Abstract
Approximately 300 million people worldwide are estimated to be affected by asthma, and the number of patients affected is growing exponentially—with potential for an additional 100 million people affected by the condition by 2025. With this increasing burden of disease, there is high motivation to discover effective prevention strategies. Strategies aimed at stalling the atopic progression, modifying the microbiome, preventing respiratory viral infections, and reducing the impact of toxin/pollutant exposure through dietary supplements have had limited success in the prevention of asthma. This is likely because asthma is heterogenous and is influenced by different genetic and environmental factors. Genes underlie a predisposition to asthma and allergic sensitization, whereas exposure to allergens, respiratory infections, and pollution may modify asthma pathogenesis and the variation in severity seen among individuals. Future advances in asthma prevention may include a more personalized approach: genetic variations among susceptible individuals with distinct asthma phenotypes or different biomarkers of disease may help individualize prevention strategies and render them more . In this article, we summarize interventions that have been studied for the prevention of asthma and identify some of the clinical trials that are actively underway in asthma prevention.
Key Words: asthma, immunotherapy, omalizumab, prevention, vitamin D
Abbreviations: AR, allergic rhinoconjunctivitis; COAST, Childhood Origins of ASThma; HDM, house dust mite; IT, immunotherapy; LRTI, lower respiratory tract infection; RCT, randomized controlled trial; RSV, respiratory syncytial virus; RV, rhinovirus; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy
Asthma disproportionately affects children and represents the most common non-communicable disease of childhood.1 Approximately 300 million people worldwide are estimated to be affected by asthma, and the number of patients affected is growing—with the potential for an additional 100 million people affected by 2025.2 Abnormalities in the lung function of patients with asthma can be seen early in life and often persist. Asthma can predispose a child to COPD, which is a common cause of mortality in adulthood.3 Given the significant burden asthma imposes on a person’s health, the health care system, and the global economy, effective interventions to prevent asthma are desperately needed.
The earliest strategies aimed at asthma prevention stem from limiting the atopic march. The “atopic march” refers to IgE-mediated allergic progression beginning with eczema and food allergy in infancy, followed by aeroallergen sensitization in the preschool age, and culminating in the development of allergic rhinoconjunctivitis (AR) and chronic asthma.4 Asthma prevention strategies, including allergen avoidance and allergen immunotherapy, have targeted steps in the atopic march with varying success.5
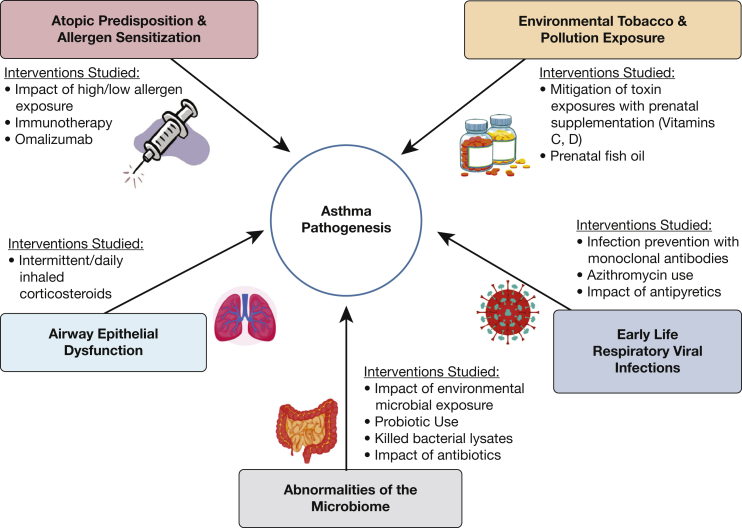
The influence of environmental triggers in asthma pathogenesis, such as pollution exposure and respiratory viral infections, is well established.6,7 Our ability to intervene in these contributing factors through intrauterine and early life interventions can alter the course of the disease and is the focus of ongoing investigation. Attention to asthma prevention has also included modification of the microbiome (Fig 1). Promising, ongoing studies involve modulation of the microbiome,8,9 administration of antiinflammatory medications to children with viral respiratory infections, and implementation of biologic therapy in high-risk toddlers.10,11
Figure 1.
Factors involved in asthma pathogenesis and attempted strategies at prevention.
Avoidance of Allergic Sensitization
Links between allergic sensitization and asthma pathogenesis have been reported in the literature. In a 2017 study, 127 infants hospitalized or seen in the ED for first severe wheeze were followed-up to determine asthma prevalence at 8 years. The 17% who were sensitized to allergens at study entry had 12 times higher risk of developing asthma by age 8 than their nonsensitized counterparts.12 Information from the Childhood Origins of ASThma (COAST) cohort further informs the relationship between allergen exposure and asthma development. Sensitization to specific perennial allergens, such as cat and dog, was highly associated with increased asthma risk. Interestingly, dog exposure at birth was associated with reduced risk of asthma, regardless of sensitization status, indicating that allergic sensitization alone may not be enough to cause asthma, and other factors are involved.13
Given the association between allergic sensitization and the development of atopy and asthma, allergen avoidance was studied as a means of asthma prevention, yielding conflicting results. In the Isle of Wight prevention study, infants at high risk of atopy were randomized to an intervention group that included reduced ingested and inhaled allergen exposure during early development. As compared with control subjects, a significant decrease was found in atopic dermatitis, asthma, AR, and atopy in the prevention group.14 By age 18, a 75% reduced risk of asthma was seen in the intervention group, but the prevalence of allergic sensitization had equalized between the groups.15 Conversely, another cohort study demonstrated that high allergen (dog, cat, cockroach, and mouse) exposure early in life was associated with lower rates of asthma later in life.16 Multiple other studies have been equivocal or have shown an increase in atopy through allergen avoidance (Table 1).13, 14, 15,17, 18, 19
Table 1.
