Abstract
Background
Fatigue is commonly reported by ARDS survivors, but empirical data are scarce.
Research Question
This study evaluated fatigue prevalence and associated variables in a prospective study of ARDS survivors.
Study Design and Methods
This analysis is part of the ARDSNet Long-Term Outcomes Study (ALTOS) conducted at 38 US hospitals. Using age- and sex-adjusted, time-averaged random effects regression models, we evaluated associations between the validated Functional Assessment of Chronic Illness Therapy-Fatigue Scale with patient and critical illness variables, and with physical, cognitive, and mental health status at 6 and 12 months following ARDS.
Results
Among ARDS survivors, 501 of 711 (70%) and 436 of 659 (66%) reported clinically significant symptoms of fatigue at 6 and 12 months, respectively, with 41% and 28% reporting clinically important improvement and worsening (n = 638). At 6 months, the prevalence of fatigue (70%) was greater than that of impaired physical functioning (50%), anxiety (42%), and depression (36%); 46% reported both impaired physical function and fatigue, and 27% reported co-existing anxiety, depression, and fatigue. Fatigue was less severe in men and in those employed prior to ARDS. Critical illness variables (eg, illness severity, length of stay) had little association with fatigue symptoms. Worse physical, cognitive, and mental health symptoms were associated with greater fatigue at both the 6- and 12-month follow-up.
Interpretation
During the first year following ARDS, more than two-thirds of survivors reported clinically significant fatigue symptoms. Due to frequent co-occurrence, clinicians should evaluate and manage survivors’ physical, cognitive, and mental health status when fatigue is endorsed.
Key Words: acute lung injury, cognitive function, depression, disability, rehabilitation
Abbreviations: APACHE III, Acute Physiology and Chronic Health Evaluation III; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue Scale; SF-36v2, Short Form-36 Version 2
FOR EDITORIAL COMMENT, SEE PAGE 848
Advances in critical care medicine have translated into decreased mortality due to ARDS, but survivors often experience significant long-lasting impairments in physical, cognitive, and mental health.1, 2, 3, 4, 5, 6, 7, 8 Accompanying this constellation of morbidities, survivors frequently endorse fatigue in the months following hospital discharge.9,10 Although ICU-acquired weakness and negative psychological symptoms are recognized as important and common sequelae of ARDS,11 there are few empirical data on the course of fatigue and associations with these other morbidities. This omission was identified in the Intensive Care Medicine Research Agenda on Intensive Care Unit-Acquired Weakness, in which the authors recommend that future studies should “evaluate the prevalence and severity of fatigue in ICU survivors and define its association with psychiatric disorders, pain, cognitive impairment, and axonal loss.”12
The objective of the current study was to evaluate the prevalence of self-reported fatigue and its association with physical, cognitive, and mental health status over a 6- and 12-month follow-up in a national cohort of patients surviving ARDS.
Patients and Methods
Participants
Data used in this analysis are part of the ARDSNet Long-Term Outcomes Study (ALTOS), a national, multicenter prospective study of ARDS survivors13,14 recruited from 38 hospitals in the United States.15, 16, 17, 18 In ALTOS, telephone-based follow-up assessments, conducted at 6 and 12 months following ARDS, occurred from 2008 to 2014.13,14,19 Committee IRB-5 of the Institutional Review Board of Johns Hopkins University School of Medicine and all participating institutions approved these studies, and patients or their surrogates provided informed consent (Approval: NA_00013113).
Fatigue Measure
The Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-F) instrument is a valid and reliable self-reported measure of fatigue, evaluated in patients from diverse populations, including those with anemia,20 rheumatoid arthritis,21 critical illness,22 and cancer.23 The 13-item FACIT-F evaluates fatigue symptoms experienced over the past 7 days with scoring via a five-point Likert scale ranging from “not at all” to “very much.” The raw total score ranges from 0 (severe fatigue) to 52 (negligible fatigue). Raw scores are converted to a transformed scale (range, 0-100), with a score ≤ 68 representing a clinically significant threshold for fatigue compared with the general population.23
Physical, Cognitive, and Mental Health Measures
Other patient-reported outcome measures obtained by ALTOS at 6- and 12-month follow-up assessments included: (1) the Short Form-36 Version 2 (SF-36v2) Physical Component Summary and Mental Component Summary scales24 (standardized score; range, 0-100; mean ± SD, 50 ± 10; higher score = better function); (2) the Functional Performance Inventory-Short Form25 (range, 0-3; higher scores = better physical function; score ≤ 2 = physical dysfunction)26; (3) the Mini-Mental State Examination27 (range, 0-30; higher scores = better cognitive function; score ≤ 24 = cognitive impairment)28; (4) Impact of Events Scale-Revised for Post Traumatic Distress Syndrome symptoms (range, 0-4; higher score = more symptoms; score ≥ 1.6 = clinically significant symptoms)29; and Hospital Anxiety and Depression Scale anxiety and depression subscales30 (range, 0-21; higher score = more symptoms; score ≥ 8 = clinically significant symptoms).31 The Physical Component Summary (SF-36v2) and the Functional Performance Inventory-Short Form were used to measure physical health outcomes; MMSE was used for cognitive outcomes; and the Mental Component Summary (SF-36v2), Impact of Events Scale-Revised, and Hospital Anxiety and Depression Scale were used for mental health outcomes. All patient outcome measures were purchased or used with appropriate permission from copyright holders.
