. [DOI] [PubMed] [Google Scholar]
- 20.Kolte D, Khera S, Aronow WS, Palaniswamy C, Mujib M, Ahn C, et al. Regional variation in the incidence and outcomes of in-hospital cardiac arrest in the United States. Circulation 2015;131:1415–25. [DOI] [PubMed] [Google Scholar]
- 21.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. ; Resuscitation Outcomes Consortium Investigators Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 2008;300:1423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandroni C, Nolan J, Cavallaro F, Antonelli M. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med 2007;33:237–45. [DOI] [PubMed] [Google Scholar]
- 23.Dumas F, Grimaldi D, Zuber B, Fichet J, Charpentier J, Pène F, et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients?: insights from a large registry. Circulation 2011;123:877–86. [DOI] [PubMed] [Google Scholar]
- 24.Phelps R, Dumas F, Maynard C, Silver J, Rea T. Cerebral performance category and long-term prognosis following out-of-hospital cardiac arrest. Crit Care Med 2013;41:1252–7. [DOI] [PubMed] [Google Scholar]
- 25.Mikkelsen ME, Christie JD, Abella BS, Kerlin MP, Fuchs BD, Schweickert WD, et al. ; American Heart Association’s Get With the Guidelines-Resuscitation Investigators Use of therapeutic hypothermia after in-hospital cardiac arrest. Crit Care Med 2013;41:1385–95. [DOI] [PubMed] [Google Scholar]
- 26.Graham R, McCoy MA, Schultz AM. Strategies to improve cardiac arrest survival: a time to act. 2015Washington, DC: National Academies Press; [PubMed] [Google Scholar]
- 27.Callaway CW, Soar J, Aibiki M, Böttiger BW, Brooks SC, Deakin CD, et al. ; Advanced Life Support Chapter Collaborators Part 4: advanced life support: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2015;13216 Suppl 1S84–145. [DOI] [PubMed] [Google Scholar]
- 28.Gueret RM, Bailitz JM, Sahni AS, Tulaimat A. Therapeutic hypothermia at an urban public hospital: development, implementation, experience and outcomes. Heart Lung 2017;46:40–5. [DOI] [PubMed] [Google Scholar]
- 29.Huang CH, Tsai MS, Ong HN, Chen W, Wang CH, Chang WT, et al. Association of hemodynamic variables with in-hospital mortality and favorable neurological outcomes in post-cardiac arrest care with targeted temperature management. Resuscitation 2017;120:146–52. [DOI] [PubMed] [Google Scholar]
- 30.Kjaergaard J, Nielsen N, Winther-Jensen M, Wanscher M, Pellis T, Kuiper M, et al. Impact of time to return of spontaneous circulation on neuroprotective effect of targeted temperature management at 33 or 36 degrees in comatose survivors of out-of hospital cardiac arrest. Resuscitation 2015;96:310–6. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds JC, Frisch A, Rittenberger JC, Callaway CW. Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: when should we change to novel therapies? Circulation 2013;128:2488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wibrandt I, Norsted K, Schmidt H, Schierbeck J. Predictors for outcome among cardiac arrest patients: the importance of initial cardiac arrest rhythm versus time to return of spontaneous circulation, a retrospective cohort study. BMC Emerg Med 2015;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bulkley GB. Free radical-mediated reperfusion injury: a selective review. Br J Cancer Suppl 1987;8:66–73. [PMC free article] [PubMed] [Google Scholar]
- 34.González-Ibarra FP, Varon J, López-Meza EG. Therapeutic hypothermia: critical review of the molecular mechanisms of action. Front Neurol 2011;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polderman KH. Application of therapeutic hypothermia in the ICU: opportunities and pitfalls of a promising treatment modality. Part 1: indications and evidence. Intensive Care Med 2004;30:556–75. [DOI] [PubMed] [Google Scholar]
- 36.Webster CM, Kelly S, Koike MA, Chock VY, Giffard RG, Yenari MA. Inflammation and NFkappaB activation is decreased by hypothermia following global cerebral ischemia. Neurobiol Dis 2009;33:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao H, Wang JQ, Shimohata T, Sun G, Yenari MA, Sapolsky RM, et al. Conditions of protection by hypothermia and effects on apoptotic pathways in a rat model of permanent middle cerebral artery occlusion. J Neurosurg 2007;107:636–41. [DOI] [PubMed] [Google Scholar]
- 38.Wallmüller C, Testori C, Sterz F, Stratil P, Schober A, Herkner H, et al. Limited effect of mild therapeutic hypothermia on outcome after prolonged resuscitation. Resuscitation 2016;98:15–9. [DOI] [PubMed] [Google Scholar]
- 39.Kirkpatrick AW, Chun R, Brown R, Simons RK. Hypothermia and the trauma patient. Can J Surg 1999;42:333–43. [PMC free article] [PubMed] [Google Scholar]
- 40.Kim KH, Shin SD, Song KJ, Ro YS, Kim YJ, Hong KJ, et al. Cooling methods of targeted temperature management and neurological recovery after out-of-hospital cardiac arrest: a nationwide multicenter multi-level analysis. Resuscitation 2018;125:56–65. [DOI] [PubMed] [Google Scholar]
- 41.Aibiki M, Kawaguchi S, Maekawa N. Reversible hypophosphatemia during moderate hypothermia therapy for brain-injured patients. Crit Care Med 2001;29:1726–30. [DOI] [PubMed] [Google Scholar]
- 42.Clifton GL, Miller ER, Choi SC, Levin HS. Fluid thresholds and outcome from severe brain injury. Crit Care Med 2002;30:739–45. [DOI] [PubMed] [Google Scholar]
- 43.Bednarczyk JM, White CW, Ducas RA, Golian M, Nepomuceno R, Hiebert B, et al. Resuscitative extracorporeal membrane oxygenation for in hospital cardiac arrest: a Canadian observational experience. Resuscitation 2014;85:1713–9. [DOI] [PubMed] [Google Scholar]
- 44.Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 2008;372:554–61. [DOI] [PubMed] [Google Scholar]
- 45.Kagawa E, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Kurisu S, et al. Assessment of outcomes and differences between in- and out-of-hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation 2010;81:968–73. [DOI] [PubMed] [Google Scholar]
- 46.Smith K, Andrew E, Lijovic M, Nehme Z, Bernard S. Quality of life and functional outcomes 12 months after out-of-hospital cardiac arrest. Circulation 2015;131:174–81. [DOI] [PubMed] [Google Scholar]
- 47.Paul M, Bougouin W, Dumas F, Geri G, Champigneulle B, Guillemet L, et al. Comparison of two sedation regimens during targeted temperature management after cardiac arrest. Resuscitation 2018;128:204–10. [DOI] [PubMed] [Google Scholar]
- 48.Giraud R, Siegenthaler N, Bendjelid K. Cardiac index during therapeutic hypothermia: which target value is optimal? Crit Care 2013;17:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee BK, Jeung KW, Jung YH, Lee DH, Lee SM, Cho YS, et al. Relationship between timing of cooling and outcomes in adult comatose cardiac arrest patients treated with targeted temperature management. Resuscitation 2017;113:135–41. [DOI] [PubMed] [Google Scholar]
- 50.Lu X, Ma L, Sun S, Xu J, Zhu C, Tang W. The effects of the rate of postresuscitation rewarming following hypothermia on outcomes of cardiopulmonary resuscitation in a rat model. Crit Care Med 2014;42:e106–13. [DOI] [PubMed] [Google Scholar]