Selected Studies Evaluating the Association Between Allergen Exposure and Asthma Development
| Study | Study Design | Asthma and Other Clinically Important Outcomes |
|---|---|---|
| Cullinan et al17 (2004) | Prospective cohort study of children followed from birth to 5.5 y. Home Der p 1 and Fel d 1 measured at 8 wks. Report of wheeze assessed yearly. SPT completed at 5.5 y. | 7% had atopic wheeze. Risk of asthma rose at low exposure level and then was attenuated thereafter. 10% sensitized to HDM or cat. No relation between allergen exposure and sensitization or wheeze found. |
| Marks et al18 (2006) | Prospective RCT of children with family history of asthma were randomized antenatal to HDM avoidance vs control subject and reduced allergen diet vs control subject. Assessed for asthma and SPT at age 5. | No significant difference in the prevalence of asthma, eczema, or atopy. |
| Arshad et al14 (2007) Scott et al15 (2012) |
Prospective single-blind RCT of infants recruited prenatally from atopic families. Low allergen infants breast-fed by mother on low allergen diet or hydrolyzed formula. HDM exposure reduced by acaricide and mattress covers vs control subject. | At age 8, statistically significant decrease in asthma, allergic sensitization to foods and HDM. Less AR, AD, and atopy in the intervention group. Follow-up study at age 18, statistically significant decrease in asthma, but no difference in allergic sensitization to foods, HDM, or atopy in the intervention group. |
| Stoltz et al13 (2013) | Prospective longitudinal cohort study of high-risk children enrolled in the COAST study. Specific IgE measured at 1, 3, 6, and 9 y. Asthma and AR diagnosed at 6 and 8 y. | Sensitization to cat and dog associated with increased asthma risk. Sensitization to perennial vs seasonal allergens also associated with asthma risk. Dog exposure at birth associated with decreased asthma risk, regardless of sensitization. Sensitization to seasonal allergens more associated with rhinitis risk. |
| Belgrave et al19 (2018) | Prospective longitudinal cohort study of patients from ages 5-24 from MAAS and ALSPAC cohorts followed with spirometry to ascertain trajectories of FEV1. | Participants with severe recurrent wheeze and allergic sensitization at 3 y had increased likelihood of belonging to the subgroup with persistently low FEV1. |
ALSPAC = Avon Longitudinal Study of Parents and Children; AR = allergic rhinoconjunctivitis; HDM = house dust mite; IT = immunotherapy; MAAS = Manchester Asthma and Allergy Study; RCT = randomized controlled trial; SCIT = subcutaneous immunotherapy; SLIT = sublingual immunotherapy; SPT = skin prick test.
Recent studies confirm that the relationship between allergen exposure and asthma development is complicated, mediated by genes and gene methylation.20 Gene-environment interactions, such as the effects of pet exposure on those with different genotypes, have been studied. For example, those with a high-risk variant in the chromosome 17q21 locus may benefit from early exposure to cat allergen, more than those with other genotypes.20 Additionally, patterns of gene methylation, for example, of the gene encoding CD14, may be impacted by environmental allergen and pollutant exposure and underscore development of allergic disease.21 Recent analyses suggest that clusters of exposure combined with specific sensitization can augment asthma risk, highlighting the complexity of this progression.16
Allergen Immunotherapy
Aside from allergen avoidance for the prevention of asthma, targeting allergic sensitization through immunotherapy (IT) has been studied (Table 2).22, 23, 24, 25, 26 IT interferes with the pathophysiology that mediates allergic and asthmatic disease, and can impact the long-term trajectory of atopy and asthma. IT augments specific IgG4, reducing late-phase airway hyperreactivity and allergic inflammation.27 Several randomized controlled trials (RCTs) have demonstrated that IT minimizes new allergic sensitization in individuals already sensitized to other allergens.23,24,28 However, efficacy of IT for prevention of asthma has varied. Jacobsen and colleagues22 evaluated 3 years of subcutaneous IT (SCIT) in children with seasonal AR and found that at 10 years, AR and reported asthma rates were significantly lower in the SCIT group. The study had several limitations: it was not blinded or placebo-controlled, and although reported diagnoses of asthma were significantly different, bronchial hyperresponsiveness was not.22
Table 2.