Study Procedures
Trained research staff administered the FACIT-F and the other aforementioned measures at 6- and 12-month telephone-based follow-up assessments. Each research staff member underwent initial training, consisting of didactic sessions, observation of survey administration, and then supervised practice in administering the survey. Thereafter, there were initial quality assurance reviews with simulated and then real participants; ongoing interval quality assurance reviews were subsequently conducted throughout the study.
Statistical Analysis
STATA version 15 (StataCorp) was used for statistical analyses.32 Exploratory analyses included inspection of histograms and spaghetti plots of patient outcomes over time and included change in raw FACIT-F scores between 6- and 12-month follow-up. We constructed Venn diagrams that included the overlap of patients reporting clinically significant fatigue (transformed FACIT-F ≤ 68) with clinically significant physical dysfunction (Functional Performance Inventory-Short Form score ≤ 2), cognitive dysfunction (Mini-Mental State Examination score ≤ 24), and anxiety and depressive symptoms (Hospital Anxiety and Depression Scale subscale scores ≥ 8) at the 6-month follow-up.
Covariates for regression models were chosen a priori based on hypothesized clinical relationships with fatigue. We fit random intercept linear mixed effects models, after confirming that FACIT-F scores were normally distributed. This approach allowed the use of all data points from each individual, with a random intercept to capture patient heterogeneity at baseline. In exploratory data analysis, histograms and scatterplots of the proposed covariates were plotted vs fatigue at each time point. These exploratory analyses showed no difference in associations over time. Hence, the regression models did not include a term for time. As such, coefficients could be interpreted as the average relationship across time points (“time-averaged”). This model, adjusted for age and sex, was used to test individual associations of each covariate with fatigue (as measured using the transformed FACIT-F scores). This same regression model was used to evaluate a single post hoc analysis evaluating baseline ARDS severity and fatigue. We fit separate age- and sex-adjusted random effects regression models to evaluate the associations between physical, cognitive, and mental health status at 6 and 12 months with fatigue at the same time point. We also modeled the lagged associations of physical, cognitive, and mental health status variables at 6 months with fatigue at 12 months with a random intercept.
We chose 0.5 SD as a standardized measure of change in physical, cognitive, and mental health variables. An SD of 0.5 is often used as an estimate of a distribution-based minimally important difference.33,34 For the outcome variable (FACIT-F), based on prior literature, three to four points were considered as a clinically important difference.21
Results
A total of 732 ARDS survivors were included in this evaluation; 52% were female, 82% were white, and their mean ± SD age was 49 ± 15 years (Table 1). Prior to admission to the ICU, 91% of the patients were living independently, and 49% had either full- or part-time employment. Patients had mean Acute Physiology and Chronic Health Evaluation III (APACHE III) scores of 86 ± 26. Mean duration of mechanical ventilation was 11 ± 10 days, and ICU and hospital lengths of stay were 14 ± 11 and 22 ± 15 days, respectively. Pneumonia was identified as the primary risk factor for ARDS in the majority of patients (n = 447 [61%]). Shock at baseline occurred in 41% of the sample. Mean mechanical ventilation-related parameters were: positive end-expiratory pressure, 9 ± 4 mm Hg; inspiratory plateau pressure, 24 ± 6 mm Hg; and Pao2:Fio2 ratio, 166 ± 70. According to the Berlin definition of ARDS severity,35 29% (n = 206) were mild, 53% (n = 382) were moderate, and 18% (n = 129) were severe; the Pao2:Fio2 ratio was missing for 15 patients. Overall, 732 patients completed FACIT-F at either the 6- or 12-month follow-up, with 711 and 659 participants completing the FACIT-F at 6 and 12 months, respectively, representing 94% and 95% response rates among eligible participants.
Table 1.
Characteristics of the Patient Cohort (N = 732)
| Characteristic | Valuea |
|---|---|
| Baseline status prior to ICU admission | |
| Age, mean ± SD, y | 49 ± 15 |
| Male sex | 352 (48) |
| White race | 584 (82) |
| Residence, living independently | 666 (91) |
| Employment, full- or part-time | 344 (49) |
| BMI, mean ± SD, kg/m2 | 31 ± 8 |
| Diabetes comorbidity | 166 (23) |
| Prior stroke with sequelae | 11 (2) |
| End-stage renal disease requiring dialysis | 14 (2) |
| Critical illness status | |
| Medical ICU admission | 409 (56) |
| APACHE III score, mean ± SD | 86 ± 26 |
| Primary lung injury risk factor | |
| Pneumonia | 447 (61) |
| Sepsis | 129 (18) |
| Aspiration | 76 (10) |
| Trauma | 32 (4) |
| Transfusion | 14 (2) |
| Other | 39 (5) |
| Treatment in ICU with | |
| Opioid | 471 (95) |
| Vasopressor | 390 (53) |
| Corticosteroidb | 197 (32) |
| Neuromuscular blocker | 91 (18) |
| Duration of mechanical ventilation, mean ± SD, d | 11 ± 10 |
| ICU length of stay, mean ± SD, d | 14 ± 11 |
| Hospital length of stay, mean ± SD, d | 22 ± 15 |
Data are presented as No. (%), unless otherwise indicated. Proportions might not add to 100% due to rounding. APACHE III = Acute Physiology and Chronic Health Evaluation III severity of illness score.