Selected Studies Evaluating the Role of Immunotherapy in Asthma Prevention
| Study | Study Design | Asthma and Clinically Important Outcomes |
|---|---|---|
| Novembre et al23 (2004) | Prospective randomized open controlled study of children aged 5-14 y with AR and grass allergy, no prior signs of asthma. Intervention group received 3 y of SLIT vs standard therapy. | Fourfold increased rate of asthma in the grass-allergic children not treated with IT. Treated children had reduced symptoms and used less medication in the second and third years. |
| Jacobsen et al22 (2007) | Prospective open, partially RCT involving those 16-25 y, with grass ± birch allergy. Intervention arm received SCIT birch ± grass SCIT for 3 y vs standard medical therapy. | In this open, non-placebo-controlled study of older patients, the SCIT-treated group had significantly lower rates of asthma, and their AR improved more than that of the control group. |
| Zolkipli et al24 (2015) | Prospective, double-blind RCT of infants at high risk of atopy, but not yet sensitized. Intervention involved HDM extract given orally twice daily for 12 mo vs placebo. | SLIT had no significant preventative effect on wheeze. Sensitization to any allergen 25.5% in control group vs 16% in the intervention group. No effect on AD or food allergy. |
| Marogna et al25 (2017) | Prospective open, nonrandomized, trial of patients with AR aged 8-57 y mono-sensitized to HDM. Interventions included SLIT, adjuvanted SLIT, vs standard pharmacotherapy only (control group). | Over the 5 y of the study, control patients had a decline in FEV1, IT group had stable FEV1. After 5 y, 58% of control patients developed new sensitization, compared with 13.2% in the SLIT group and 8.1% in the adjuvanted SLIT group. |
| Valovirta et al26 (2018) | Prospective, double-blind RCT of children, aged 5-12 y with AR caused by grass pollen, but no asthma. Intervention involved 3 y of treatment with grass SLIT vs placebo. | Primary outcome not reached: no difference in time to asthma diagnosis. SLIT reduced asthma symptoms, use of asthma medications at the end of the trial (5 y). Total IgE, grass pollen-specific IgE and SPT reactivity reduced in the intervention group. |
See Table 1 legend for expansion of abbreviations.
In addition to SCIT, sublingual IT (SLIT) has been studied for asthma and atopy prevention.23,25,26,28 In an RCT involving high-risk infants, the intervention group was treated with twice-daily house dust mite (HDM) SLIT for 1 year, while the control group was treated with placebo. Although the study confirmed decreased sensitization to common allergens in the intervention group, no effect was found on the development of eczema, food allergy, or wheeze.24 Another open RCT was more promising. Children between ages 5 and 14 with AR who were sensitized to grass received 3 years of grass SLIT vs standard medical therapy. The control group had a fourfold increased rate of asthma, suggesting that SLIT may have a protective effect.23
In patients sensitized to HDM who were treated with SLIT, spirometry and bronchoprovocation via methacholine were studied in an open trial, which concluded that SLIT, over a 5-year period, had a protective effect on pulmonary function, as compared with a control group of sensitized individuals who did not receive IT.25 A meta-analysis evaluated IT in those with AR and found a significant reduction in the development of asthma in the 2 years after IT.29 Whether this benefit is pervasive is unclear. The results of these studies must be interpreted carefully, because some were open studies, and in some cases they were not placebo-controlled. RCTs are needed to rigorously study which patients will benefit from IT, and for how long.
Inhaled Corticosteroids for Asthma Prevention
Lung function abnormalities and airway remodeling can be seen in early life in those who develop persistent asthma. The degree of FEV1/FVC ratio impairment in childhood was found to be the largest predictor of asthma remission by adulthood. Decreased airway responsiveness in childhood was also a predictor of remission of asthma.30 Interventions to improve the lung function of young children at high risk of developing asthma have been evaluated in asthma prevention. In an extension of the Prevention of Early Asthma in Kids study, high-risk 2- to 3-year-olds were treated with 2 years of inhaled fluticasone and observed for 1 year after.43 During the treatment period, the intervention increased episode-free days and reduced asthma exacerbations. However, after cessation of therapy, no evidence was found that indicated that treatment improved lung function or modified asthma development.31 This study, and others evaluating the use of inhaled corticosteroids in at-risk children, did not support the use of inhaled corticosteroids in modifying the course of disease.31, 32, 33
Inherent airway epithelial cell dysfunction is now considered integral in asthma pathogenesis.34 Previous publications have identified discrepancies in the airway epithelium in patients with asthma and people who do not have asthma. However, although differences in nasal airway epithelial cells between are apparent at birth, this alone is not entirely predictive, indicating that asthma onset is multifactorial. A study is underway that evaluates the interplay of genetics, the immune system, and the airway microbiome, with the objective of understanding the effect of each on the airway epithelium.35
Modifications of the Intrauterine Environment: Supplements During Pregnancy
The intrauterine period may represent a critical window in the development of atopy and asthma. Vitamin C supplementation during pregnancy has been studied for asthma prevention and has shown benefit to offspring of mothers who smoked during pregnancy. Children born to mothers who smoked during pregnancy demonstrate lifelong decreases in pulmonary function and increased risk of childhood asthma.7 Approximately half of smokers continue smoking while pregnant; approximately 12% of infants born in the United States are exposed to maternal smoking in utero.36 A double-blind RCT of pregnant smokers evaluated vitamin C supplementation and found that infants born to the vitamin C intervention group had improved neonatal pulmonary function and decreased wheeze in the first year of life. However, there was no difference in lung function at 1 year. Mothers who were homozygous for a polymorphism in the gene encoding the alpha-5 nicotinic acetylcholine receptor had a greater response to the vitamin C than heterozygotes or those without the polymorphism.37 Mechanistically, DNA methylation is altered by maternal smoke exposure in utero.6 Vitamin C administration during pregnancy was associated with a reduction or reversal in smoking-related methylation changes across the genome and across tissues.7 Follow-up investigation is required to determine whether supplementation with vitamin C reduces asthma rates.
Prenatal supplementation with vitamin D has also been evaluated. Vitamin D may be important for pulmonary and immunologic development during the prenatal period and early life.38 It may attenuate airway inflammation in the setting of pollution, and it may be useful for secondary prevention of asthma. Bolcas and colleagues39 reported that vitamin D protects against asthma development in the setting of exposure to air pollution: Maintenance of normal vitamin D status mitigated airway hyperresponsiveness in allergic asthma that was worsened by diesel exhaust, possibly by reducing pulmonary Th2/TH17 cells.39 In an RCT, the Vitamin D Antenatal Asthma Reduction Trial, either 400 or 4,400 IU vitamin D3 was administered during weeks 10 through 18 of pregnancy to expectant mothers with offspring projected to be at high risk of asthma. In both an intention-to-treat analysis and an analysis with stratification according to maternal vitamin D level during pregnancy, there was no effect of maternal vitamin D supplementation on asthma development, recurrent wheeze, or both.40
Antenatal fish oil supplementation has also been studied in asthma prevention. A systematic review and meta-analysis showed an inverse relationship between expectant mothers’ and infants’ fish intake and the development of childhood asthma.41 Bisgaard and colleagues42 completed an RCT involving expectant mothers and concluded that supplementation with 2.4 g omega-3 fish oil in the third trimester reduced persistent wheeze/asthma and lower respiratory tract infections (LRTIs) in offspring by approximately one third. The effects were strongest in mothers with low baseline dietary intake of long-chain omega-3 fatty acids, and those with a genotype associated with low levels of these fatty acids.42 Given these effect modifiers, whether this intervention could be universally helpful in preventing asthma remains controversial.
Modification of the Microbiome
Environmental microbial exposures may influence asthma development. Literature suggests that diverse microbial exposure, as in agricultural communities, may decrease asthma and allergic disease. However, the nuances of which microbes and how many is poorly understood. A recent study of house dust samples from children with and without asthma showed that bacterial richness was inversely associated with ever having asthma.43 Specifically, of the 658 genera detected in the dust samples, abundance of Lactococcus genus was considered to be an independent risk factor for asthma, whereas 12 other genera (mostly from order Actinomycetales) were identified as protective. Diversity of ingested, inhaled, and cutaneous bacterial exposures may be integral in immunologic maturation and protective against asthma.43
The intestinal microbiome is the most diverse and largest microbial environment in the body and includes approximately 40 trillion bacteria.44 Neonates at risk of asthma exhibit dysbiosis of the gut, which increases 12,13-diHOME (a lipid) concentration in their feces. In a murine model, abdominal treatment with 12,13-diHOME impeded immune tolerance by decreasing pulmonary Treg cells and increasing pulmonary inflammation. This finding offers a mechanistic link between abnormalities of the gut microbiome and asthma pathogensis.45 Antibiotics, known to alter the GI microbiome, also have been linked in a dose-responsive manner to increased asthma diagnosis in childhood, even after exclusion of children prescribed antibiotics for respiratory infection.46 Shifting the intestinal flora through the use of probiotics has been evaluated in asthma prevention in humans. A meta-analysis concluded that probiotics administered to pregnant women, breastfeeding mothers, or infants may prevent atopic dermatitis, but not prevent other allergic conditions such as asthma.47 A double-blind RCT involving Lactobacillus supplementation over the first 6 months of life in high-risk infants was conducted, and it showed no significant reduction in the rate of asthma development.48
The airway microbiome has been studied in asthma pathogenesis. Bisgaard and colleagues49 found that neonatal nasopharyngeal colonization by Moraxella catarrhalis, Haemophilus influenzae, or Streptococcus pneumoniae was associated with a higher risk of developing asthma.49 Dysfunctional mucosal immune responses due to bacterial colonization may underscore these different outcomes.50
OM-85 is an oral immunomodulator containing a bacterial lysate of pathogenic bacteria that can stimulate immune defenses, is used to prevent recurrent infections, and is being studied in asthma prevention. Data supporting the use of bacterial lysates for asthma prevention vary. In one mouse model of asthma, OM-85 did not reduce pulmonary eosinophilic response or improve lung resistance, but it decreased IL-5 and IL-13 in BAL fluid.9 In another murine model, oral administration of OM-85 attenuated airway inflammation as measured by airway wall thickness, luminal stenosis, mucus plugging, and eosinophilic infiltration.8 In humans, metanalysis involving 4,851 pediatric patients confirmed that OM-85 reduced the frequency of respiratory infection.51 An RCT of 59 infants at high risk of asthma found that time to first significant LRTI was longer, and number of days with severe symptoms was lower in the OM-85 arm; however, frequency of significant LRTI was not different between the two groups.52 A clinical trial, NCT02148796, is ongoing in high-risk infants, to evaluate the efficacy of killed bacterial lysates. In this double-blind RCT, OM-85 or placebo is administered for 10 days per month over a 2-year period. The time to occurrence of first LRTI with wheezing is studied during a third observation year.53
Preventing Early Life Viral Respiratory Infections
Virus-induced wheezing often precedes asthma diagnosis. Respiratory syncytial virus (RSV) in infancy is associated with wheezing in early life and more than doubles risk of asthma by age 6.54 Scheltema and colleagues55 studied the effect of RSV prevention on asthma development. Preterm infants received palivizumab (RSV monoclonal antibody) vs placebo in their first RSV season. The treatment reduced RSV infection and wheezing though 1 year, but by 6 years, there was no significant difference in pulmonary health.55 Similarly, the American Indian RSV prevention trial, which studied full-term infants using motavizumab (RSV monoclonal antibody), showed no improvement in rates of medically attended wheezing in children aged 1 to 3 years. The authors concluded their findings “did not support a direct, generalizable, causal association between RSV LRTI and subsequent long-term wheezing in term infants.”56 Mitigation of RSV is still being studied as a means of wheeze or asthma prevention (Table 3).10,11,53,57, 58, 59
Table 3.