Missing data: race, n = 22; employment, n = 36; BMI, n = 2; APACHE III score, n = 21; duration of mechanical ventilation, n = 1; ICU length of stay, n = 3; and hospital length of stay, n = 5. Not all of the parent studies collected data for opioid, corticosteroid, or neuromuscular blocker use. For opioid and neuromuscular blocker, N = 495, no missing data; for corticosteroid, N = 634 with missing data, n = 22.
Defined as receiving > 20 mg of methylprednisolone-equivalents on one or more days in the ICU.
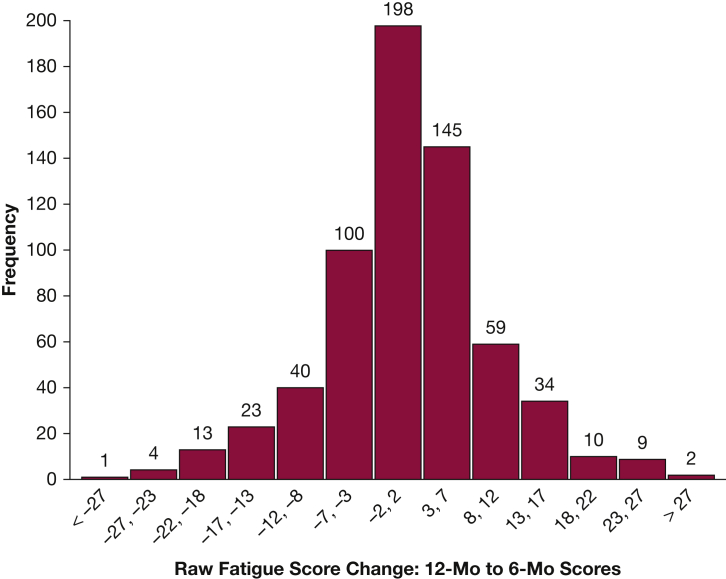
The mean transformed FACIT-F score at the 6- and 12-month follow-up was 60 ± 17 and 62 ± 18, respectively. Clinically important fatigue (transformed FACIT-F ≤ 68) was reported by 70% (n = 501) and 66% (n = 436) at 6 and 12 months. Clinically important changes (defined as ≥ 3 points using raw FACIT-F scores36) between the 6- and 12-month follow-up were observed, with 41% of all patients reporting a clinically important decrease, 28% an increase, and 31% no change (Fig 1).
Figure 1.
Histogram of change in raw fatigue scores between 6- and 12-month follow-up (n = 638). Shown are the raw scores from the validated Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-F) scale (range, 0-52), with higher scores representing less fatigue. Minimally clinically important difference was indicated by a 3-point change in raw score; 181 of 638 (28%) patients had a decrease in score ≥ 3 points (representing increased fatigue); and 259 of 638 (41%) patients had an increase in score ≥ 3 points (representing decreased fatigue) from 6- to 12-month follow-up. A total of 94 FACIT-F scores at 6 and/or 12 months were missing.
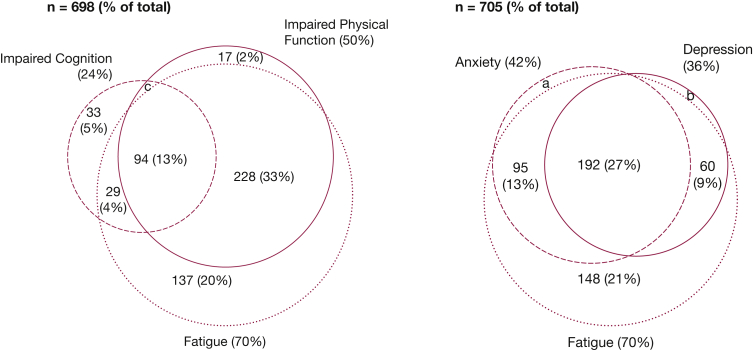
Figure 2 displays the overlap and frequency of clinically important fatigue (transformed FACIT-F score ≤ 68) along with clinically important impairment in physical, cognitive, and mental health status at the 6-month follow-up. The 70% prevalence of clinically important fatigue was greater than impaired physical function (50%), impaired cognition (24%), anxiety (42%), and depression (36%). Almost half (46%) of the cohort reported both impaired physical function and fatigue, and 27% reported co-occurrence of clinically significant symptoms of anxiety, depression, and fatigue.
Figure 2.