Selected Ongoing Clinical Trials in the Prevention of Asthma
| Trial Number | Study Design | Primary Outcome of Study |
|---|---|---|
| NCT0214879653 | Double-blind, RCT of Broncho-Vaxom vs placebo for 10 d/mo for 2 y in children 6-18 mo old at high risk of asthma. Followed by observation year. | Time to LRTI with wheeze in the third year of study. |
| NCT0257098410 | Double-blind RCT of omalizumab vs placebo every 4 wks for 2 y starting at 24-47 mo of age in wheezing children at high risk of asthma because of sensitization to aeroallergens. | Asthma diagnosis during the second of 2 y of observation. |
| NCT0262494758 | Observer-blind, RCT of RSV F vaccine vs placebo in 4,636 third-trimester pregnant women. Maternal subjects studied for ∼ 9 mo after the first dose and the infant studied for 1 y of life. | Incidence of significant RSV LRTI (hypoxia or tachypnea) in the first 90 d of life. Also evaluating wheeze in the first year. |
| NCT0287833058 | Double-blind, RCT evaluating MEDI8897, an anti-RSV monoclonal antibody with an extended half-life in healthy preterm infants. | Number of infants hospitalized with LRTI due to RSV. |
| NCT0291193511 | Double-blind RCT of children aged 1-18 mo hospitalized with RSV bronchiolitis and treated with azithromycin vs placebo × 2 wks and observed for up to 48 mo. | Occurrence of recurrent wheeze during preschool years. |
| U1111-1203-196164 | RCT of infants randomized to receive either exclusively paracetamol vs ibuprofen prn fever/pain in the first year of life and asthma rates evaluated. | Asthma diagnosis at age 6. |
LRTI = lower respiratory tract infection; RSV = respiratory syncytial virus. See Table 1 legend for expansion of other abbreviations.
Early life rhinovirus (RV) infection may be an even stronger contributor to asthma pathogenesis than RSV.60 It is postulated that RV disrupts the airway epithelium, upregulates IL-25 and IL-33, and catalyzes type 2 airway inflammation and remodeling.61 In COAST, outpatient wheezing due to RV through infancy was the most significant predictor of wheeze through age 3 years.62 Examining a high-risk birth cohort, Jackson and colleagues60 determined that wheezing due to RV was associated with a higher risk of developing asthma by age 6 than wheezing due to RSV. In infancy, wheezing due to RV and aeroallergen sensitization both independently increased asthma risk by age 6. However, by age 3, wheezing with RV was more strongly associated with asthma than sensitization. Wheezing due to RV in the first 3 years of life was associated with a nearly 10-fold increased risk of asthma at age 6. Nearly 90% of the cohort who wheezed with RV in the third year of life had asthma at age 6. They concluded that among early viral wheezing episodes, those precipitated by RV predict asthma most significantly.60
Ability to study the impact of viral infection on asthma pathogenesis is complicated by host as well as viral factors. Strong evidence suggests that allergic sensitization and viral infection interact synergistically in asthma pathogenesis. An association between wheezing RV/RSV illnesses in infancy and persistent wheeze at 5 years of age has been found and was most significant in those with comorbid allergic sensitization.63 Different strains of the same virus may induce different sequelae in different hosts. Genetic polymorphisms in antiviral innate immune genes, and confounding environmental exposures may impact viral susceptibility and complicate our understanding of the impact of viral infections on asthma development.64
Medications used for symptomatic treatment of viral illnesses may confound the link between viruses and asthma development. An observational study suggested a positive association between early life acetaminophen use and asthma development that could not be fully explained by confounding by indication for use.65 However, a double-blind RCT of 300 patients with asthma 12 to 59 months of age receiving exclusively acetaminophen or ibuprofen for fever or pain over the course of 48 weeks showed no significant differences in asthma exacerbations requiring glucocorticoids.66 Another RCT in New Zealand is studying whether the exclusive use of either paracetamol or ibuprofen in the first year of life is linked to asthma at age 6.59
Future Advances
Antiinflammatory and Immunobiological Therapies for Asthma Prevention
Abrogation of the inflammatory response to respiratory viruses is currently being studied. Azithromycin, an antibiotic with antiinflammatory properties, is being considered for prevention of recurrent wheeze and asthma. A proof-of-concept study found that infants hospitalized with bronchiolitis and treated with azithromycin had reduced wheezing in the following year.67
Omalizumab is also being studied as an immunomodulator in asthma prevention. Preschool is considered a “critical period” in the maturation of the immune and pulmonary systems. The Preventing Asthma in High Risk Kids study (NCT02570984) is underway to determine whether 2 years of omalizumab in predisposed preschoolers can prevent asthma development.10 Omalizumab binds IgE, inhibits engagement with high-affinity IgE receptor and downregulates its expression on mast cells, basophils, and plasmacytoid dendritic cells.68 It may inhibit the development of atopy, target multiple allergens simultaneously, and mitigate the impact of viral infections on asthma pathogenesis. In the Preventive Omalizumab or Step-up Therapy for Fall Exacerbation study, treatment with omalizumab decreased the risk and duration of RV infections and minimized viral shedding.69 It significantly decreased asthma-related symptom days, fall and spring exacerbations, hospitalizations, and need for inhaled corticosteroids.70 Mechanistically, blocking IgE may inhibit allergic sensitization and restore virus-induced interferon-alpha responses in plasmacytoid dendritic cells, which are often lacking in patients with asthma,71 and it may be effective in asthma prevention.70
Use of Biomarkers to Predict Development of Persistent Asthma
Biomarkers are being used to personalize asthma therapy and also to predict which patients with transient wheeze will develop persistent asthma. A study of wheezing toddlers concluded that the presence of exhaled volatile organic compounds improved asthma prediction at age 6 to 90% when combined with the asthma predictive index and gene expression markers.72 Another study combined an exacerbation clinical score with an assessment of serum circulating microRNAs and found that the combination predicted exacerbation more accurately than either parameter alone.73 Once understood more completely, biomarkers and genetic studies may be instrumental in predicting which toddlers are at highest risk of developing asthma and help investigators target preventative therapies to those most likely to benefit while minimizing risk of a potential intervention to children who are less likely to benefit.
Conclusions
Given the significant burden of asthma, effective prevention strategies are desperately needed. Early-life intervention may be instrumental in limiting initial deficits in lung function. Strategies aimed at stalling the atopic progression, modifying the microbiome, preventing respiratory viral infections, and reducing the impact of toxin/pollutant exposure through dietary supplements have had limited success in the prevention of asthma. Distinct endotypes and phenotypes may require different strategies for prevention. Asthma is heterogenous, influenced by both genetic and environmental factors. Genes underlie a predisposition to sensitization, whereas exposure to allergens, respiratory infections, and pollution may modify asthma severity. For example, allergic sensitization, particularly to HDM and mouse, combined with early-life viral infection increases the risk of developing asthma and its severity.74 Individual differences make finding a universally applicable prevention strategy challenging. Future advances in asthma prevention may include a more personalized approach. Understanding genetic variations among susceptible individuals and different biomarkers of disease may help individualize prevention strategies and improve efficacy. Agents such as omalizumab, which target multiple factors in the pathogenesis of asthma simultaneously, may prove effective in asthma prevention and are being studied in RCTs.10
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: W. P. has served as a consultant/advisor within the last 3 years for Novartis, Genentech, Regeneron Pharmaceuticals, GlaxoSmithKline, Teva Pharmaceutical Industries, Ltd, and AstraZeneca.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: Dr Maciag is funded under National Institutes of Health (NIH) grant T32AI007512. Dr Phipatanakul is funded under NIH grants K-24AI106822, U01AI110397, and U01AI126614.
References
- 1.Masoli M., Fabian D., Holt S., Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Gur M., Hakim F., Bentur L. Better understanding of childhood asthma, towards primary prevention: are we there yet? Consideration of pertinent literature. F1000Res. 2017;6:2152. doi: 10.12688/f1000research.11601.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGeachie M.J., Yates K.P., Zhou X. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374(19):1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhodes H.L., Sporik R., Thomas P., Holgate S.T., Cogswell J.J. Early life risk factors for adult asthma: a birth cohort study of subjects at risk. J Allergy Clin Immunol. 2001;108(5):720–725. doi: 10.1067/mai.2001.119151. [DOI] [PubMed] [Google Scholar]
- 5.Beigelman A., Bacharier L.B. Early-life respiratory infections and asthma development: role in disease pathogenesis and potential targets for disease prevention. Curr Opin Allergy Clin Immunol. 2016;16(2):172–178. doi: 10.1097/ACI.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joubert B.R., Felix J.F., Yousefi P. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98(4):680–696. doi: 10.1016/j.ajhg.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shorey-Kendrick L.E., McEvoy C.T., Ferguson B. Vitamin C prevents offspring DNA methylation changes associated with maternal smoking in pregnancy. Am J Respir Crit Care Med. 2017;196(6):745–755. doi: 10.1164/rccm.201610-2141OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C., Huang R., Yao R., Yang A. The immunotherapeutic role of bacterial lysates in a mouse model of asthma. Lung. 2017;195(5):563–569. doi: 10.1007/s00408-017-0003-8. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues A., Gualdi L.P., de Souza R.G. Bacterial extract (OM-85) with human-equivalent doses does not inhibit the development of asthma in a murine model. Allergol Immunopathol. 2016;44(6):504–511. doi: 10.1016/j.aller.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Preventing Asthma in High Risk Kids (PARK) https://clinicaltrials.gov/ct2/show/NCT02570984
- 11.Azithromycin to prevent wheezing following severe RSV bronchiolitis-II (APW-RSV-II) https://clinicaltrials.gov/ct2/show/NCT02911935 [DOI] [PMC free article] [PubMed]
- 12.Lukkarinen M., Koistinen A., Turunen R., Lehtinen P., Vuorinen T., Jartti T. Rhinovirus-induced first wheezing episode predicts atopic but not nonatopic asthma at school age. J Allergy Clin Immunol. 2017;140(4):988–995. doi: 10.1016/j.jaci.2016.12.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoltz D.J., Jackson D.J., Evans M.D. Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin Exp Allergy. 2013;43(2):233–241. doi: 10.1111/cea.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arshad S.H., Bateman B., Sadeghnejad A., Gant C., Matthews S.M. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol. 2007;119(2):307–313. doi: 10.1016/j.jaci.2006.12.621. [DOI] [PubMed] [Google Scholar]
- 15.Scott M., Roberts G., Kurukulaaratchy R.J., Matthews S., Nove A., Arshad S.H. Multifaceted allergen avoidance during infancy reduces asthma during childhood with the effect persisting until age 18 years. Thorax. 2012;67(12):1046–1051. doi: 10.1136/thoraxjnl-2012-202150. [DOI] [PubMed] [Google Scholar]
- 16.Bacharier L.B., Beigelman A., Calatroni A. Longitudinal phenotypes of respiratory health in a high-risk urban birth cohort. Am J Respir Crit Care Med. 2019;199(1):71–82. doi: 10.1164/rccm.201801-0190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullinan P., MacNeill S.J., Harris J.M. Early allergen exposure, skin prick responses, and atopic wheeze at age 5 in English children: a cohort study. Thorax. 2004;59(10):855–861. doi: 10.1136/thx.2003.019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks G.B., Mihrshahi S., Kemp A.S. Prevention of asthma during the first 5 years of life: a randomized controlled trial. J Allergy Clin Immunol. 2006;118(1):53–61. doi: 10.1016/j.jaci.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Belgrave D.C.M., Granell R., Turner S.W. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018;6(7):526–534. doi: 10.1016/S2213-2600(18)30099-7. [DOI] [PubMed] [Google Scholar]