Venn diagrams of fatigue symptoms with impaired physical function and cognition, and with depression and anxiety symptoms, at 6-month follow-up. a, n = 10 (1%); b, n = 4 (1%); c, n = 9 (1%). Fatigue, assessed by using the Functional Assessment of Chronic Illness Therapy-Fatigue Scale score (percentage of cohort with score ≤ 68). Impaired physical function, assessed by using the Functional Performance Inventory-Short Form (percentage of cohort with score ≤ 2). Impaired cognition, assessed by using Mini-Mental State Examination (percentage of cohort with score ≤ 24). Anxiety and depression, assessed by using the Hospital Anxiety and Depression Scale (percentage of cohort with subscale scores ≥ 8). Missing data among 732 patients comprised the following: fatigue, n = 11; anxiety, n = 27; depression, n = 27; Functional Performance Inventory, n = 26; and Mini-Mental State Examination, n = 31.
Table 2 reports associations with fatigue at 6- and 12-month follow-up for each baseline and critical illness variable. Adjusting for age, men (vs women) reported less fatigue, with a mean difference of 7.4 (95% CI, 5.1-9.8; P < .001) points on the transformed FACIT-F. Moreover, after adjusting for age and sex, patients employed on a full- or part-time basis prior to onset of ARDS reported less fatigue, with a mean difference of 6.0 (95% CI, 3.6-8.5; P < .001) points. The APACHE III score had no clinically important association with fatigue, with a difference of 10 points in APACHE III being associated with a mean difference of 0.5 (95% CI, 0.03-1.0; P = .04) points on the transformed FACIT-F. Other medical and treatment variables, such as ICU length of stay, presence of diabetes, or types of medications administered during the ICU admission, were not significantly associated with fatigue symptoms at follow-up. A post hoc analysis of the association of ARDS severity with fatigue revealed no statistically significant association (severe vs mild ARDS, P = 0.41; moderate vs mild ARDS, P = .71).
Table 2.
Age and Sex-Adjusted Associations of Individual Baseline and Critical Illness Variables With Fatigue Symptoms Over 6- and 12-Month Follow-up (N = 732)
| Variable | Mean Difference (95% CI) in Fatiguea [Positive Value = Less Fatigue] |
|---|---|
| Baseline status prior to ICU admission | |
| Age (per 10 y) | –0.7 (–1.5 to 0.1) |
| Male sex | 7.4 (5.1 to 9.8) |
| White race | –2.7 (–5.8 to 0.5) |
| Employment (full- or part-time vs unemployed) | 6.0 (3.6 to 8.5) |
| Diabetes comorbidity | –0.3 (–3.1 to 2.6) |
| Critical illness status | |
| Medical ICU | 0.2 (–2.2 to 2.6) |
| APACHE III score (per 10 points) | 0.5 (0.03 to 1.0) |
| Treatment in ICU stay with | |
| Vasopressor | –1.9 (–4.5 to 0.6) |
| Corticosteroidb | –2.1 (–5.1 to 1.0) |
| Neuromuscular blocker | 1.4 (–2.4 to 5.2) |
| ICU length of stay (per 5 d) | –0.5 (–1.1 to 0.1) |
Each row is a separate regression model evaluating the age- and sex-adjusted association of the variable named in each row with fatigue, using a longitudinal time-averaged random effects regression model. For the variables of age and male sex, the regression model only adjusted for sex and age, respectively. Fatigue was measured by using the transformed score from the validated Functional Assessment of Chronic Illness Therapy scale (range, 0-100), with higher scores representing less fatigue. See Table 1 legend for expansion of abbreviation.
Missing data: race, n = 22; employment, n = 36; APACHE III score, n = 21; ICU length of stay, n = 3. Not all of the parent studies collected data for opioid, corticosteroid, or neuromuscular blocker use. For opioid and neuromuscular blocker, N = 495 with no missing data; and for corticosteroid, N = 634 with missing data, n = 22.
Defined as receiving > 20 mg of methylprednisolone-equivalents on one or more days in the ICU.
Table 3 reports associations of physical, cognitive, and mental health status scores with fatigue symptoms at both the 6- and 12-month follow-up. Every model showed a statistically significant and clinically important association between each measure of health status and fatigue symptoms. For example, a 0.5 SD increase in physical functioning status, evaluated via the SF-36v2 Physical Component Summary, and Functional Performance Inventory-Short Form, was associated with less fatigue, with a mean difference of 5.0 (95% CI, 4.6 to 5.4; P < .001) and 4.7 (95% CI, 4.3 to 5.1; P < .001) points, respectively. Similarly, a 0.5 SD increase in anxiety and depressive symptoms was associated with greater fatigue, with a mean difference of –4.9 (95% CI, –5.2 to –4.5; P < .001) and –5.9 (95% CI, –6.2 to –5.6; P < .001).
Table 3.
Age- and Sex-Adjusted Associations of Individual Physical, Cognitive, and Mental Health Status Variables With Fatigue Symptoms at 6- and 12-Month Follow-up (N = 732)
| Variable (Scaled by 0.5 SD) | Mean Difference (95% CI) in Fatiguea [Positive Value = Less Fatigue] | P |
|---|---|---|
| Physical Component summary (SF-36v2) [∼6 points]b | 5.0 (4.6 to 5.4) | < .001 |
| Physical Functioning (FPI-SF) [∼0.5 point] | 4.7 (4.3 to 5.1) | < .001 |
| Cognition (MMSE) [∼1 point] | 0.9 (0.6 to 1.3) | < .001 |
| Mental Component summary (SF-36v2) [∼7 points] | 5.1 (4.7 to 5.5) | < .001 |
| PTSD symptoms (IES-R) [∼0.5 points] | –4.3 (–4.7 to –3.9) | < .001 |
| Anxiety symptoms (HADS-Anxiety subscale) [∼2.5 points] | –4.9 (–5.2 to –4.5) | < .001 |
| Depression symptoms (HADS-Depression subscale) [2.5 points] | –5.9 (–6.2 to –5.6) | < .001 |
For SF-36, FPI-SF, and MMSE, higher scores = better function; for IES-R and HADS, higher scores = greater symptoms. FPI-SF = Functional Performance Inventory-Short Form; HADS = Hospital Anxiety and Depression Scale; IES-R = Impact of Events Scale-Revised; MMSE = Mini-Mental State Examination; PTSD = posttraumatic stress disorder; SF-36v2 = Short Form-36 Version 2.
Each row reports the results of a separate regression model that evaluates the age- and sex-adjusted association of fatigue symptoms with the variable named in that row. All analyses evaluate fatigue symptoms and the variable named in the row at the same follow-up time point. Analyses were conducted by using a longitudinal time-averaged random effects regression model. Fatigue symptoms were measured by using the transformed score from the validated Functional Assessment of Chronic Illness Therapy-Fatigue Scale (range, 0-100), with higher scores representing less fatigue. Values presented represent the estimated mean difference in fatigue score for a 0.5 SD difference in the variable named in the row, over both 6- and 12-month follow-up time points.
Interpretation of this first row is as follows: “If Patient A had a Physical Component Summary score that was 0.5 standard deviations higher than Patient B, then Patient A’s expected fatigue score would be 5.0 points higher than Patient B.”
Table 4 reports on associations of physical, cognitive, and mental health status at 6 months with subsequent fatigue symptoms at 12 months. Although all models showed a statistically significant association, the magnitude of these associations was not clinically important.
Table 4.
Age- and Sex-Adjusted Longitudinal Associations of Individual Physical, Cognitive, and Mental Health Status Variables at 6 Months With Fatigue Symptoms at 12-Month Follow-up (N = 732)
| Variable at 6 Months Follow-up [Scaled by 0.5 SD] | Mean Difference (95% CI) in Fatigue at 12 Monthsa [Positive Value = Less Fatigue] | P |
|---|---|---|
| Physical Component summary (SF-36v2) [∼6 points]b | 1.0 (0.3 to 1.5) | .002 |
| Physical Functioning (FPI –SF) [∼0.5 point] | 1.2 (0.6 to 1.7) | < .001 |
| Cognition (MMSE) [∼1 point] | 0.6 (0.2 to 1.1) | .006 |
| Mental Component summary (SF-36v2) [∼7 points] | 1.0 (0.4 to 1.6) | < .001 |
| PSTD symptoms (IES-R) [∼0.5 points] | –1.1 (–1.6 to –0.5) | < .001 |
| Anxiety symptoms (HADS-Anxiety Subscale) [∼2.5 points] | –1.0 (–1.6 to –0.4) | < .001 |
| Depression symptoms (HADS-Depression Subscale) [2.5 points] | –1.7 (–2.3 to –1.0) | < .001 |
For SF-36, FPI-SF and MMSE higher scores = better function; for IES-R and HADS higher scores = greater symptoms. See Table 1 legend for expansion of abbreviations.
Each row reports the results of a separate regression model that evaluates the age- and sex-adjusted association of the variable named in the row at 6 months, with fatigue symptoms at 12 months. Analyses were conducted by using a longitudinal random effects regression model. Fatigue symptoms were measured by using the transformed score from the validated Functional Assessment of Chronic Illness Therapy-Fatigue Scale (range: 0 to 100), with higher scores representing less fatigue (range, 0-100). Values presented represent the estimated mean difference in 12-month fatigue score for a 0.5 SD difference in the variable named in the row at 6 months.
The interpretation of first row is as follows: “If Patient A had a Physical Component Summary score that was 0.5 standard deviations higher than Patient B at 6 month follow-up, then Patient A’s expected fatigue score would be 1.0 point higher than Patient B’s at 12 month follow-up.”
Discussion
Over the first 12 months after ARDS, clinically important fatigue symptoms were very common in survivors, with 70% prevalence at 6-month follow-up, 31% reporting no clinically important change, and 28% reporting worsening symptoms by the 12-month follow-up. The prevalence of fatigue symptoms is greater than impairment in physical function, cognition, or clinically important symptoms of anxiety or depression in the year following ARDS; fatigue symptoms frequently co-occur and are strongly associated with all of these other impairments. Men and patients employed prior to ARDS reported lower levels of fatigue during follow-up, but critical illness variables during admission for ARDS had little association with fatigue symptoms.