- 20.Stokholm J., Chawes B.L., Vissing N., Bonnelykke K., Bisgaard H. Cat exposure in early life decreases asthma risk from the 17q21 high-risk variant. J Allergy Clin Immunol. 2018;141(5):1598–1606. doi: 10.1016/j.jaci.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 21.Munthe-Kaas M.C., Bertelsen R.J., Torjussen T.M. Pet keeping and tobacco exposure influence CD14 methylation in childhood. Pediatr Allergy Immunol. 2012;23(8):747–754. doi: 10.1111/pai.12021. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen L., Niggemann B., Dreborg S. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62(8):943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 23.Novembre E., Galli E., Landi F. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114(4):851–857. doi: 10.1016/j.jaci.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Zolkipli Z., Roberts G., Cornelius V. Randomized controlled trial of primary prevention of atopy using house dust mite allergen oral immunotherapy in early childhood. J Allergy Clin Immunol. 2015;136(6):1541–1547. doi: 10.1016/j.jaci.2015.04.045. [DOI] [PubMed] [Google Scholar]
- 25.Marogna M., Massolo A., Passalacqua G. Effect of adjuvanted and standard sublingual immunotherapy on respiratory function in pure rhinitis due to house dust mite over a 5-year period. World Allergy Org J. 2017;10(1):7. doi: 10.1186/s40413-016-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valovirta E., Petersen T.H., Piotrowska T. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. 2018;141(2):529–538. doi: 10.1016/j.jaci.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen L., Wahn U., Bilo M.B. Allergen-specific immunotherapy provides immediate, long-term and preventive clinical effects in children and adults: the effects of immunotherapy can be categorised by level of benefit -the centenary of allergen specific subcutaneous immunotherapy. Clin Transl Allergy. 2012;2:8. doi: 10.1186/2045-7022-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marogna M., Spadolini I., Massolo A., Canonica G.W., Passalacqua G. Long-lasting effects of sublingual immunotherapy according to its duration: a 15-year prospective study. J Allergy Clin Immunol. 2010;126(5):969–975. doi: 10.1016/j.jaci.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 29.Kristiansen M., Dhami S., Netuveli G. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28(1):18–29. doi: 10.1111/pai.12661. [DOI] [PubMed] [Google Scholar]
- 30.Wang A.L., Datta S., Weiss S.T., Tantisira K.G. Remission of persistent childhood asthma: early predictors of adult outcomes. J Allergy Clin Immunol. 2019;143(5):1752–1759. doi: 10.1016/j.jaci.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guilbert T.W., Morgan W.J., Zeiger R.S. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354(19):1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 32.Devulapalli C.S., Lodrup Carlsen K.C., Haland G. No evidence that early use of inhaled corticosteroids reduces current asthma at 10 years of age. Respir Med. 2007;101(8):1625–1632. doi: 10.1016/j.rmed.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Bisgaard H., Hermansen M.N., Loland L., Halkjaer L.B., Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354(19):1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 34.Holgate S.T. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242(1):205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 35.Turner S., Custovic A., Ghazal P. Pulmonary epithelial barrier and immunological functions at birth and in early life: key determinants of the development of asthma? A description of the protocol for the Breathing Together study. Wellcome Open Res. 2018;3:60. doi: 10.12688/wellcomeopenres.14489.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alshaarawy O., Anthony J.C. Month-wise estimates of tobacco smoking during pregnancy for the United States, 2002-2009. Maternal Child Health J. 2015;19(5):1010–1015. doi: 10.1007/s10995-014-1599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEvoy C.T., Schilling D., Clay N. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311(20):2074–2082. doi: 10.1001/jama.2014.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zosky G.R., Berry L.J., Elliot J.G., James A.L., Gorman S., Hart P.H. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med. 2011;183(10):1336–1343. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 39.Bolcas P.E., Brandt E.B., Zhang Z., Biagini Myers J.M., Ruff B.P., Khurana Hershey G.K. Vitamin D supplementation attenuates asthma development following traffic-related particulate matter exposure. J Allergy Clin Immunol. 2019;143(1):386–394. doi: 10.1016/j.jaci.2018.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litonjua A.A., Carey V.J., Laranjo N. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382(6):525–533. doi: 10.1056/NEJMoa1906137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H., Xun P., He K. Fish and fish oil intake in relation to risk of asthma: a systematic review and meta-analysis. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0080048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bisgaard H., Stokholm J., Chawes B.L. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530–2539. doi: 10.1056/NEJMoa1503734. [DOI] [PubMed] [Google Scholar]
- 43.Karvonen A.M., Kirjavainen P.V., Taubel M. Indoor bacterial microbiota and development of asthma by 10.5 years of age. J Allergy Clin Immunol. 2019;144(5):1402–1410. doi: 10.1016/j.jaci.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 44.Sender R., Fuchs S., Milo R. Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Levan S.R., Stamnes K.A., Lin D.L. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nature Microbiol. 2019;4(11):1851–1861. doi: 10.1038/s41564-019-0498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patrick D.M., Sbihi H., Dai D.L.Y. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30052-7. [Published online ahead of print March 24, 2020] [DOI] [PubMed] [Google Scholar]