To our knowledge, this study is the first large-scale longitudinal evaluation of fatigue symptoms over the first year after critical illness due to ARDS. A prior study, specifically validating the FACIT-F in ICU patients, evaluated 130 one-year survivors from a single mixed ICU (64% surgical) in Italy.22 This study reported a mean ± SD FACIT-F transformed score of 66 ± 12, similar in magnitude to our score of 62 ± 18. By way of comparison, the mean FACIT-F scores from our ARDS study and the prior Italian ICU study are worse than the mean ± SD score of 68 ± 15 reported in nonanemic patients with solid and hematologic tumors prior to chemotherapy or radiation therapy, and of 75 ± 15 reported by the normal population, but better than scores of 50 ± 14 reported by oncology patients with anemia.23
Another ICU study evaluated fatigue symptoms using a different instrument, the Multidimensional Fatigue Inventory in 195 sepsis survivors admitted to a single German ICU at 6 months following discharge.37 This study reported a 45% prevalence of clinically relevant fatigue symptoms and significant associations with a diagnosis of major depressive disorder or posttraumatic stress disorder at the 6-month follow-up. Due to use of differing fatigue instruments, we cannot directly compare these scores. However, this single-site German sepsis study supports our national US-based ARDS study findings by reinforcing that clinically important fatigue symptoms are very common in critical illness survivors and co-occur with other post-ICU morbidities.
A recent study of 1,290 patients with COPD reported that three-quarters had fatigue, evaluated by using the Checklist Individual Strength-Fatigue instrument.38 Although fatigue was significantly associated with lung function (eg, FEV1), 70% of the variance in fatigue scores could not be explained by demographic characteristics, clinical features, or COPD severity. This is similar to our study and emphasizes that, in addition to physical status, fatigue symptoms are associated with cognitive and mental health status. This observation suggests that a comprehensive evaluation of patient status is indicated when evaluating symptoms of fatigue.
Despite robust associations at the same follow-up time points, we found that physical, cognitive, and mental health status at 6 months was not strongly associated with subsequent fatigue symptoms at 12 months. This finding may be due to heterogeneity of fatigue symptom trajectories from the 6- to 12-month follow-up, along with dynamic changes in physical, cognitive, and mental health status also occurring during this stage of recovery. These findings emphasize the importance of broadly evaluating patient status at each follow-up assessment.
Understanding the many correlates with fatigue symptoms is important when considering treatment options. Evidence in other medical populations suggests that a comprehensive, multicomponent treatment may be most effective. For example, fatigue management in patients recovering from cancer,39, 40, 41, 42, 43 traumatic brain injury,44, 45, 46 HIV,47,48 and multiple sclerosis49, 50, 51 include development of an exercise program, proper nutrition/hydration, mood management, activity pacing, medication review, and sleep hygiene; each represents issues that are frequently disrupted during critical illness recovery.
The strengths of the current study include the large number of patients recruited from numerous study sites across the United States, along with very low loss to follow-up and high completion rates of multiple well-validated outcome measures, despite high levels of participant fatigue. Compared with our follow-up of 94% and 95% at 6 and 12 months, respectively, previous ICU studies had 47% and 43% follow-up.22,37
The current study has potential limitations. First, although the FACIT-F was previously validated in ICU survivors,22 most data used to interpret FACIT-F scores are extrapolated from other populations.20,21,23 However, our fatigue findings in ARDS survivors are consistent with these other populations. Second, the evolution of health status during post-ARDS recovery could directly affect the functional measures and/or reports of fatigue. We suggest that future work include more detailed evaluations of such hypotheses. Third, the use of validated surveys in this study provided patient-reported perspectives on fatigue along with physical, cognitive, and mental health status. Future research should consider performance-based physical testing (eg, electromyography/nerve conduction testing, muscle strength testing, 6-min walk test), detailed cognitive testing, and psychiatric diagnosis (eg, semi-structured interview by a trained clinician). Research including such performance-based tests may be helpful in delineating potential interventions that might target physical, cognitive, and mental health status, as well as fatigue symptoms in survivors in the year following ARDS.
Interpretation
In the first year following ARDS, more than two-thirds of survivors report clinically significant and persistent fatigue symptoms. Such symptoms should prompt clinicians to broadly evaluate physical, cognitive, and mental health status among survivors due to frequent co-occurrence of impairments in health status with fatigue, and they should prompt researchers to design and evaluate multicomponent interventions to address this common problem in an effort to improve the outcomes of ARDS survivors.
Acknowledgments
Author contributions: The senior author (D. M. N.) is the guarantor of the content of the manuscript, including the data and analysis. K. J. N. conceived of the design of the study, directed the data interpretation, and wrote the manuscript. J.-M. S. L. designed and supervised the analysis, and H. Y. performed the statistical analyses. J.-M. S. L., H. Y., S. L., J. S. Z., V. D. D., M. M. H., A. M. P., R. O. H., and D. M. N. contributed substantially to the interpretation and review of the data and review of the manuscript, critically revising it for important intellectual content. D. M. N. designed the original study, had full access to all of the data, and takes responsibility for the integrity of the data. All authors gave final approval of the submitted version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: K. J. N. has received research grant funds from Hitachi Corporation for the study of Near Infra-red spectroscopy in delirium; and has been a consultant to Merck & Co. None declared (J.-M. S. L., H. Y., S. L., J. S. Z., V. D. D., M. M. H., A. M. P., R. O. H., D. M. N.).
Role of the sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This research was supported by the National Heart, Lung, and Blood Institute [Grants R24 HL111895, R01HL091760, and R01HL091760-02S1], the Johns Hopkins Institute for Clinical and Translational Research [Grant UL1 TR 000424-06], and the Albuterol for Treatment of Acute Lung Injury Trial (ALTA), Early Versus Delayed Enteral Nutrition Trial (EDEN), Omega Nutrition Supplement Trial (OMEGA), and Statins for Acutely Injured Lungs from Sepsis Trial (SAILS) [National Heart, Lung, and Blood Institute contracts HHSN268200536165C to HHSN268200536176C and HHSN268200536179C].
References
- 1.Desai S.V., Law T.J., Needham D.M. Long-term complications of critical care. Crit Care Med. 2011;39(2):371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins R.O., Weaver L.K., Pope D., Orme J.F., Bigler E.D., Larson-Lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 3.Davydow D.S., Desai S.V., Needham D.M., Bienvenu O.J. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70(4):512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson J.C., Pandharipande P.P., Girard T.D. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikayin S., Rabiee A., Hashem M.D. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23–29. doi: 10.1016/j.genhosppsych.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabiee A., Nikayin S., Hashem M.D. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744–1753. doi: 10.1097/CCM.0000000000001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker A.M., Sricharoenchai T., Raparla S., Schneck K.W., Bienvenu O.J., Needham D.M. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 8.Herridge M.S., Moss M., Hough C.L. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42(5):725–738. doi: 10.1007/s00134-016-4321-8. [DOI] [PubMed] [Google Scholar]
- 9.Chaboyer W., Grace J. Following the path of ICU survivors: a quality-improvement activity. Nurs Crit Care. 2003;8(4):149–155. doi: 10.1046/j.1478-5153.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 10.Steenbergen S., Rijkenberg S., Adonis T., Kroeze G., van Stijn I., Endeman H. Long-term treated intensive care patients outcomes: the one-year mortality rate, quality of life, health care use and long-term complications as reported by general practitioners. BMC Anesthesiol. 2015;15:142. doi: 10.1186/s12871-015-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azoulay E., Vincent J.L., Angus D.C. Recovery after critical illness: putting the puzzle together—a consensus of 29. Crit Care. 2017;21(1):296. doi: 10.1186/s13054-017-1887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latronico N., Herridge M., Hopkins R.O. The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med. 2017;43(9):1270–1281. doi: 10.1007/s00134-017-4757-5. [DOI] [PubMed] [Google Scholar]
- 13.Dinglas V.D., Hopkins R.O., Wozniak A.W. One-year outcomes of rosuvastatin versus placebo in sepsis-associated acute respiratory distress syndrome: prospective follow-up of SAILS randomised trial. Thorax. 2016;71(5):401–410. doi: 10.1136/thoraxjnl-2015-208017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Needham D.M., Dinglas V.D., Bienvenu O.J. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ. 2013;346:f1532. doi: 10.1136/bmj.f1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Rice TW, Wheeler AP, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice T.W., Wheeler A.P., Thompson B.T., deBoisblanc B.P., Steingrub J., Rock P. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581. doi: 10.1001/jama.2011.1435. [published correction appears in JAMA. 2012;307(6):563] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Matthay MA, Brower RG, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Truwit J.D., Bernard G.R. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Needham D.M., Colantuoni E., Dinglas V.D. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med. 2016;4(3):203–212. doi: 10.1016/S2213-2600(16)00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acaster S., Dickerhoof R., DeBusk K., Bernard K., Strauss W., Allen L.F. Qualitative and quantitative validation of the FACIT-fatigue scale in iron deficiency anemia. Health Qual Life Outcomes. 2015;13:60. doi: 10.1186/s12955-015-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cella D., Yount S., Sorensen M., Chartash E., Sengupta N., Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005;32(5):811–819. [PubMed] [Google Scholar]
- 22.Spadaro S., Capuzzo M., Valpiani G. Fatigue in intensive care survivors one year after discharge. Health Qual Life Outcomes. 2016;14(1):148. doi: 10.1186/s12955-016-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella D., Lai J.S., Chang C.H., Peterman A., Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 24.Ware J.E., Jr., Kosinski M., Dewey J.E. Quality Metric Incorporated; Lincoln, RI: 2000. How to Score Version 2 of the SF-36 Health Survey. [Google Scholar]