- 47.Cuello-Garcia C.A., Brozek J.L., Fiocchi A. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136(4):952–961. doi: 10.1016/j.jaci.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 48.Cabana M.D., McKean M., Caughey A.B. Early probiotic supplementation for eczema and asthma prevention: a randomized controlled trial. Pediatrics. 2017;140(3) doi: 10.1542/peds.2016-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bisgaard H., Hermansen M.N., Buchvald F. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 50.Larsen J.M., Brix S., Thysen A.H., Birch S., Rasmussen M.A., Bisgaard H. Children with asthma by school age display aberrant immune responses to pathogenic airway bacteria as infants. J Allergy Clin Immunol. 2014;133(4):1008–1013. doi: 10.1016/j.jaci.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Yin J., Xu B., Zeng X., Shen K. Broncho-Vaxom in pediatric recurrent respiratory tract infections: a systematic review and meta-analysis. Int Immunopharmacol. 2018;54:198–209. doi: 10.1016/j.intimp.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 52.Sly P.D., Galbraith S., Islam Z., Holt B., Troy N., Holt P.G. Primary prevention of severe lower respiratory illnesses in at-risk infants using the immunomodulator OM-85. J Allergy Clin Immunol. 2019;144(3):870–872. doi: 10.1016/j.jaci.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 53.Oral Bacterial Extract for the prevention of wheezing lower respiratory tract illness (ORBEX) clinicaltrials.gov/ct2/show/NCT02148796
- 54.Regnier S.A., Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32(8):820–826. doi: 10.1097/INF.0b013e31829061e8. [DOI] [PubMed] [Google Scholar]
- 55.Scheltema N.M., Nibbelke E.E., Pouw J. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. Lancet Respir Med. 2018;6(4):257–264. doi: 10.1016/S2213-2600(18)30055-9. [DOI] [PubMed] [Google Scholar]
- 56.O’Brien K.L., Chandran A., Weatherholtz R. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis. 2015;15(12):1398–1408. doi: 10.1016/S1473-3099(15)00247-9. [DOI] [PubMed] [Google Scholar]
- 57.A study to determine the safety and efficacy of the rsv f vaccine to protect infants via maternal immunization. https://clinicaltrials.gov/ct2/show/NCT02624947
- 58.A study to evaluate the safety and efficacy of MEDI8897 for the prevention of medically attended RSV LRTI in healthy preterm infants. (MEDI8897 Ph2b) https://clinicaltrials.gov/ct2/show/NCT02878330
- 59.Randomised controlled trial of paracetamol or ibuprofen, as required for fever and pain in the first year of life, for prevention of asthma at age six years. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374403 [DOI] [PMC free article] [PubMed]
- 60.Jackson D.J., Gangnon R.E., Evans M.D. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jartti T., Gern J.E. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. 2017;140(4):895–906. doi: 10.1016/j.jaci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lemanske R.F., Jr., Jackson D.J., Gangnon R.E. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 63.Kusel M.M., de Klerk N.H., Kebadze T. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119(5):1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altman M.C., Beigelman A., Ciaccio C. Evolving concepts in how viruses impact asthma. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2019.12.904. [published online ahead of print January 9, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magnus M.C., Karlstad O., Haberg S.E., Nafstad P., Davey Smith G., Nystad W. Prenatal and infant paracetamol exposure and development of asthma: the Norwegian Mother and Child Cohort Study. Int J Epidemiol. 2016;45(2):512–522. doi: 10.1093/ije/dyv366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheehan W.J., Mauger D.T., Paul I.M. Acetaminophen versus ibuprofen in young children with mild persistent asthma. N Engl J Med. 2016;375(7):619–630. doi: 10.1056/NEJMoa1515990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beigelman A., Isaacson-Schmid M., Sajol G. Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135(5):1171–1178. doi: 10.1016/j.jaci.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cardet J.C., Casale T.B. New insights into the utility of omalizumab. J Allergy Clin Immunol. 2019;143(3):923–926. doi: 10.1016/j.jaci.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teach S.J., Gill M.A., Togias A. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–1485. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Busse W.W., Morgan W.J., Gergen P.J. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gill M.A., Liu A.H., Calatroni A. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. 2018;141(5):1735–1743. doi: 10.1016/j.jaci.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klaassen E.M., van de Kant K.D., Jobsis Q. Exhaled biomarkers and gene expression at preschool age improve asthma prediction at 6 years of age. Am J Respir Crit Care Med. 2015;191(2):201–207. doi: 10.1164/rccm.201408-1537OC. [DOI] [PubMed] [Google Scholar]
- 73.Kho A.T., McGeachie M.J., Moore K.G. Circulating microRNAs and prediction of asthma exacerbation in childhood asthma. Respir Res. 2018;19(1):128. doi: 10.1186/s12931-018-0828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kantor D.B., Stenquist N., McDonald M.C. Rhinovirus and serum IgE are associated with acute asthma exacerbation severity in children. J Allergy Clin Immunol. 2016;138(5):1467–1471. doi: 10.1016/j.jaci.2016.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]