- 25.Leidy N.K. Psychometric properties of the functional performance inventory in patients with chronic obstructive pulmonary disease. Nurs Res. 1999;48(1):20–28. doi: 10.1097/00006199-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Leidy N.K., Hamilton A., Becker K. Assessing patient report of function: content validity of the Functional Performance Inventory-Short Form (FPI-SF) in patients with chronic obstructive pulmonary disease (COPD) Int J Chron Obstruct Pulmon Dis. 2012;7:543–554. doi: 10.2147/COPD.S32032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folstein M.F., Folstein S.E., McHugh P.R. "Mini-mental state." A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Crum R.M., Anthony J.C., Bassett S.S., Folstein M.F. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- 29.Bienvenu O.J., Williams J.B., Yang A., Hopkins R.O., Needham D.M. Posttraumatic stress disorder in survivors of acute lung injury evaluating the impact of event scale-revised. Chest. 2013;144(1):24–31. doi: 10.1378/chest.12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zigmond A.S., Snaith R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Snaith R.P. The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton L.C. Duxbury Thompson Learning; Belmont, CA: 2017. Statistics with STATA 15. [Google Scholar]
- 33.Norman G.R., Sloan J.A., Wyrwich K.W. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 34.Chan K.S., Aronson F.L., Bienvenu O.J. Distribution-based estimates of minimal important difference for hospital anxiety and depression scale and impact of event scale-revised in survivors of acute respiratory failure. Gen Hosp Psychiatry. 2016;42(4):32–35. doi: 10.1016/j.genhosppsych.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 36.Cella D., Eton D.T., Lai J.S., Peterman A.H., Merkel D.E. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 37.Wintermann G.B., Rosendahl J., Weidner K., Strauß B., Hinz A., Petrowski K. Self-reported fatigue following intensive care of chronically critically ill patients: a prospective cohort study. J Intensive Care. 2018;6:27. doi: 10.1186/s40560-018-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goërtz Y.M.J., Spruit M.A., Van‘t Hul A.J. Fatigue is highly prevalent in patients with COPD and correlates poorly with the degree of airflow limitation. Ther Adv Respir Dis. 2019;13 doi: 10.1177/1753466619878128. 1753466619878128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson E.J.M., Morris M.E., McKinstry C.E. Cancer related fatigue: implementing guidelines for optimal management. BMC Health Serv Res. 2017;17(1):496. doi: 10.1186/s12913-017-2415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebede C.C., Jang Y., Escalante C.P. Cancer-related fatigue in cancer survivorship. Med Clin North Am. 2017;101(6):1085–1097. doi: 10.1016/j.mcna.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Escalante C.P., Manzullo E.F. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412–S416. doi: 10.1007/s11606-009-1056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohandas H., Jaganathan S.K., Mani M.P., Ayyar M., Rohini Thevi G.V. Cancer-related fatigue treatment: an overview. J Cancer Res Ther. 2017;13(6):916–929. doi: 10.4103/jcrt.JCRT_50_17. [DOI] [PubMed] [Google Scholar]
- 43.Berger A.M., Mitchell S.A., Jacobsen P.B., Pirl W.F. Screening, evaluation, and management of cancer-related fatigue: ready for implementation to practice? CA Cancer J Clin. 2015;65(3):190–211. doi: 10.3322/caac.21268. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen S., McKay A., Wong D. Cognitive behavior therapy to treat sleep disturbance and fatigue after traumatic brain injury: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98(8):1508–1517.e2. doi: 10.1016/j.apmr.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 45.Marshall S., Bayley M., McCullagh S. Updated clinical practice guidelines for concussion/mild traumatic brain injury and persistent symptoms. Brain Inj. 2015;29(6):688–700. doi: 10.3109/02699052.2015.1004755. [DOI] [PubMed] [Google Scholar]
- 46.Lequerica A.H., Botticello A.L., Lengenfelder J. Factors associated with remission of post-traumatic brain injury fatigue in the years following traumatic brain injury (TBI): a TBI model systems module study. Neuropsychol Rehabil. 2017;27(7):1019–1030. doi: 10.1080/09602011.2016.1231120. [DOI] [PubMed] [Google Scholar]
- 47.Barroso J., Madisetti M., Mueller M. A feasibility study to develop and test a cognitive behavioral stress management mobile health application for HIV-related fatigue. J Pain Symptom Manage. 2020;59(2):242–253. doi: 10.1016/j.jpainsymman.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perazzo J., Webel A., Voss J., Prince-Paul M. Fatigue symptom management in people living with human immunodeficiency virus. J Hospice Palliative Nursing. 2017;19(2):122–127. doi: 10.1097/NJH.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan F., Amatya B., Galea M. Management of fatigue in persons with multiple sclerosis. Front Neurol. 2014;5:177. doi: 10.3389/fneur.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tur C. Fatigue management in multiple sclerosis. Curr Treat Options Neurol. 2016;18(6):26. doi: 10.1007/s11940-016-0411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finlayson M., Akbar N., Turpin K., Smyth P. A multi-site, randomized controlled trial of MS INFoRm, a fatigue self-management website for persons with multiple sclerosis: rationale and study protocol. BMC Neurol. 2019;19(1):142. doi: 10.1186/s12883-019-1367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]